Abstract
Cartilage canals (CCs) are microscopic structures involved in secondary ossification centers (SOCs) development. The features of CCs were investigated in the humeral and femoral proximal epiphyses of small-sized newborn dogs (from premature to 28 days after birth) with histochemical and immunohistochemical approaches. Masson’s Trichrome revealed a ring-shaped area around CCs, which changes in colour from green (immature collagen) to red (mature collagen) as ossification progresses; perichondrium staining always matched the ring color. Safranin-O was always negative. Immunohistochemical analysis revealed immunopositivity for both collagen type I and V around the CCs; collagen type II was negative. CCs count showed a tendency to be higher in the humerus than in the femur. This work enlightened for the first time changes in composition of CCs surrounding matrix during SOCs development in dogs, paving the way to further investigations.
Key words: Cartilage canals, secondary ossification center, collagens, newborn small-sized dog
Introduction
Mammalian long bones develop via endochondral ossification, by formation of primary ossification centers and secondary ossification centers (SOCs). A crucial event preceding the formation of SOCs is the initial generation of cartilage canals (CCs). Cartilage canals have been described in the long bones of birds and mammals, as well as in human tarsal bones.1 Their growth and development have been detailed mostly in mice2,3 and chickens.4,5 Cartilage canals are tube-shaped formations containing a central arteriole which branches out into an anastomosing network of capillaries; a single venule follows the course of the arteriole back to the perichondral plexus6 from which the CCs derives as an invagination.2 They also house lymphatics,6 unmyelinated nerve fibers6-8 and mesenchymal stem cells, embedded within a loose extracellular matrix (ECM).1 The primary function of CCs is to supply nutrition to the growing cartilage and eliminate waste products. A wide variety of CCs size and shape was described in the humeral head of the dog, reflecting the metabolic needs of specific areas. Cartilage canals varied from short unbranched channels to channels coursing from one side of the epiphysis to the other,6 and they were tightly associated with SOCs.9 Other functions of CCs are still under discussion: some authors suggest they may contribute to the formation and maintenance of SOCs2,3,5,10 and they may serve the cartilage growth itself, turning perivascular cells into matrix-producing chondrocytes as cartilage physiologically regresses.11 Evidence demonstrated that defects of cartilage canal blood supply lead to disturbance of endochondral ossification, in human being as well as in animal species, including dogs.12 The way in which these diseases is initiated is still debated. The aim of this work was to continue and to expand the researches on CCs behavior in the dog. Previous researches, in fact, although very extensive, only concern the first week of age and medium and large breeds.6,9 Humerus and femur proximal epiphyses were investigated with morphological, histochemical and immunohistochemical analyses in prematurely born and up-to-28-days-aged. Expected result could provide anatomical basis for a better understanding of the onset of such endochondral disturbances, in the light of dog health, translational and/or comparative research.
Materials and Methods
Animals
Sixteen spontaneously dead puppies aged up to 28 days and belonging to breeds categorized as small-sized according to the standard breed adult body weight <7 kg13 were examined (Supplementary Table 1) and stored at -20°C.
Puppies were full term and died as a consequence of hypoxia during birth and/or bacterial diseases in the first days of life. Their body weight was normal in relation to the breed and the age at the time of death. Two puppies were premature (49 days of gestation). A spontaneously dead skeletally mature small-sized dog (15-year-old) was enrolled as positive control of ossification. The presence of skeletal abnormalities was excluded by macroscopic examination and confirmed by radiographic investigation: the expected appearance of the ossification centers was confirmed and no sign of degenerative joint disease in the shoulder and in the hip joint was detected in the mature dog.
All cadavers were obtained from breeder prior their informed consent signature; the research was approved by the Ethic Committee of the University of Milan (OPBA, 58/2016).
