Abstract
We report the clinical findings, epidemiology, and risk factors for moderate-to-severe diarrhea (MSD) associated with Aeromonas species in children 0–59 months of age, from the Global Enteric Multicenter Study, conducted at three sites in south Asia and four sites in sub-Saharan Africa. Children with MSD were enrolled along with controls matched for age, gender, and neighborhood. Pooled, age-stratified conditional logistic regression models were applied to evaluate the association of Aeromonas infection controlling for coinfecting pathogens and sociodemographic variables. A pooled, age-stratified, multivariate logistic regression analysis was done to identify risk factors associated with Aeromonas positivity in MSD cases. A total of 12,110 cases and 17,291 matched controls were enrolled over a period of 48 months. Aeromonas was identified as a significant pathogen in 736 cases of MSD in Pakistan and Bangladesh (22.2%). Aeromonas remained a significant pathogen even after adjustment for the presence of other pathogens and sociodemographic factors. Odds ratio (OR) for Aeromonas were higher in the presence of Shigella (matched OR: 6.2, 95% confidence interval [CI]: 1.9–20.2). Cases of Aeromonas were likely to present with dysentery, particularly in the 0–11 months (OR: 1.4, 95% CI 1.0–2.0) and 12–23 months (OR: 1.8, 95% CI: 1.3–2.5) age group. The odds of Aeromonas increased with increasing degree of stunting, being highest for severe stunting (OR: 10.1, 95% CI: 3.6–28.9). Aeromonas is a significant pathogen for MSD in Pakistan and Bangladesh. Presence of dysentery and co-occurrence with other pathogens, notably Shigella spp. are significant features of Aeromonas-associated diarrhea.
Introduction
Aeromonas species are motile, gram-negative facultative anaerobic bacilli that cause gastroenteritis. They are widespread in nature, found in soil as well as aquatic environments. They are emerging as human pathogens, and are commonly isolated from diarrheal specimens in tropical countries.1 Aeromonas was first identified in human feces in 1961.2 Some case–control studies have demonstrated an epidemiologic association between diarrhea in young infants and children3,4 while others have not.5 Methodological issues (e.g. short period of surveillance, controls not matched for age or season), asymptomatic shedding, and failure to consider differences in pathogenicity according to genospecies of known virulence factors may have contributed to variation in results. In various case–control studies, Aeromonas has been identified as a pathogen in 1–88% of children with diarrhea and in 0–45% of controls.6 The reported rates differ depending on the use of culture methods, species identification methods, and inclusion or exclusion of other diarrheal co-pathogens.1 There is also a wide geographical variation in the reported frequency of isolation and association with diarrhea.6
In most cases of gastroenteritis, Aeromonas is a co-pathogen, isolated along with other enteric pathogens.7 Most (90%) cases of acute Aeromonas-associated diarrhea (AAD) are transient and self-limiting, but dysentery and severe cholera-like diarrhea due to Aeromonas have been described and may require treatment with antibiotics.6 Despite these anecdotal descriptions, Aeromonas pathogenicity and virulence still remains in question.8
There is a need for well-designed epidemiological studies. AAD was described recently by the Global Enteric Multicenter Study (GEMS), which was designed to measure burden, microbial etiology, and nutritional effects of moderate-to-severe diarrhea (MSD) in developing countries.9 Attributable fractions (AFs) for enteropathogens were derived from the first 36 months of GEMS data collection. Aeromonas spp. were a major pathogen for diarrhea in Pakistan and Bangladesh with the highest AF among children of 24–59 months of age, where the AF for AAD in Bangladesh was 18.3 (95% confidence interval [CI]: 10.8–25.9) and for Pakistan it was 24.1 (95% CI: 16.0–32.3).10 We present the distribution of Aeromonas among cases and controls in children 0–59 months of age in all seven GEMS sites over 48 months and odds ratios (ORs) for Aeromonas MSD adjusted for other pathogens and socioeconomic factors. We also describe clinical and sociodemographic predictors for AAD among MSD cases.
Methods
Primary study.
The GEMS was an age-stratified, case–control study. Three age strata of 0–11, 12–23, and 24–59 months were studied. Each of seven sites in Asia and sub-Saharan Africa (Bangladesh, India, Pakistan, Mozambique, Mali, The Gambia, and Kenya) included one to 11 community Sentinel Health Centers serving censused populations. Children were defined as having diarrhea if they fit the World Health Organization definition of > 3 loose stools per day, and were included as cases if diarrhea represented a new episode (no episodes in preceding 7 days) and was moderate to severe (with any one of the following: sunken eyes, loss of skin turgor, dysentery, need for intravenous rehydration, or hospitalization). For each case of MSD, —one to three matched community controls were randomly selected from the censused population at each site. Controls were children with no episode of diarrhea in the 7 days preceding enrollment. In addition, they were selected on the basis of the following matching criteria: age + 2 months for age group 0–11 months, +4 months for age group 12–59 months, and not exceeding stratum boundaries for age group of case, same gender, residence in same catchment area, and enrollment within 14 days of that of corresponding case. Further details regarding clinical and epidemiological design of the GEMS and its Health Care Utilization and Attitudes Surveys have been described by Kotloff and others.9
GEMS was carried out for a total of 48 months, initially from 2009 to 2011 and for an additional 12 months in 2012.
