Abstract
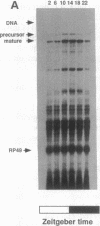
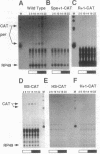
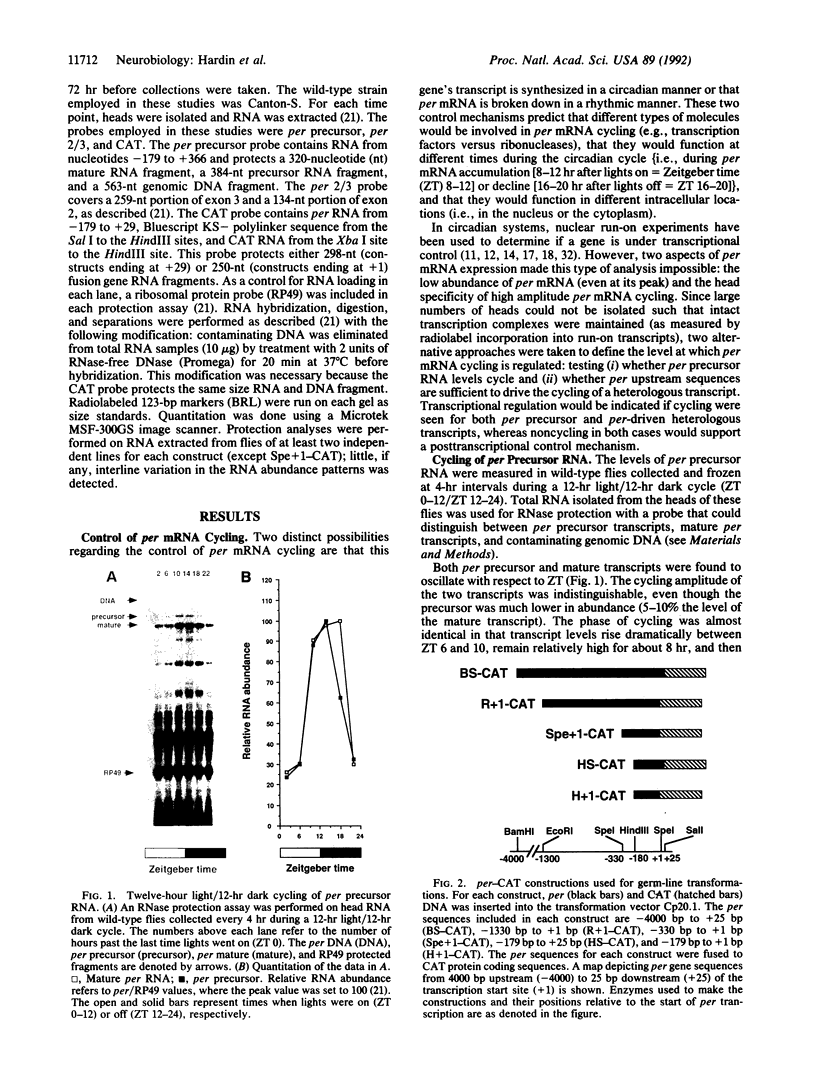
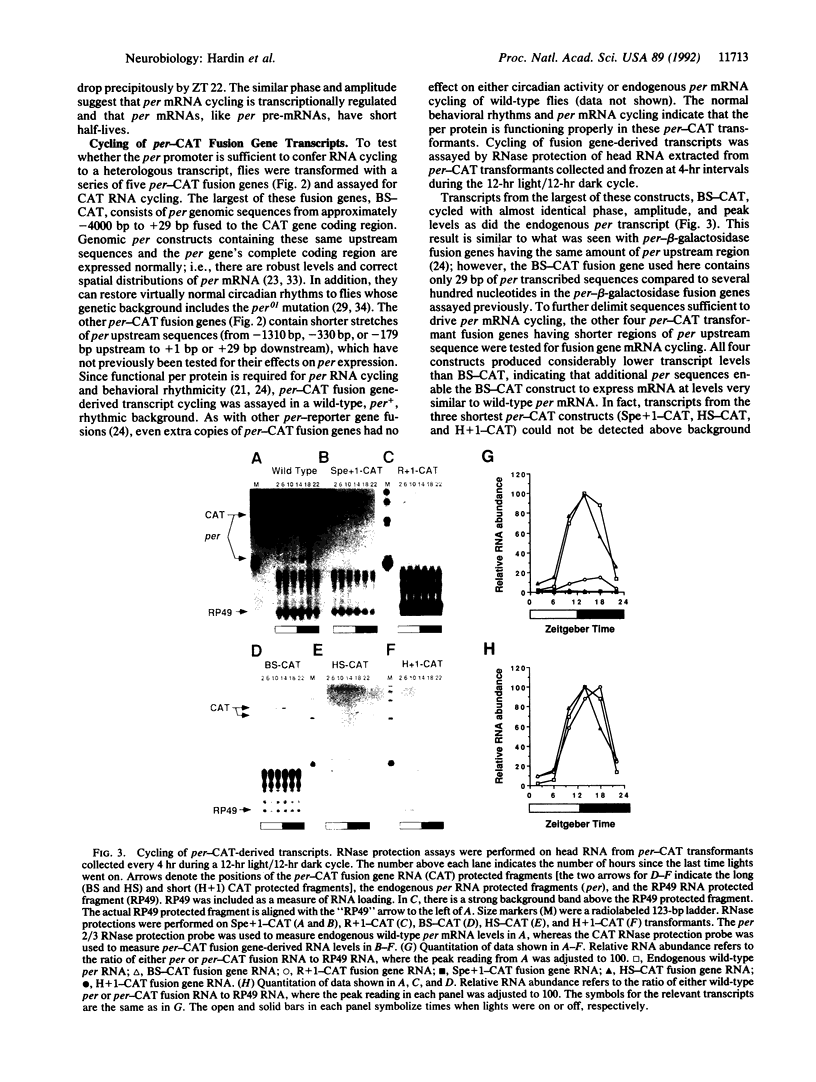
The period (per) gene is involved in regulating circadian rhythms in Drosophila melanogaster. The per gene is expressed in a circadian manner, where fluctuations in per mRNA abundance are influenced by its own translation product, which also cycles in abundance. Since per gene expression is necessary for circadian rhythmicity, we sought to determine how certain features of this feedback loop operate. The results of this study reveal that fluctuations in per mRNA are primarily controlled by fluctuations in per gene transcription, that per mRNA has a relatively short half-life, and that sequences sufficient to drive per mRNA cycling are present in 1.3 kilobases of 5' flanking sequences. These and other results indicate that the per feedback loop has all of the basic properties necessary to be a component of a circadian oscillator.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barberis A., Superti-Furga G., Busslinger M. Mutually exclusive interaction of the CCAAT-binding factor and of a displacement protein with overlapping sequences of a histone gene promoter. Cell. 1987 Jul 31;50(3):347–359. doi: 10.1016/0092-8674(87)90489-2. [DOI] [PubMed] [Google Scholar]
- Baylies M. K., Bargiello T. A., Jackson F. R., Young M. W. Changes in abundance or structure of the per gene product can alter periodicity of the Drosophila clock. 1987 Mar 26-Apr 1Nature. 326(6111):390–392. doi: 10.1038/326390a0. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Cohen L. V. Diurnal expression of transducin mRNA and translocation of transducin in rods of rat retina. Science. 1987 Jan 30;235(4788):585–587. doi: 10.1126/science.3101175. [DOI] [PubMed] [Google Scholar]
- Citri Y., Colot H. V., Jacquier A. C., Yu Q., Hall J. C., Baltimore D., Rosbash M. A family of unusually spliced biologically active transcripts encoded by a Drosophila clock gene. Nature. 1987 Mar 5;326(6108):42–47. doi: 10.1038/326042a0. [DOI] [PubMed] [Google Scholar]
- Crews S. T., Thomas J. B., Goodman C. S. The Drosophila single-minded gene encodes a nuclear protein with sequence similarity to the per gene product. Cell. 1988 Jan 15;52(1):143–151. doi: 10.1016/0092-8674(88)90538-7. [DOI] [PubMed] [Google Scholar]