Histology and histochemistry
Cadavers were thawed at room temperature. The proximal epiphyses of the left humerus and femur (total number of specimens = 32) were separated from the remaining bone and fixed in buffered 10% formalin (Bio-Optica, Milan, Italy) for 24 h as a whole. Samples were decalcified with 45% formic acid (Sigma Chemical Co., St. Louis, MO, USA), for 2-3 days and then with 15% 0.5 M EDTA solution (pH 8.0 - Sigma) for 7-10 days14 and embedded in paraffin. Serial sections (4 μm thickness) were mounted on glass slides. Sections corresponding to the sagittal median plane of each proximal epiphysis were stained with standard Hematoxylin-Eosin (HE), Masson’s Trichrome (MT) and Safranin-O (SO) (Bio-Optica) in order to follow ossification progression. Quantitative analysis of CCs was performed on both HE and MT stained sections, with the Olympus DP-software program at 20x magnification: the area corresponding to the sagittal median plane of each proximal epiphysis was measured, and the number of CCs in each of them contained calculated for each dog (total CCs: humerus vs femur; green (immature collagen fibers) or red (mature collagen fibers) CCs: humerus vs femur).
Immunohistochemistry
Collagen type I and II were localized with immunohistochemical analyses according to Di Giancamillo et al.15 A mouse anti-collagen type V (ab-112551, Abcam, Cambridge, UK) antibody was used too, utilizing the same procedure previously described. Briefly, all the primary antisera were diluted 1:200 with a 0.05 M pH 7.4 Tris–HCl saline buffer (TBS: 0.05 M, pH 7.4, 0.55 M NaCl). Antigene-antibody complexes were detected with a ready-to-use secondary antibody (Dako REALTM EnVisionTM/Horseradish Peroxidase, Rabbit/Mouse) and with 3,3'-diaminobenzidine (DAB, Dako Cytomation) as substrate.15 Sections from the humerus of the adult dog were used as positive control; negative controls were performed replacing primary and secondary antibodies. Photomicrographs were captured with an Olympus BX51 microscope (Olympus, Milan, Italy) equipped with a digital camera.
Statistical analysis
Statistical analysis was performed with SAS statistical software (version 9.3, Cary Inc., NC). Data from the CCs counts were analyzed using 2-way ANOVA with bone (humerus/femur) and color (green/red) as main factors, and co-variated for the area corresponding to the sagittal median plane of each humeral and femoral proximal epiphysis. Values from each dog were considered as the experimental unit of all response variables. The data are presented as least-square means (SEM). Differences between means were considered significant at P<0.05.
Results
Histology and histochemistry
Analyses showed the appearance and the gradual development of the SOCs of the humeral and femoral proximal epiphyses, according with the age. Therefore, animals were categorized on the basis of the total number of the examined proximal epiphyses of both humerus and femur, as follows: No Ossification Center (NO, 43.75% humerus vs 37.50% femur; animals age: preterm up to 7 days); Early Ossification Center (EO, 18.75% humerus and 50% femur; animals age: 7-15 days) and Ossification Center (OC 37.50% humerus vs 12.50% femur; animals age: 15-28 days).
Hematoxylin-Eosin
In both humerus and femur, CCs were scattered in the chondroepiphysis in NO group (Figure 1a), scattered both in the mineralized ECM (Figure 1b) and surrounding resting cartilage in EO group, both scattered and entering the SOC in the OC group (Figure 1c). Quantitative analysis revealed a tendency of the total CCs number in the humerus, which was higher than in femur (27.32±2.52 vs 22.15±2.27; P=0.078). As expected, the adult dog showed no CCs.
Figure 1.
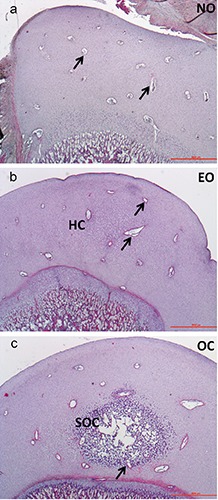
Representative image of the SOC formation in the left humerus of growing dogs - HE staining; a) at 0 days of age; b) at 7 days of age; c) at 21 days of age. NO, no ossification; EO, early ossification; OC, ossification center; arrow, cartilage canal; HC, hypertrophic chondrocytes; SOC, secondary ossification center. Scale bar: 1000 µm.