Microbiological methods for detection of Aeromonas species.
In GEMS, an extensive workup for a wide array of putative and established viral, bacterial, and protozoal diarrheal pathogens was undertaken on each case and control specimen at the local site laboratories, as described by Panchalingam and others.11 These laboratories followed standard protocols, which were ensured by site supervisors and site visits by study personnel. Rigorous quality control and quality assurance procedures were implemented to ensure robustness of procedures.
In brief, stool specimens for detection of Aeromonas were collected at the field sites in Cary Blair tubes, plated on Ryan medium, and incubated aerobically at 35–37°C for 48 hours. After 48 hours, all dark green colonies with darker centers on Ryan medium were characterized further by testing for presence of oxidase, catalase, salt tolerance, and O129 susceptibility. Oxidase-positive, catalase-producing, salt-intolerant, and O129-resistant colonies were identified as Aeromonas spp. Antimicrobial susceptibility tests were not performed.
Box 1. Lessons from the GEMS Aeromonas experience.

Statistical analysis.
Operational definitions.
For the purpose of statistical analysis, Aeromonas positivity among cases was defined either as AAD or Aeromonas-only diarrhea (AOD).
AAD: All MSD cases where Aeromonas was isolated from stool cultures, regardless of the co-occurrence of other pathogens.
AOD: All MSD cases where Aeromonas was isolated as a sole pathogen.
For the parent study, a matched case–control analysis using conditional logistic regression (CLR) modeling and MSD as outcome was carried out to identify pathogens associated with MSD across the seven sites and three age strata. Adjusted ORs (AORs) were used to calculate the AF of each pathogen identified as a risk factor. These have been reported elsewhere.10 Statistical analytic methods used in the parent study have been described by Blackwelder and others.12
Matched case–control analysis.
To evaluate the association of Aeromonas stool positivity with MSD, and to determine how this association is affected by other factors, we performed an age-stratified CLR analysis for Bangladesh and Pakistan. Matched AORs were calculated for Aeromonas. Three models were built stratified by age groups with Aeromonas as the main exposure variable. Model 1 was adjusted for pathogens, Model 2 was adjusted for socioeconomic variables, and Model 3 was adjusted for both pathogens and sociodemographic variables. All covariates that occurred greater than five times and had P < 0.10 in CLR model for individual covariate were put in the multivariable model. A backward elimination process was used to derive a parsimonious model with all variables having a P value ≤ 0.05. All variables that co-occurred with Aeromonas ≥ 10 times in cases or controls were tested for interaction. All interaction terms with a P value of ≤ 0.05 were retained in the model.
Within-case logistic regression analysis.
To determine clinical, microbiological, and environmental predictors of AAD, we performed a within-case logistic regression analysis for Aeromonas positivity among MSD cases stratified by age. We also pooled MSD cases from Bangladesh and Pakistan within their respective age strata. All variables with a P value ≤ 0.1 in the univariate analysis were used to build a multivariable model. A backward selection procedure was used to derive a parsimonious model for each age stratum, retaining only variables significant at P value ≤ 0.05.
Ethical approval.
The parent study was approved by the Ethical Review Committee of all sites and the Institutional Review Board of the University of Maryland, Baltimore, MD. Signed and written informed consents were collected from all participants. The parent or primary caretaker received a copy of the signed consent form to keep, and the original was stored in the regulatory files at the study site. Data were stored anonymously to ensure confidentiality.