- Ewer J., Frisch B., Hamblen-Coyle M. J., Rosbash M., Hall J. C. Expression of the period clock gene within different cell types in the brain of Drosophila adults and mosaic analysis of these cells' influence on circadian behavioral rhythms. J Neurosci. 1992 Sep;12(9):3321–3349. doi: 10.1523/JNEUROSCI.12-09-03321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewer J., Hamblen-Coyle M., Rosbash M., Hall J. C. Requirement for period gene expression in the adult and not during development for locomotor activity rhythms of imaginal Drosophila melanogaster. J Neurogenet. 1990 Nov;7(1):31–73. doi: 10.3109/01677069009084151. [DOI] [PubMed] [Google Scholar]
- Giuliano G., Hoffman N. E., Ko K., Scolnik P. A., Cashmore A. R. A light-entrained circadian clock controls transcription of several plant genes. EMBO J. 1988 Dec 1;7(12):3635–3642. doi: 10.1002/j.1460-2075.1988.tb03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbeter A. A minimal cascade model for the mitotic oscillator involving cyclin and cdc2 kinase. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9107–9111. doi: 10.1073/pnas.88.20.9107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblen M., Zehring W. A., Kyriacou C. P., Reddy P., Yu Q., Wheeler D. A., Zwiebel L. J., Konopka R. J., Rosbash M., Hall J. C. Germ-line transformation involving DNA from the period locus in Drosophila melanogaster: overlapping genomic fragments that restore circadian and ultradian rhythmicity to per0 and per- mutants. J Neurogenet. 1986 Sep;3(5):249–291. doi: 10.3109/01677068609106855. [DOI] [PubMed] [Google Scholar]
- Hardin P. E., Hall J. C., Rosbash M. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature. 1990 Feb 8;343(6258):536–540. doi: 10.1038/343536a0. [DOI] [PubMed] [Google Scholar]
- Hoffman E. C., Reyes H., Chu F. F., Sander F., Conley L. H., Brooks B. A., Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991 May 17;252(5008):954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Konopka R. J., Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. I., Fernald R. D. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989 Feb 2;337(6206):454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas P. L., Brody S., Coté G. G. Amplitude model for the effects of mutations and temperature on period and phase resetting of the Neurospora circadian oscillator. J Biol Rhythms. 1991 Winter;6(4):281–297. doi: 10.1177/074873049100600401. [DOI] [PubMed] [Google Scholar]
- Lausson S., Segond N., Milhaud G., Staub J. F. Circadian rhythms of calcitonin gene expression in the rat. J Endocrinol. 1989 Aug;122(2):527–534. doi: 10.1677/joe.0.1220527. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Staudt L., Robbins P., Kuang A., Mulligan R. C., Baltimore D. Repression of the IgH enhancer in teratocarcinoma cells associated with a novel octamer factor. Science. 1989 Jan 27;243(4890):544–546. doi: 10.1126/science.2536195. [DOI] [PubMed] [Google Scholar]