Masson’s Trichrome
In NO group all CCs were surrounded by a light green matrix, and the same reactivity was always observed in the perichondrium indicating that these areas were ‘immature’ concerning the collagen fibers detected. Cartilage canals were surrounded by a basal membrane and hosted capillaries embedded by an amorphous matrix in the lumen. No sign of chondrification has been observed (Figure 2 a,i). In the EO group, 50% of the CCs appeared surrounded by green matrix, while the other 50% by red matrix (Figure 2 b,c, respectively). In the perichondrium the histochemical reactivity revealed both a green and red color indicating a gradual passage from an ‘immature’ to a ‘mature’ collagen composition in both localizations. In the OC group the presence of a well-structured SOC was accompanied by areas of matrix reactivity in red color, also when the CCs were incorporated within the SOC (Figure 2d). Perichondrium appeared in its definitive histochemical red reactivity (Figure 2j). In the skeletally mature dog, articular cartilage stained light green, subchondral bone stained red (Supplementary Figure 1a). The quantitative analysis conducted on the three new born studied ages revealed that total green CCs number was significantly higher in the humerus compared to the red CCs of the humerus and to the green/red CCs of the femur (P<0.05 all comparisons, Figure 2).
Figure 2.
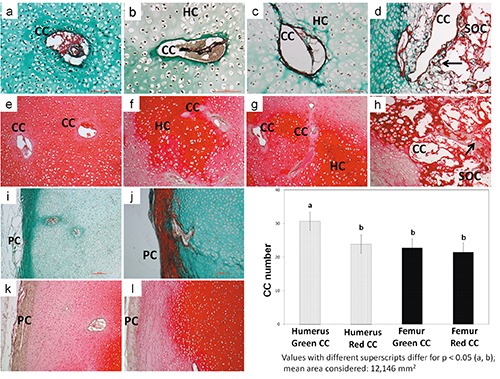
Images of CCs and perichondrium in NO, EO and OC groups - Masson’s Trichrome and Safranin-O staining (left humerus). Masson’s Trichrome staining: in NO group a homogeneously green ECM surrounds a CC (a); an amorphous matrix surrounds the capillaries hosted in its lumen (a); the perichondrium stains green (i); in EO group a subtle green ring of matrix surrounds 50% of the CC (b) and a red ring surrounds the other 50% (c); the number of CCs tends to decrease as the matrix ring changes in colour from green to red (b,c); perichondrium colour always reflects the ring color (i,j); in OC group a red ring matrix surrounds all CC (d); the content of SOC CCs is markedly decreased (d); the perichondrium stains red (j). Safranin-O staining: in all groups the staining of ECM around CCs is always paler than in the surrounded areas (e-h); the perichondrium is negative (k,l). Green ring CCs number was significantly higher in the humerus compared to the red rings CCs of the humerus and to the green/red rings CC of the femur (P<0.05 all comparisons). All described features are observed both in the humeral and femoral head. CC, cartilage canal; HC, hypertrophic chondrocytes; arrows, trabeculae; SOC, secondary ossification center; PC, perichondrium. Scale bar: 100 µm.
Safranin-O
Staining around CCs was evident, and it was paler than the surrounding ECM in all groups, irrespective of the ossification stage (Figure 2 e-h); perichondrium was always negative (Figure 2 k,l). Very intense staining was evident in the deep layers of the articular cartilage, close to the tight mark line in the adult dog (Supplementary Figure 1b).
Immunohistochemistry
Collagen type I was localized in a thin ring surrounding the CCs in all groups (Figure 3 a-c); the perichondrium showed an immunopositive staining in all groups (Figure 3d).
Figure 3.