Results
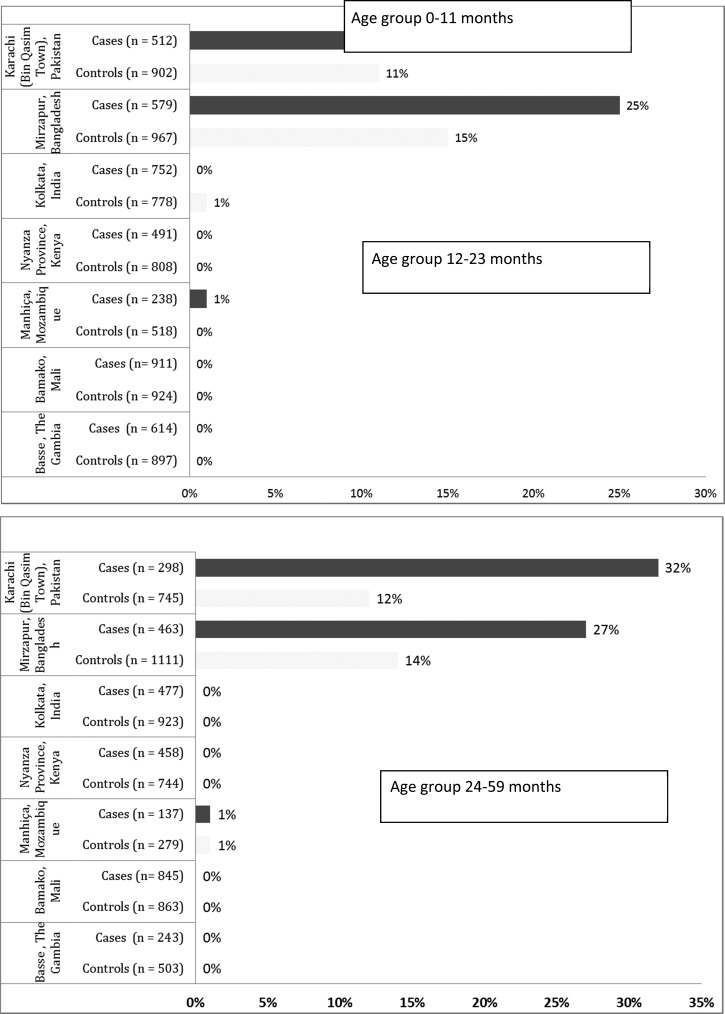
A total of 12,110 children with MSD plus 17,291 matched controls without diarrhea were enrolled at seven sites over a period of 60 months. Figure 1 describes the distribution of Aeromonas spp. in stool samples of cases and controls across all sites, stratified by age group. Aeromonas spp. were recovered from stool cultures in > 1% of cases and controls from Pakistan and Bangladesh sites only.1 Results of the matched case–control analysis from all seven GEMS sites are presented elsewhere.12
Figure 1.
Distribution of Aeromonas among cases with moderate to severe diarrhea and their matched controls across seven sites and three age strata.
During the study 1,460, 1,091, and 761 MSD cases in age groups of 0–11, 12–23, and 24–59 months, respectively, were enrolled in both Pakistan and Bangladesh combined. Stool culture positivity rate for Aeromonas spp. among diarrheal specimens from cases was 18.7% (N = 273) in the 0–11 months age group, 22.5% (N = 245) in the 12–23 months age group, and 28.6% (N = 218) in the 24–59 months age group in Pakistan and Bangladesh. Aeromonas spp. were isolated as a sole pathogen from only 2.3% (N = 35) of cases aged 0–11 months, 1.8% (N = 20) of cases aged 12–23 months, and 4.2% (N = 32) of cases aged 24–59 months. Of these AOD cases, 65.7% (N = 23), 80% (N = 16), and 59.4% (N = 19) presented with blood in their stools from among the 0–11, 12–23, and 24–59 months age strata. Clinical features of AOD compared with MSD due to all other pathogens are provided in Supplemental Appendix, Supplemental Tables 1 and 2. Diarrheal stool specimens from cases were negative for all enteropathogens in 9.1% (N = 133) of 0–11 months of age group, 4.5% (N = 50) of 12–23 months age group, and 6.0% (N = 46) of 24–59 months age group.
Association of Aeromonas with MSD and effect of pathogens and SE factors.
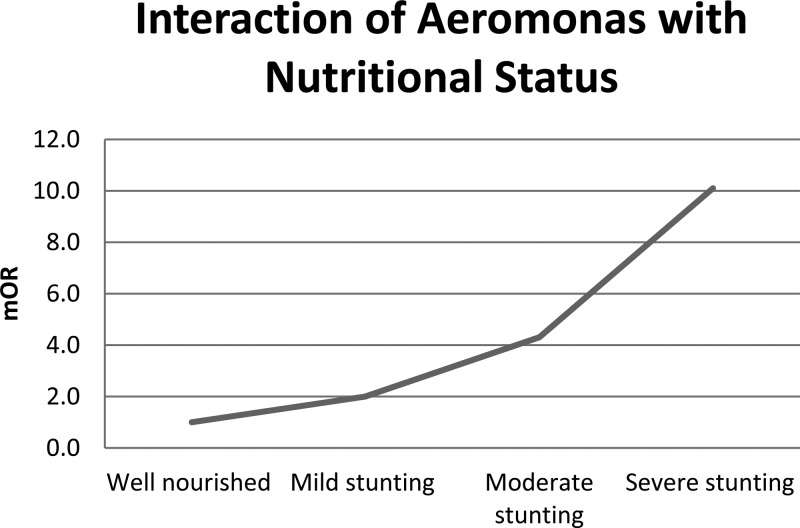
Table 1 presents matched ORs (mORs)for AAD derived from four separate models. After adjusting for other enteropathogens and socioeconomic factors, Aeromonas remained a significant pathogen across all age strata in both sites as well as in the pooled analysis. In the model adjusted for both other pathogens and sociodemographic variables (Model 3), Aeromonas had an interaction with Shigella species driven by the Pakistan site in age group 2. The mORs for Aeromonas were higher in the presence of Shigella (mOR: 1.4, 95% CI: 0.9–2.2 versus mOR: 8.0, 95% CI: 1.5–43.8) in Pakistan. This interaction was also seen when data from Bangladesh and Pakistan were pooled. In age group 3, there was an interaction with nutritional status at Pakistan site, mOR for Aeromonas increased with increasing degree of stunting (Figure 2 ).