- Liu X., Lorenz L., Yu Q. N., Hall J. C., Rosbash M. Spatial and temporal expression of the period gene in Drosophila melanogaster. Genes Dev. 1988 Feb;2(2):228–238. doi: 10.1101/gad.2.2.228. [DOI] [PubMed] [Google Scholar]
- Liu X., Zwiebel L. J., Hinton D., Benzer S., Hall J. C., Rosbash M. The period gene encodes a predominantly nuclear protein in adult Drosophila. J Neurosci. 1992 Jul;12(7):2735–2744. doi: 10.1523/JNEUROSCI.12-07-02735.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loros J. J., Denome S. A., Dunlap J. C. Molecular cloning of genes under control of the circadian clock in Neurospora. Science. 1989 Jan 20;243(4889):385–388. doi: 10.1126/science.2563175. [DOI] [PubMed] [Google Scholar]
- Loros J. J., Dunlap J. C. Neurospora crassa clock-controlled genes are regulated at the level of transcription. Mol Cell Biol. 1991 Jan;11(1):558–563. doi: 10.1128/mcb.11.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambu J. R., Lewis J. O., Wharton K. A., Jr, Crews S. T. The Drosophila single-minded gene encodes a helix-loop-helix protein that acts as a master regulator of CNS midline development. Cell. 1991 Dec 20;67(6):1157–1167. doi: 10.1016/0092-8674(91)90292-7. [DOI] [PubMed] [Google Scholar]
- Piechulla B., Gruissem W. Diurnal mRNA fluctuations of nuclear and plastid genes in developing tomato fruits. EMBO J. 1987 Dec 1;6(12):3593–3599. doi: 10.1002/j.1460-2075.1987.tb02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju U., Koumenis C., Nunez-Regueiro M., Eskin A. Alteration of the phase and period of a circadian oscillator by a reversible transcription inhibitor. Science. 1991 Aug 9;253(5020):673–675. doi: 10.1126/science.1871602. [DOI] [PubMed] [Google Scholar]
- Redinbaugh M. G., Sabre M., Scandalios J. G. Expression of the maize Cat3 catalase gene is under the influence of a circadian rhythm. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6853–6857. doi: 10.1073/pnas.87.17.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosbash M., Hall J. C. The molecular biology of circadian rhythms. Neuron. 1989 Oct;3(4):387–398. doi: 10.1016/0896-6273(89)90199-2. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. Drosophila melanogaster as an experimental organism. Science. 1988 Jun 10;240(4858):1453–1459. doi: 10.1126/science.3131880. [DOI] [PubMed] [Google Scholar]