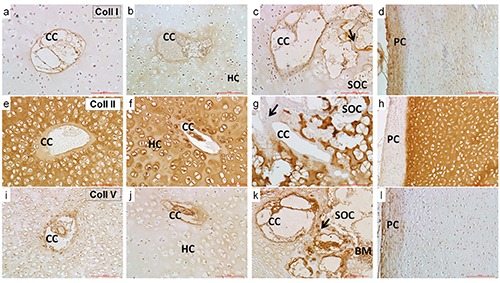
Images of CCs and perichondrium in NO (a,e,i), EO (b,f,j) and OC (c,g.k) groups - Collagen type I, II and V immunolocalization. A thin CC ring positive signal for collagen type I is evident in all groups (a-c) as well as in the perichondrium (d). The signal for collagen type II around the CC is very pale in all groups (e-g) and always negative in the perichondrium (h). The immunopositivity for collagen type V is evident in the inner side of the CC and in the structures inside them in all groups (i-k). The perichondrium is weakly immunopositive (l). A positive signal is also observed in the bone marrow, when present (k). A positive signal was observed in the trabeculae of OC group for all collagen types (c,g,k). All described features are observed both in the humeral and femoral head.CC, cartilage canal; arrows, trabeculae; HC, hypertrophic chondrocytes; SOC, secondary ossification center; PC, perichondrium; BM, bone marrow. Scale bar: 100 µm.
Collagen type II showed a very pale signal in the ring around the CCs in all groups and a more intense reactivity in the ECM (Figure 3 e-g); the perichondrium revealed to be unreactive in all groups (Figure 3h).
Collagen type V was localized in a very subtle ring surrounding CCs, in all the structures inside them in all groups and in bone marrow (Figure 3 i-k); the perichondrium revealed a very slight immunopositivity in all groups (Figure 3l). A clear immunolocalisation of collagen type I in the subchondral bone, of collagen type II in the articular cartilage and collagen type V inside the bone marrow was observed in the adult dog (Supplementary Figure 2 a,c, respectively).
Discussion
To date, the timing of SOCs appearance in limb bones has only been described in medium and large breed dogs.6,9,16 The present work indicates that this pattern is confirmed in small-sized dogs; it also suggests that SOC matures faster in the humerus than in the femur (OC humerus = 37.50% vs OC femur =12.50%). Since CCs proved to be involved in SOC development,1 a more precise characterization of these structures was attempted. In rabbits,17-19 mice,3,17,20,21 rats22 and pigs,23 CCs appear long before the SOC formation; in medium and large breed dogs they have been identified at birth.6,9 In this study their presence before birth was proved, as in humans24 and rabbits.18,25 Cartilage canals scattering in all humeral and femoral heads were observed, irrespective of the ossification stage. Age-matched CCs number analysis was not performed due to the small number of animals per group, however the CCs number tended to be higher in the humerus.
This value, identified at the level of the sagittal median plane of each proximal epiphysis, may be put in relation with a higher vascularization in the humerus than in the femur. Nonetheless, CCs number may also partially refer to the same canal, considering that canal itself becomes curved and branched thorough the humeral epiphyses as SOC develops, inducing the formation of several capillary glomeruli.9 Authors opinion is that CCs numbers or CCs possible repeated counts, caused by branched canals, is always to be referred to a higher vascularization. So that the humerus receives a greater metabolic intake that in turn may explain the more rapid SOC maturation. This is also confirmed by another hypothesis: from birth to the age of about 4 weeks, the new-born puppy ability to stand and to walk increases, by the rudimentary crawling mainly on its hind limbs to the well-coordinated walking on both hind and fore limb.26 This implies that humeral epiphysis earlier undergoes to mechanical stimuli compared to the femur. This could stimulate CCs formation and expansion and the release of hypertrophic factors27 and in turn a earliest SOC formation. Masson’s Trichrome staining showed that the subtle area surrounding CCs changed in color from green to red as age increased, indicating that SOC formation is accompanied by a maturation of collagen fibers surrounding CCs. The same color change accordingly occurred in the perichondrium.