Table 1.
Site-specific and pooled (both Pakistan and Bangladesh) mAORs for association of MSD with Aeromonas adjusted for pathogens and socioeconomic variables
| Bangladesh | Pakistan | Pooled | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Model 0 | ||||||
| 0–11 | 2.0 (1.5–2.6) | < 0.001 | 2.1 (1.5–2.9) | < 0.001 | 2.0 (1.7–2.5) | < 0.001 |
| 12–23 | 2.0 (1.5–2.7) | < 0.001 | 2.2 (1.6–3.0) | < 0.001 | 2.1 (1.7–2.6) | < 0.001 |
| 24–59 | 2.4 (1.8–3.2) | < 0.001 | 3.6 (2.5–5.0) | < 0.001 | 2.8 (2.3–3.5) | < 0.001 |
| Model 1 | ||||||
| 0–11 | 1.7 (1.3–2.4) | 0.001 | 2.3 (1.5–3.4) | < 0.001 | 1.9 (1.5–2.5) | < 0.001 |
| 12–23 | 1.9 (1.2–3.0) | 0.005 | 2.0 (1.4–2.9) | < 0.001 | 2.0 (1.5–2.6) | |
| Aeromonas × Shigella | Aeromonas × Shigella | |||||
| Shigella negative, 1.8 (1.2–2.6) | 0.002 | Shigella negative, 1.8 (1.4–2.4) | < 0.001 | |||
| Shigella positive, 9.6 (1.8–51.3) | 0.008 | Shigella positive, 5.8 (1.9–18.1) | 0.003 | |||
| 24–59 | 2.4 (1.5–3.9) | < 0.001 | 3.9 (2.6–5.9) | < 0.001 | 3.3 (2.4–4.5) | < 0.001 |
| Aeromonas × site | ||||||
| Bangladesh, 2.3 (1.5–3.5) | < 0.001 | |||||
| Pakistan, 4.8 (3.0–7.5) | < 0.001 | |||||
| Model 2 | ||||||
| 0–11 | 1.9 (1.5–2.5) | < 0.001 | 2.2 (1.6–3.0) | < 0.001 | 2.0 (1.6–2.5) | < 0.001 |
| Aeromonas × age | ||||||
| ≤ 6 month, 3.8 (1.7–8.2) | < 0.001 | |||||
| > 6 months, 1.1 (0.6–2.3) | 0.706 | |||||
| 12–23 | 2.1 (1.6–2.8) | < 0.001 | 1.8 (1.3–2.6) | 0.001 | 2.0 (1.6–2.6) | < 0.001 |
| 24–59 | 2.5 (1.8–3.4) | < 0.001 | 3.6 (2.3–5.4) | 0.001 | 2.7 (2.1–3.5) | < 0.001 |
| Aeromonas × HAZ | ||||||
| Well-nourished, 1.3 (0.3–4.8) | 0.705 | |||||
| Mild stunting, 1.9 (0.8–4.4) | 0.128 | |||||
| Mod stunting, 3.6 (1.8–7.0) | < 0.001 | |||||
| Severe stunting, 11.8 (4.4–32.0) | < 0.001 | |||||
| Model 3 | ||||||
| 0–11 | 1.8 (1.3–2.4) | 0.001 | 2.3 (1.6–3.5) | < 0.001 | 1.9 (1.4–2.5) | < 0.001 |
| 12–23 | 2.0 (1.2–3.1) | 0.003 | 1.7 (1.1–2.5) | 0.013 | 1.8 (1.3–2.5) | < 0.001 |
| Aeromonas × Shigella | Aeromonas × Shigella | |||||
| Shigella negative, 1.4 (0.9–2.2) | 0.097 | Shigella negative, 1.6 (1.2–2.3) | 0.002 | |||
| Shigella positive, 8.0 (1.5–43.8) | 0.016 | Shigella positive, 6.2 (1.9–20.2) | 0.002 | |||
| 24–59 | 2.3 (1.4–3.9) | 0.002 | 3.7 (2.3–6.0) | < 0.001 | 3.0 (2.1–4.2) | < 0.001 |
| Aeromonas × HAZ | Aeromonas × site | |||||
| Well nourished, 1.0 (0.3–4.2) | 0.950 | Bangladesh, 2.2 (1.3–3.5) | 0.002 | |||
| Mild stunting, 2.0 (0.8–5.3) | 0.141 | Pakistan, 4.0 (2.5–6.6) | < 0.001 | |||
| Mod stunting, 4.3 (2.1–9.0) | < 0.001 | |||||
| Severe stunting, 10.1 (3.6–28.9) | < 0.001 | |||||
CI = confidence interval; ETEC = enterotoxigenic E. coli; EHEC = enterohaemorrhagic E. coli; EAEC = entero-adherent E. coli; EPEC = enteropathogenic E. coli; HAZ = height-for-age Z score; mAORs = matched adjusted odds ratios; MSD = moderate-to-severe diarrhea; OR = odds ratio. Model 0 represents unadjusted analysis. Model 1 is adjusted for Shigella, Salmonella, Campylobacter, ETEC, EHEC, EAEC, EPEC, Adenovirus, Vibrio cholera, Cryptosporidium, Giardia, Entameoba histolytica, Norovirus GI, Norovirus GII, Astrovirus, Sapovirus, and Rotavirus (cumulative list of pathogens that were adjusted across all models). Model 2 is adjusted for sociodemographic variables (wealth quintiles, water supply, treatment of drinking water, type of flooring, number of children under 5 years in household, breastfeeding, HAZ, animals in household). Model 3 is adjusted for pathogens and sociodemographic variables.