- Siwicki K. K., Eastman C., Petersen G., Rosbash M., Hall J. C. Antibodies to the period gene product of Drosophila reveal diverse tissue distribution and rhythmic changes in the visual system. Neuron. 1988 Apr;1(2):141–150. doi: 10.1016/0896-6273(88)90198-5. [DOI] [PubMed] [Google Scholar]
- Spiller S. C., Kaufman L. S., Thompson W. F., Briggs W. R. Specific mRNA and rRNA Levels in Greening Pea Leaves during Recovery from Iron Stress. Plant Physiol. 1987 Jun;84(2):409–414. doi: 10.1104/pp.84.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. C. Transcriptional regulation by a circadian rhythm. Plant Cell. 1989 Feb;1(2):259–264. doi: 10.1105/tpc.1.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl G. R., Reppert S. M. Suprachiasmatic nucleus vasopressin messenger RNA: circadian variation in normal and Brattleboro rats. Science. 1986 Apr 18;232(4748):390–393. doi: 10.1126/science.3961487. [DOI] [PubMed] [Google Scholar]
- Van Doren M., Ellis H. M., Posakony J. W. The Drosophila extramacrochaetae protein antagonizes sequence-specific DNA binding by daughterless/achaete-scute protein complexes. Development. 1991 Sep;113(1):245–255. doi: 10.1242/dev.113.1.245. [DOI] [PubMed] [Google Scholar]
- Wuarin J., Schibler U. Expression of the liver-enriched transcriptional activator protein DBP follows a stringent circadian rhythm. Cell. 1990 Dec 21;63(6):1257–1266. doi: 10.1016/0092-8674(90)90421-a. [DOI] [PubMed] [Google Scholar]
- Yu Q., Jacquier A. C., Citri Y., Hamblen M., Hall J. C., Rosbash M. Molecular mapping of point mutations in the period gene that stop or speed up biological clocks in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1987 Feb;84(3):784–788. doi: 10.1073/pnas.84.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehring W. A., Wheeler D. A., Reddy P., Konopka R. J., Kyriacou C. P., Rosbash M., Hall J. C. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 1984 Dec;39(2 Pt 1):369–376. doi: 10.1016/0092-8674(84)90015-1. [DOI] [PubMed] [Google Scholar]
- Zerr D. M., Hall J. C., Rosbash M., Siwicki K. K. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J Neurosci. 1990 Aug;10(8):2749–2762. doi: 10.1523/JNEUROSCI.10-08-02749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiebel L. J., Hardin P. E., Liu X., Hall J. C., Rosbash M. A post-transcriptional mechanism contributes to circadian cycling of a per-beta-galactosidase fusion protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3882–3886. doi: 10.1073/pnas.88.9.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]