Moreover, the number of humerus green CCs was significantly higher than the number of red CCs, and also higher than the number of red CCs in both humerus and femur. In order to find a functional meaning for such changes in histochemical reactivity, the expression of collagen type I and II have been immunoisto-chemically investigated, together with presence of collagen type V. Collagen type V is a minor, but important component of connective tissues that are rich in collagen type I.28 In mice, a strong expression of pro-a I (V) has been observed concomitantly with the appearance of the ossification centers, both in long bones and vertebrae.29 Little is known about the role of collagen V in developing bones: it might play a role in the brittleness of the bone by interfering with the process of mineraliza-tion30 and/or be involved in osteogenesis.29,31,32 It has been hypothesized that fibrillar components of the collagen matrix contribute to the formation of a firm shell around the CCs, in order to protect the structures located in their lumen from the density of the cartilage matrix.33 A positive label for collagen type I and V in CCs was found, while the ring around each CC was devoid of collagen type II. This was in agreement with the pale Safranin-O staining. These results suggest that CCs mesenchymal cells are involved in bone ECM production rather than cartilage ECM production, according to Blumer et al.4 This happens in dogs even before the SOC formation, as previously demonstrated in pre-5 and post hatching chickens.3 The perichondrium collagen type I and V staining confirmed that mesenchymal cells could directly derive from the perichondrium.2 Collagen V was also localized in the bone marrow:28,32 it is therefore possible to speculate that bone marrow cells could be involved in type V collagen production as well as type I.34 This is the first time that collagen type V is described near CCs. Further studies are needed to better characterize the histochemical changes. Unfortunately, our results did not prove any differences related to the color changes that we observed. Future studies will be performed in order to investigate collagen structural organization and detect other collagen types. These data, although preliminary, lay the basis for more extensive studies on the delicate mechanisms that modulate endochondral bone development also in the context of the growth disorders affecting the proximal femoral epiphysis of small-sized breeds dogs.35 Moreover, it could be useful to study the role of CCs in the pathogenesis of the joint disorders affecting growing large sized dogs, such as the humeral osteochondritis12 as well as the fracture of the medial coronoid process.36,37
This study finally suggests that cadaver may be considered a useful tool, not only in gross anatomy research,38 but also a convincing alternative for histochemical and immunohistochemical investigation to the in vivo animal models.
Acknowledgments
The authors would like to thank Mr. Luca Cerri for the technical support and Prof. Cristiano Rumio and Ms. Patrizia Luchini for the setup of bone processing.
References
- 1.Blumer MJ, Longato S, Fritsch H. Structure, formation and role of cartilage canals in the developing bone. Ann Anat 2008;190:305-15. [DOI] [PubMed] [Google Scholar]
- 2.Blumer MJ, Longato S, Richter E, Perez MT, Konakci KZ, Fritsch H. The role of cartilage canals in endochondral and perichondral bone formation: are there similarities between these two processes? J Anat 2005;206:359-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blumer MJ, Longato S, Schwarzer C, Fritsch H. Bone development in the femoral epiphysis of mice: the role of cartilage canals and the fate of resting chondrocytes. Dev Dyn 2007;236:2077-88. [DOI] [PubMed] [Google Scholar]