Figure 2.
Interaction of moderate-to-severe diarrhea due to Aeromonas with nutritional status (height-for-age Z score) in children aged 24–59 months in Pakistan.
In age group 3, mOR for Aeromonas was higher in Pakistan than in Bangladesh (mOR: 3.7, 95% CI: 2.3–6.0 versus mOR: 2.3, 95% CI: 1.4–3.9). This also manifested as an interaction with site in the pooled analysis.
Clinical, microbiological, and environmental predictors of AAD.
Table 2 presents results for the multivariate analysis for predictors of AAD, pooled for Bangladesh and Pakistan. Site-specific analysis is presented as a Supplemental Appendix, Supplemental Tables 1 and 2. In the 0–11 months age group, AAD cases were more likely to be not breastfed (OR: 0.4, 95% CI: 0.3–0.6), present with dysentery (OR: 1.4, 95% CI: 1.0–2.0) and abdominal pain (OR: 1.5, 95% CI: 1.1–2.1), more likely to have received home-based fluid therapy (OR: 2.6, 95% CI: 1.1–6.0), and more likely to co-occur with nontyphoidal Salmonella (NTS) (OR: 2.1, 95% CI: 1.1–4.0). In 12–23 months age group, AAD cases were again more likely to be not breastfed (OR: 0.6, 95% CI: 0.4–0.9) and more likely to present with dysentery (OR: 1.8, 95% CI: 1.3–2.5). There were no significant predictors identified in the third age group.
Table 2.
Multivariate model of predictors of Aeromonas positivity among MSD cases in Bangladesh and Pakistan
| 0–11 months cases (N = 1,460) | 12–23 months cases (N = 1,091) | 24–59 months cases (N = 761) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
| Model 1 | ||||||||||||
| Shigella species | 1.6 | 1.1 | 2.3 | 0.023 | 1.5 | 1.1 | 2.0 | 0.010 | – | – | – | – |
| NTS | 2.87 | 1.62 | 5.09 | < 0.001 | – | – | – | – | – | – | – | – |
| Model 2 | ||||||||||||
| Breastfed | 0.4 | 0.3 | 0.6 | < 0.001 | 0.6 | 0.4 | 0.9 | 0.008 | – | – | – | – |
| Dysentery | 1.5 | 1.1 | 2.1 | 0.022 | 2.1 | 1.5 | 2.9 | < 0.001 | – | – | – | – |
| Abdominal pain | 1.5 | 1.1 | 2.1 | 0.019 | – | – | – | – | – | – | – | – |
| Rehydrated with homemade fluid | 2.8 | 1.2 | 6.4 | 0.016 | – | – | – | – | – | – | – | – |
| Model 3 | ||||||||||||
| NTS | 2.1 | 1.1 | 4.0 | 0.026 | – | – | – | – | – | – | – | – |
| Breastfed | 0.4 | 0.3 | 0.6 | < 0.001 | 0.6 | 0.4 | 0.9 | 0.024 | – | – | – | – |
| Dysentery | 1.4 | 1.0 | 2.0 | 0.04 | 1.8 | 1.3 | 2.5 | 0.001 | – | – | – | – |
| Abdominal pain | 1.5 | 1.1 | 2.1 | 0.026 | – | – | – | – | – | – | – | – |
| Rehydrated with homemade fluid | 2.6 | 1.1 | 6.0 | 0.025 | – | – | – | – | – | – | – | – |
CI = confidence interval; NTS = nontyphoidal Salmonella; OR = odds ratio.
Speciation of Aeromonas was performed by the Sanger Institute, and 131 Aeromonas isolates were sequenced. Preliminary data show that they belong to the following species: Aeromonas caviae (42 cases and 42 controls), Aeromonas dhakensis (7 cases and 3 controls), Aeromonas veronii (11 cases and 17 controls), Aeromonas enteropelogenes (4 cases and 2 controls), and Aeromonas sanarelli (1 case and 0 control). The sequence of one isolate did not match any of the 141 references present in the databases.
