Abstract
Fibrolamellar hepatocellular carcinoma is a rare hepatocellular tumor usually arising in noninfected and noncirrhotic livers. Only 2 cases accompanied by hyperammonemia due to intrahepatic shunting have been reported. A 23-year-old white woman presented with a 2-week history of nausea, vomiting, generalized weakness, and intermittent right upper quadrant pain. Abdominal computerized tomography revealed a 13 x 9-cm hepatic mass. Core-needle biopsy revealed fibrolamellar hepatocellular carcinoma. She presented with coma due to hyperammonemia levels (peak at 437 mcg/dL) but without metastatic disease. She was urgently transplanted, started on daily sorafenib 8 weeks after transplantation, and was free of disease at 1 year after transplantation.
Introduction
Fibrolamellar hepatocellular carcinoma (FL-HCC) is a rare variant of hepatocellular tumor arising in noninfected and noncirrhotic liver as a primary tumor. Fibrolamellar hepatocellular carcinoma is a very uncommon tumor rarely encountered, accounting for only 0.3% of all primary HCC cases.1 Histologically, it is characterized by large polygonal hepatocytes with eosinophilic and granular cytoplasm surrounded by numerous thick fibrous bands in a parallel or lamellar distribution.2 Median age at diagnosis is 25, with no clearly-defined gender disposition, and conventional tumor markers for HCC (eg, alpha-fetoprotein) are usually normal.2 The most important prognostic feature is whether the tumor is resectable. Only 2 cases of FL-HCC accompanied by hyperammonemia due to intrahepatic shunting have been published, none of which were successfully treated. The liver transplant literature on FL-HCC is outdated and not favorable. Despite this, young patients with unresectable FL-HCC and without evidence of metastatic disease should be considered for transplant.
Case Report
A 23-year-old female presented with a 2-week history of nausea, vomiting, generalized weakness, and intermittent right upper quadrant pain. She had a family history of liver and breast cancer. Following admission, abdominal computerized tomography revealed a large hepatic mass measuring 13 x 9 cm. Multiple internal enhancing vessels and altered vascular architecture were visualized (Figure 1). A core needle biopsy revealed FL-HCC, demonstrating neoplastic cells with features of hepatocyctes arranged in thick cores and sheets. Cells demonstrated eosinophilic granular abundant cytoplasm with enlarged and prominent nucleoli (Figure 2). Immunohistochemistry was strongly positive for alpha 1 antitrypsin, CD31 and CD34 within the endothelial cells, and cytokeratin 7. Cytokeratin 19/20 were negative. As hyperammonemia worsened, the patient began to show signs of altered mentation. She was treated with lactulose and rifaximin, followed by intubation on the 4th day of admission. Ammonia levels peaked at 437 mcg/dL from the time of presentation. MRI of the brain revealed areas of restricted diffusion corresponding to an abnormal T2 signal. This raised concern towards an ischemic process or hyperammonemic encephalopathy. The patient began to respond to painful stimuli. Liver function tests included aspartate aminotransferase (47 U/I), alanine aminotransferase (41 U/I), total bilirubin (2.2 mg/dL), and international normalized ratio (1.5).
Figure 1.
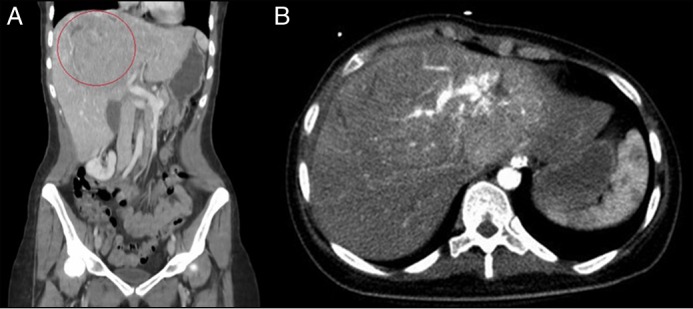
(A) Coronal computerized tomography showing a 13 x 9-cm mass in the liver, and (B) axial computerized tomography showing multiple internal enhancing vessels and altered vascular architecture.
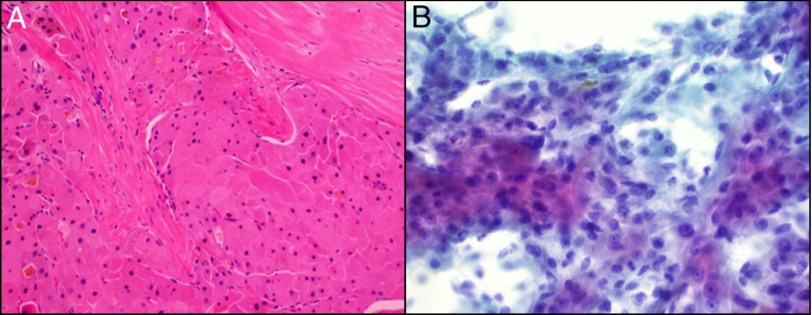
Figure 2.
(A) Hematoxylin and eosin stains (200x) demonstrating dense fibrous bands surrounded by eosinophilic hepatocytes with extensive bile pigment. (B) Papanicolaou-stained cytologic smears (400x) demonstrating neoplastic hepatocytes with transgressing vessels and focal bile pigment.