- 4.Blumer MJ, Fritsch H, Pfaller K, Brenner E. Cartilage canals in the chicken embryo: ultrastructure and function. Anat Embryol (Berl) 2004;207(6):453-62. [DOI] [PubMed] [Google Scholar]
- 5.Blumer MJ, Schwarzer C, Perez MT, Konakci KZ, Fritsch H. Identification and location of bone-forming cells within cartilage canals on their course into the secondary ossification centre. J Anat 2006;208: 695-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilsman NJ, Van Sickle DC. Cartilage canals, their morphology and distribution. Anat Rec 1972;173:79-93. [DOI] [PubMed] [Google Scholar]
- 7.Stockwell RA. The ultrastructure of cartilage canals and the surrounding cartilage in the sheep fetus. J Anat 1971;109:397-410. [PMC free article] [PubMed] [Google Scholar]
- 8.Hedberg A, Messner K, Persliden J, Hildebrand C. Transient local presence of nerve fibers at onset of secondary ossification in the rat knee joint. Anat Embryol (Berl) 1995;192:247-55. [DOI] [PubMed] [Google Scholar]
- 9.Wilsman NJ, Van Sickle DC. The relationship of cartilage canals to the initial osteogenesis of secondary centers of ossification. Anat Rec 1970;168:381-91. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez J, Costales L, Lopez-Muniz A, Lopez JM. Chondrocytes are released as viable cells during cartilage resorption associated with the formation of intrachondral canals in the rat tibial epiphysis. Cell Tissue Res 2005;320:501-7. [DOI] [PubMed] [Google Scholar]
- 11.Olstad K, Ytrehus B, Ekman S, Carlson CS, Dolvik NI. Early lesions of osteochondrosis in the distal tibia of foals. J Orthop Res 2007;25:1094-105. [DOI] [PubMed] [Google Scholar]
- 12.Ytrehus B, Carlson CS, Ekman S. Etiology and pathogenesis of osteochondrosis. Vet Pathol 2007;44:429-48. [DOI] [PubMed] [Google Scholar]
- 13.Brianza SZ, D’Amelio P, Pugno N, Delise M, Bignardi C, Isaia G. Allometric scaling and biomechanical behavior of the bone tissue: an experimental intraspecific investigation. Bone 2007;40:1635-42. [DOI] [PubMed] [Google Scholar]
- 14.Ozaki S, Toida K, Suzuki M, Nakamura Y, Ohno N, Ohashi T, et al. Impaired olfactory function in mice with allergic rhinitis. Auris Nasus Larynx 2010;37575-83. [DOI] [PubMed] [Google Scholar]
- 15.Di Giancamillo A, Deponti D, Addis A, Domeneghini C, Peretti GM. Meniscus maturation in the swine model: changes occurring along with anterior to posterior and medial to lateral aspect during growth. J Cell Mol Med 2014;18:1964-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hare WC. The ages at which the centers of ossification appear roentgenographically in the limb bones of the dog. Am J Vet Res 1961;22:825-35. [PubMed] [Google Scholar]
- 17.Cole AA, Wezeman FH. Morphometric analysis of cartilage canals in the developing mouse epiphysis. Acta Anat 987;128:93-7. [DOI] [PubMed] [Google Scholar]
- 18.Rivas R, Shapiro F. Structural stages in the development of the long bones and epiphyses: a study in the New Zealand white rabbit. J Bone Joint Surg Am 2002;84-A:85-100. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro F. Epiphyseal and physeal cartilage vascularization: a light microscopic and tritiated thymidine autoradiographic study of cartilage canals in newborn and young postnatal rabbit bone. Anat Rec 1998;252:140-8. [DOI] [PubMed] [Google Scholar]
- 20.Kugler JH, Tomlinson A, Wagstaff A, Ward SM. The role of cartilage canals in the formation of secondary centres of ossification. J Anat 1979;129):493-506. [PMC free article] [PubMed] [Google Scholar]
- 21.Blumer MJ, Longato S, Fritsch H. Localization of tartrate-resistant acid phosphatase (TRAP), membrane type-1 matrix metalloproteinases (MT1-MMP) and macrophages during early endochondral bone formation. J Anat 2008;213:431-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delgado-Baeza E, Gimenez-Ribotta M, Miralles-Flores C, Nieto-Chaguaceda A, Santos-Alvarez I. Morphogenesis of cartilage canals: experimental approach in the rat tibia. Acta Anat 1991;142:132-7. [DOI] [PubMed] [Google Scholar]