Discussion
Aeromonas was significantly associated with MSD among children less than 5 years of age at two Asian sites: Karachi, Pakistan, and Mirzapur, Bangladesh. Aeromonas was significantly associated with MSD in all age groups and after controlling for the presence of co-pathogens and for sociodemographic variables that might increase the risk of MSD. Our findings further illustrate a remarkable geographic variation in the importance of Aeromonas as a diarrheal pathogen. While Aeromonas was identified in < 1% of cases and controls in all age strata from the African sites and Kolkata, India, it was identified in 18.7% of infants, 22.5% of toddlers 12–23 months of age, and 28.6% of children 24–59 months of age. The association was seen at the two sites individually and when the data were pooled, all with a trend toward increasing frequency with age.
Past studies evaluating whether Aeromonas is significantly associated with childhood diarrhea have yielded conflicting results. Some case–control studies have demonstrated an epidemiologic association with diarrhea in infants and children3 while others have not.5,13 Factors that may have contributed to study-to-study variation include methodological issues (e.g. short period of surveillance, failure to account for differences in cases and controls in factors such as age or season), carriage rates in asymptomatic children that were similar to diarrhea patients in some settings, geographic variability in disease incidence, and failure to account for differences in pathogenicity according to genospecies or known virulence factors. In addition, bias may be introduced when the impact of coinfection with other pathogens is not considered. Finally, there may be host factors that influence the pathogenicity of Aeromonas in various settings, such as genetic predilection, the prevalence of underlying stunting, a risk factor in our study, or high environmental exposure due to predisposing factors such as contamination of drinking water. GEMS overcame many of these issues by using standardized methods in a 36-month study conducted at seven sites in Africa and Asia and performing analyses that controlled for the presence of co-pathogens and evaluated associations in episodes from which Aeromonas was the only pathogen identified. The absence of these confounding factors strengthens our epidemiologic observation that Aeromonas is significantly associated with watery diarrhea and dysentery among children under 5 years of age in Bangladesh and Pakistan.
Of the several risk factors for Aeromonas evaluated in GEMS, we were able to identify lack of breastfeeding as a significant risk factor in pooled analysis from Pakistan and Bangladesh. Breast milk contains factors such as secretory IgA and oligosaccharides that prevent both pathogen-specific (e.g., Shigella and rotavirus) and nonspecific diarrheal illness. Moreover, reliance of breastfeeding may prevent exposure to contaminated food and water. A lack of breastfeeding necessitates feeding with either animal-source milk, which is diluted with water before being fed to babies, or other food prepared with water.
An interesting feature of AAD is the occurrence of co-pathogens. In the present study, NTS and Shigella spp. showed a significant association with AAD particularly in the younger age groups. In some studies Aeromonas is commonly part of a polymicrobial infection, which raises the question of whether it is behaving as a nonpathogenic “co-traveler” perhaps acquired from the same source as the true pathogen, or is interacting with other agents to cause disease. Pathogens commonly reported in literature to coexist with Aeromonas are rotavirus, Vibrio spp., Shigella, Campylobacter, and Escherichia coli O157:H7.4,13–15
Overall, Aeromonas comprised 22.7% of episodes of watery diarrhea and 26.3% of episodes of dysentery at these two sites. The proportion of cases manifesting as bloody diarrhea increased with age, from 42.4% of infants to ∼754.6% of those 1 year or older. However, Aeromonas was associated with dysentery only in the lower two age strata. This association between Aeromonas spp. and dysentery has been observed by others.16,17 Lending biological plausibility to the relationship between Aeromonas and dysentery is the observation that Aeromonas isolates from two patients with dysentery were invasive and able to replicate intracellularly in HEp-2 cell assays.18 Moreover, a cytotoxic enterotoxin Act from a human Aeromonas hydrophila isolate causes epithelial destruction in mouse ligated intestinal loops19 and elicits inflammatory responses, features that might produce dysentery in humans.
There are several limitations of this study. It is possible that there were coinfecting organisms such as Shigella that were not cultivated but were responsible for some proportion of the diarrheal symptoms observed. Further investigations using molecular diagnostics in children with MSD and their controls may shed additional light on this issue. The parent study was designed to detect pathogens for MSD and not powered for risk factors for individual pathogens. Second, although we know from reviewing the literature that A. hydrophila, Aeromonas sobria, and A. caviae are the species most frequently implicated in diseases of humans, we did not perform speciation of Aeromonas. Although this is unlikely to affect the results of the study, speciation may help to differentiate environmental from pathogenic strains.
Conclusions
Aeromonas is significantly associated with MSD, causing both watery diarrhea and dysentery among children under 5 years of age in Pakistan and Bangladesh but not in other geographic areas in Asia and sub-Saharan Africa, demonstrating its considerable geographic variability. Risk factors for AAD are similar to those of other bacterial pathogens. Presence of dysentery and co-occurrence with Shigella are significant features of AAD.