The patient was transplanted 3 days later, as she met Status 1 criteria by the Organ Procurement and Transplantation Network. On postoperative day 1, the patient was awake and extubated. Immunosuppression consisted of corticosteroids, mycophenolate mofetil, and tacrolimus. On postoperative day 7, the patient sustained a seizure. She was discharged home 20 days postoperatively. The patient fully recovered with the exception of impaired vision. She was started on 400 mg daily of sorafenib for 8 weeks after transplant. Although human studies remain inconclusive and difficult to ascertain, sorafenib adjuvant therapy after liver transplantation in rat models has inhibited reoccurrence of HCC and metastasis and improved overall survival.3 Our patient’s liver pathology revealed a 16-cm well-differentiated FL-HCC without lymphovascular invasion. One year postoperatively, the patient is alive and without evidence of recurrence.
Discussion
We present a rare case of nonmetastatic FL-HCC who presented with advanced stages of coma due to hyperammonemia. Most recent studies reveal a better survival for FL-HCC, with a median survival of 12–14 vs 8–9 months for non-FL-HCC.4 A metaanalysis of 368 FL-HCC cases between 1980 and 2013 revealed a better 5-year survival for FL-HCC, excluding the noncirrhotic FL-HCC type.5 Overall, survival was not different with liver transplant for FL-HCC vs non FL-HCC (51 vs 47 months, respectively) and resected patients fared better with FL-HCC.5 Secondly, a review of 90 FL-HCC cases from the Surveillance, Epidemiology, and End Results Program (SEER) Program revealed a female preponderance, more localized disease, and a median survival of 75 months, better than for non-FL-HCC, despite more advanced disease.6 Outcomes reported for liver transplant in FL-HCC are poor, as the data is outdated (from 1990s). Among nonmetastatic cases, like our cases, FL-HCC fared better than noncirrhotic and non-FL-HCC.7
We found only 2 cases of FL-HCC presenting with hyperammonemia, one from the United States and the other from Argentina. Neither of the cases was transplanted. In 2009, an 18-year-old male presented with a 7-day course of altered mental status, no signs of liver failure, and an elevated level of ammonia.8 The patient had diffuse metastatic disease and succumbed 3 months later after failed sorafenib treatment. The second case described a 22-year-old female with encephalopathy progressing to coma within 3 days of admission. This patient also had evidence of metastatic disease but no signs of acute liver failure.9 In these 2 cases, hyperammonemia was not found to be a consequence of acute liver failure but rather to intrahepatic shunting and lack of clearance of nitrogenous compounds by the tumor cells. In our patient, synthetic dysfunction was not proportional to the comatose state seen in acute liver failure patients. Admission lactate and INR to our institution were 1.7 and 1.3, respectively. As the imaging demonstrated shunting, we cannot be certain which mechanism predominated, as shunting and acute liver failure probably contributed. Signs of acute hepatic necrosis were not evident. In the end, young patients with unresectable FL-HCC and without evidence of metastatic disease should be considered for liver transplant.
Disclosures
Author contributions: All authors contributed to writing and editing the manuscript. AC Berry is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Bureau of Epidemiology. Florida Department of Health. Retrieved September 3, 2010 from www.doh.state.fl.us/disease_ctrl/epi/cancer/Liver_cancer.pdf.
- 2.Chagas AL, Kikuchi L, Herman P, et al. . Clinical and pathological evaluation of fibrolamellar hepatocellular carcinoma: A single center study of 21 cases. Clinics (Sao Paulo). 2015; 70(3):207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan J, Tan C, Gu F, et al. . Sorafenib delays recurrence and metastasis after liver transplantation in a rat model of hepatocellular carcinoma with high expression of phosphorylated extracellular signal-regulated kinase. Liver Transpl. 2013; 19(5):507–20. [DOI] [PubMed] [Google Scholar]
- 4.Epstein B, Pajak TF, Haulk TL, Herspt JM, Order SE, Abrams RA. Metastatiic non-resectable fibrolamellar hepatoma: Prognostic features and natural history. Am J Clin Oncol. 199(22):22–8. [DOI] [PubMed] [Google Scholar]
- 5.Njei B, Konjeti V, Ditah I. Prognosis of patients with hepatocellular carcinoma versus conventional hepatocellular carcinoma: A systematic review and meta-analysis. Gastrointest Cancer Res. 2014; 7:49–54. [PMC free article] [PubMed] [Google Scholar]
- 6.Mayo S, Mavros M, Nathan H, et al. . Treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma: A national perspective. J Am Coll Surg. 2014; 218:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakar S, Burgart LJ, Batts KP, et al. . Clinico-pathologic features and survival in fibrolamellar carcinoma: Comparison with conventional hepatocellular carcinoma with and without cirrhosis. Mod Pathol. 2005; 18:1417–23. [DOI] [PubMed] [Google Scholar]
- 8.Sethi S, Tageja N, Singh J, et al. . Hyperammonemic encephalopathy: A rare presentation of fibrolamellar hepatocellular carcinoma. Am J Med Sci. 2009; 338(6):522–4. [DOI] [PubMed] [Google Scholar]
- 9.Berger C, Dimant P, Hermida L, et al. . Encefalopatia hiperamonemicay hepatocarcinoma fibrolamelar. Medicina (Buenos Aires). 2012; 72:425–27. [PubMed] [Google Scholar]