- 23.Visco DM, Hill MA, Van Sickle DC, Kincaid SA. Cartilage canals and lesions typical of osteochondrosis in growth cartilages from the distal part of the humerus of newborn pigs. Vet Rec 1991;128:221-8. [DOI] [PubMed] [Google Scholar]
- 24.Burkus JK, Ganey TM, Ogden JA. Development of the cartilage canals and the secondary center of ossification in the distal chondroepiphysis of the prenatal human femur. Yale J Biol Med 1993;66: 193-202. [PMC free article] [PubMed] [Google Scholar]
- 25.Doschak MR, Cooper DM, Huculak CN, Matyas JR, Hart DA, Hallgrimsson B, et al. Angiogenesis in the distal femoral chondroepiphysis of the rabbit during development of the secondary centre of ossification. J Anat 2003;203:223-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson ME, Kutzler MA. Small animal pediatrics: the first 12 months of life. Elsevier-Saunders, St. Louis, MO, USA; 2011. [Google Scholar]
- 27.Peinado Cortes LM, Vanegas Acosta JC, Garzon Alvarado DA. A mechanobiological model of epiphysis structures formation. J Theor Biol 2011;287:13-25. [DOI] [PubMed] [Google Scholar]
- 28.Gelse K, Poschl E, Aigner T. Collagens-structure, function, and biosynthesis. Adv Drug Deliv Rev 2003;55:1531-46. [DOI] [PubMed] [Google Scholar]
- 29.Roulet M, Ruggiero F, Karsenty G, LeGuellec D. A comprehensive study of the spatial and temporal expression of the col5a1 gene in mouse embryos: a clue for understanding collagen V function in developing connective tissues. Cell Tissue Res 2007;327:323-32. [DOI] [PubMed] [Google Scholar]
- 30.Bonaventure J, Zylberberg L, Cohen-Solal L, Allain JC, Lasselin C, Maroteaux P. A new lethal brittle bone syndrome with increased amount of type V collagen in a patient. Am J Med Genet 1989;33:299-310. [DOI] [PubMed] [Google Scholar]
- 31.Kahai S, Vary CP, Gao Y, Seth A. Collagen, type V, alpha1 (COL5A1) is regulated by TGF-beta in osteoblasts. Matrix Biol 2004;23:445-55. [DOI] [PubMed] [Google Scholar]
- 32.Yamaguchi K, Matsuo N, Sumiyoshi H, Fujimoto N, Iyama KI, Yanagisawa S, et al. Pro-alpha3(V) collagen chain is expressed in bone and its basic N-terminal peptide adheres to osteosarcoma cells. Matrix Biol 2005;24:283-94. [DOI] [PubMed] [Google Scholar]
- 33.Le Guellec D, Mallein-Gerin F, Treilleux I, Bonaventure J, Peysson P, Herbage D. Localization of the expression of type I, II and III collagen genes in human normal and hypochondrogenesis cartilage canals. Histochem J 1994;26:695-704. [DOI] [PubMed] [Google Scholar]
- 34.Waterhouse EJ, Quesenberry PJ, Balian G. Collagen synthesis by murine bone marrow cell culture. J Cell Physiol 1986;127: 397-402. [DOI] [PubMed] [Google Scholar]
- 35.Scherzer C, Windhagen H, Nellesen J, Crostack HA, Rohn K, Witte F, et al. Comparative structural analysis of the canine femoral head in Legg-Calve-Perthes disease. Vete Radiol Ultrasound 2009;50:404-11. [DOI] [PubMed] [Google Scholar]
- 36.Wolschrijn CF, Gruys E, van der Wiel CW, Weijs WA. Cartilage canals in the medial coronoid process of young Golden Retrievers. Vet J 2008;176:333-7. [DOI] [PubMed] [Google Scholar]
- 37.Lau SF, Hazewinkel HA, Grinwis GC, Wolschrijn CF, Siebelt M, Vernooij JC, et al. Delayed endochondral ossification in early medial coronoid disease (MCD): a morphological and immunohistochemical evaluation in growing Labrador retrievers. Vet J 2013;197:731-8. [DOI] [PubMed] [Google Scholar]
- 38.Longo M, Modina SC, Bellotti A, Di Giancamillo M. Advances in the anatomic study of the interscapular region of the cat. BMC Vet Res 2015;11:249. [DOI] [PMC free article] [PubMed] [Google Scholar]