In the face of continued resource constraints and poor policy making, low-cost, “enabling” measures such as organized health education at community level to promote breastfeeding and standardized home-based water purification can be effective in AAD prevention. Furthermore, large community-based, pathogen-specific studies are important to understand the epidemiology and distribution of AAD to enable development of effective programs for disease prevention.
Supplementary Material
Footnotes
Financial support: The parent study (GEMS) was funded by the BMG foundation.
Disclosure: Farah Naz Qamar received research training support from the National Institute of Health's Fogarty International Center (1 D43 TW007585-01).
Authors' addresses: Farah Naz Qamar, Muhammad Imran Nisar, Farheen Quadri, Sadia Shakoor, Shahida Qureshi, and Anita K. M. Zaidi, Department of Paediatrics and Child Health, Aga Khan University, Karachi, Pakistan, E-mails: farah.qamar@aku.edu, imran.nisar@aku.edu, farheen.quadri@aku.edu, sadia.shakoor@aku.edu, shahida.qureshi@aku.edu, and anita.zaidi@aku.edu. Samba O. Sow, Center for Vaccine Development, Ministry of Health, Bamako, Mali, E-mail: ssow@medicine.umaryland.edu. Dilruba Nasrin, Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, E-mail: dnasrin@medicine.umaryland.edu. William C. Blackwelder, Yukun Wu, Sandra Panchalingham, Sharon M. Tennant, Karen L. Kotloff, and Myron M. Levine, Center for Vaccine Development, University of Maryland School of Medicine, Baltimore, MD, E-mails: wblackwe@medicine.umaryland.edu, statswu@gmail.com, spanchal@medicine.umaryland.edu, stennant@medicine.umaryland.edu, kkotloff@medicine.umaryland.edu, and mlevine@medicine.umaryland.edu. Tamer Farag, Vaccine Development and Surveillance, Bill & Melinda Gates Foundation, Seattle, WA, E-mail: tamer.farag@gatesfoundation.org. Dipika Sur, Division of Epidemiology, National Institute of Cholera and Enteric Diseases, Kolkata, West Bengal, E-mail: dipikasur@hotmail.com. Abu S. G. Faruque, Clinical Sciences Division, icddr,b, Dhaka, Bangladesh, E-mail: gfaruque@icddrb.org. Debasish Saha, Centre for Nutrition and Food Security (CNFS), icddr,b, Dhaka, Bangladesh. Pedro L. Alonso and Quique Bassat, International Health Research, Barcelona Centre for International Health Research (CRESIB), Hospital Clínic–Universitat de Barcelona, Barcelona, Spain, E-mail: pedro.alonso@isglobal.es. Robert F. Breiman, International Emerging Infections Program, Kenya Medical Research Institute/Centers for Disease Control and Prevention (KEMRI/CDC), Nairobi, Kenya, E-mail: rfbreiman@emory.edu. Quique Bassat, ISGlobal, Barcelona Centre for International Health Research (CRESIB), Hospital Clínic–Universitat de Barcelona, Barcelona, Spain, E-mail: quique.bassat@isglobal.org. Boubou Tamboura, Centre National d'Appui à la lutte contre la Maladie/Centre pour le Développement des Vaccins (CNAM/CVD-Mali), Bamako, Mali, E-mail: btambour@medicine.umaryland.edu. Thandavarayan Ramamurthy and Suman Kanungo, Division of Epidemiology, National Institute of Cholera and Enteric Diseases, Kolkata, India, E-mails: rama1murthy@yahoo.com and sumankanungo@gmail.com. Shahnawaz Ahmed and Sumon K. Das, Centre for Nutrition and Food Security (CNFS), icddr,b, Dhaka, Bangladesh, E-mails: shahnawz@icddrb.org and sumon@icddrb.org. Anowar Hossain, Laboratory Sciences Division, icddr,b, Dhaka, Bangladesh, E-mail: anowar@icddrb.org. Martin Antonio and M. Jahangir Hossain, Child Survival Theme, Molecular Diagnostics, Medical Research Council (MRC) Unit, Banjul, The Gambia, E-mails: mantonio@mrc.gm and jhossain@mrc.gm. Inacio Mandomando, Microbiology, Centro de Investigação em Saúde de Manhiça (CISM), Maputo, Mozambique, E-mail: inacio.mandomando@gmail.com. Eric D. Mintz, Division of Foodborne, Waterborne and Environmental Diseases, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: edm1@cdc.gov.
References
- 1.Ghenghesh KS, Ahmed SF, El-Khalek RA, Al-Gendy A, Klena J. Aeromonas-associated infections in developing countries. J Infect Dev Ctries. 2008;2:81–98. [PubMed] [Google Scholar]
- 2.Lautrop H. Aeromonas hydrophila isolated from human feces and its possible pathological significance. Acta Path Microbiol Scand. 1961;51((Suppl 144)):299–301. [Google Scholar]
- 3.Burke V, Gracey M, Robinson J, Peck D, Beaman J, Bundell C. The microbiology of childhood gastroenteritis: Aeromonas species and other infective agents. J Infect Dis. 1983;148:68–74. doi: 10.1093/infdis/148.1.68. [DOI] [PubMed] [Google Scholar]
- 4.Pazzaglia G, Sack RB, Salazar E, Yi A, Chea E, Leon-Barua R, Guerrero CE, Palomino J. High frequency of coinfecting enteropathogens in Aeromonas-associated diarrhea of hospitalized Peruvian infants. J Clin Microbiol. 1991;29:1151–1156. doi: 10.1128/jcm.29.6.1151-1156.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotloff KL, Wasserman SS, Steciak JY, Tall BD, Losonsky GA, Nair P, Morris JG, Jr, Levine MM. Acute diarrhea in Baltimore children attending an outpatient clinic. Pediatr Infect Dis J. 1988;7:753–759. doi: 10.1097/00006454-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Von Graevenitz A. The role of Aeromonas in diarrhea: a review. Infection. 2007;35:59–64. doi: 10.1007/s15010-007-6243-4. [DOI] [PubMed] [Google Scholar]
- 7.Albert MJ, Ansaruzzaman M, Talukder KA, Chopra AK, Kuhn I, Rahman M, Faruque ASG, Islam MS, Sack RB, Mollby R. Prevalence of enterotoxin genes in Aeromonas spp. isolated from children with diarrhea, healthy controls, and the environment. J Clin Microbiol. 2000;38:3785–3790. doi: 10.1128/jcm.38.10.3785-3790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23:35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, Adegbola RA, Alonso PL, Breiman RF, Faruque ASG. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis. 2012;55:S232–S245. doi: 10.1093/cid/cis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 11.Panchalingam S, Antonio M, Hossain A, Mandomando I, Ochieng B, Oundo J, Ramamurthy T, Tamboura B, Zaidi AKM, Petri W. Diagnostic microbiologic methods in the GEMS-1 case/control study. Clin Infect Dis. 2012;55:S294–S302. doi: 10.1093/cid/cis754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blackwelder WC, Biswas K, Wu Y, Kotloff KL, Farag TH, Nasrin D, Graubard BI, Sommerfelt H, Levine MM. Statistical methods in the Global Enteric Multicenter Study (GEMS) Clin Infect Dis. 2012;55:S246–S253. doi: 10.1093/cid/cis788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figura N, Marri L, Verdiani S, Ceccherini C, Barberi A. Prevalence, species differentiation, and toxigenicity of Aeromonas strains in cases of childhood gastroenteritis and in controls. J Clin Microbiol. 1986;23:595–599. doi: 10.1128/jcm.23.3.595-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dixit S, Bhandari GP, Karmacharya DB, Shrestha S, Manandhar S, Maskey MK. Molecular screening of major bacterial enteropathogens in human stool samples from diarrhoeal outbreak sites. J Nepal Health Res Counc. 2011;9:181–185. [PubMed] [Google Scholar]
- 15.Sinha S, Shimada T, Ramamurthy T, Bhattacharya SK, Yamasaki S, Takeda Y, Nair GB. Prevalence, serotype distribution, antibiotic susceptibility and genetic profiles of mesophilic Aeromonas species isolated from hospitalized diarrhoeal cases in Kolkata, India. J Med Microbiol. 2004;53:527–534. doi: 10.1099/jmm.0.05269-0. [DOI] [PubMed] [Google Scholar]
- 16.Soltan Dallal MM, Moezardalan K. Aeromonas spp associated with children's diarrhoea in Tehran: a case-control study. Ann Trop Paediatr. 2004;24:45–51. doi: 10.1179/027249304225013231. [DOI] [PubMed] [Google Scholar]
- 17.Klontz EH, Faruque AS, Das SK, Malek MA, Islam Z, Luby SP, Klontz KC. Clinical and epidemiologic features of diarrheal disease due to Aeromonas hydrophila and Plesiomonas shigelloides infections compared with Those due to Vibrio cholerae Non-O1 and Vibrio parahaemolyticus in Bangladesh. ISRN Microbiol. 2012;2012:654819. doi: 10.5402/2012/654819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson MA, Burke V, Chang BJ. Invasion of HEp-2 cells by fecal isolates of Aeromonas hydrophila. Infection and Immunity. 1985;47:680–683. doi: 10.1128/iai.47.3.680-683.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X-J, Ferguson MR, Popov VL, Houston CW, Peterson JW, Chopra AK. Role of a cytotoxic enterotoxin in Aeromonas-mediated infections: development of transposon and isogenic mutants. Infection and Immunity. 1998;66:3501–3509. doi: 10.1128/iai.66.8.3501-3509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.