Abstract
Biliary angiodysplasia is extremely rare. Our background search revealed only a few case reports in the English literature. We present a case of angiodysplasia of the proximal common bile duct in a patient with subacute upper gastrointestinal bleeding and symptomatic anemia. A standard esophagogastroduodenoscopy with subsequent dedicated duodenoscopy revealed blood-stained bile draining from the major ampulla orifice. A contrast-enhanced magnetic resonance cholangiopancreatography was unrevealing for any pancreaticobiliary pathology. The patient subsequently underwent an endoscopic retrograde cholangiopancreatography and SpyGlass® cholangioscopy, which demonstrated intermittent bleeding from angiodysplasia in the proximal common bile duct.
Introduction
Angiodysplasia of the gastrointestinal tract is one of the common causes of occult gastrointestinal tract bleeding. However, angiodysplasia of the common bile duct (CBD) is extremely rare. Our literature review revealed only a few reported cases of biliary angiodysplasia.1-3
Case Report
A 75-year-old male with coronary artery disease and indwelling coronary stents on dual antiplatelet therapy with clopridogel and aspirin for 5 years presented with chest pain, progressive dyspnea, and fatigue for a week. He also endorsed passing black tarry stools intermittently for the last 6 months. The patient had no history of epistaxis, chronic kidney disease, aortic stenosis, von Willebrand’s disease, or hereditary hemorrhagic telangiectasia.
The physical examination was unremarkable except for pallor. There was no mucocutaneous telangiectasia. Stool guaiac test was positive. The initial laboratory tests were significant for an iron deficiency anemia with a hemoglobin of 7.6 g/dL (baseline, 12-15 g/dL). Complete metabolic panel was within normal limits. The patient’s clinical presentation and supporting laboratory data were suspicious for subacute and probable insidious upper gastrointestinal bleeding. Both of his antiplatelet agents were immediately discontinued. Proton pump inhibitor therapy was initiated. He had an appropriate increase of his hemoglobin after 2 U of blood transfusion.
A diagnostic esophagogastroduodenoscopy was performed within 24 hours of admission, which demonstrated dark red blood around the major ampulla on limited tangential views. There was no evidence of varices, ulcer disease, or other mucosal vascular anomalies to the examined third portion of the duodenum. A dedicated duodenoscopy with en-face view of the major ampulla showed blood-stained bile and dark red blood emanating from the major ampulla, concerning for hemobilia or hemosuccus pancreaticus (Figure 1). There was no evidence of mass lesion. A follow-up contrast-enhanced magnetic resonance cholangiopancreatography was unrevealing for any mass lesions or intraductal filling defects. Endoscopic retrograde cholangiopancreatography and SpyGlass® (Boston Scientific, Marlborough, MA) cholangioscopy were subsequently pursued, which showed blood-stained bile during the initial inspection. After thorough irrigation and suction, clear visualization of the area showed diminutive arborizing and ectatic blood vessels with intermittent blood oozing in the proximal CBD, consistent with angiodysplasia (Figure 2). The size of the lesion was estimated to be less than 3.3 mm. Selective cannulation and inspection of the left and right main hepatic ducts, its immediate radicles, and the cystic duct did not reveal any bleeding source or similar appearing angiodysplasia. No endoscopic therapy was performed to the lesion as SpyGlass® compatible accessories for intraductal bleeding treatment were unavailable. Dual antiplatelets remained discontinued after the procedure.
Figure 1.
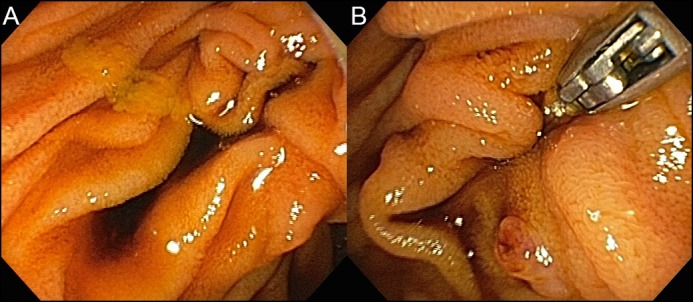
(A) Blood-stained bile pooling near to the major ampulla orifice. (B) Duodenoscopy with en-face view showed hemobilia with dark reddish blood emanating from the major ampulla orifice.
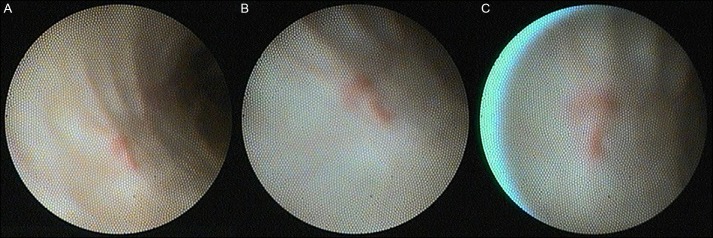
Figure 2.
(A) SpyGlass® cholangioscopy revealed ectatic blood vessels in the proximal CBD after thorough irrigation and suction. (B and C) Close up views of the angiodysplasia.
The patient’s chest pain and melena resolved during his hospitalization. His hemoglobin level remained stable at 10-10.5 g/dL. In light of stable hemoglobin and no evidence of recurrent bleeding, no further angiography or medical treatment was pursued. Dual antiplatetet therapy was not restarted on discharge. Outpatient follow-up at 1 and 3 months revealed hemoglobin of 12.2 and 12.0 g/dL, respectively. The patient later underwent an outpatient colonoscopy and terminal ileoscopy, which were negative for bleeding and mucosal pathology.
Discussion
Biliary angiodysplasia is an extremely rare clinical entity of hemobilia. Although the pathogenesis of biliary angiodysplasia is not well understood, it could be similar to gallbladder and colonic angiodysplasia.4-6 The contraction and relaxation of the CBD with resultant intermittent occlusion of the vessels may lead to focal ectatic dilation of the mucosal vessels. Biliary angiodysplasia can be seen in patients with hereditary hemorrhagic telangiectasia.1,2
Identifying the source of hemobilia is crucial to prevent potential complications. In the era of advanced endoscopic technique, cholangioscopy allows direct visualization of the biliary system to determine the source of hemobilia.1,2,3,7-11 Aydinli et al described the intraoperative application of cholangioscopy to determine the source of hemobilia in selected patient in order to guide surgical intervention.7 A study by Green et al suggests that patients who are diagnosed adequately based on endoscopic and cholangioscopic findings do not require more definitive investigations.9
Current available chalangioscopy includes SpyGlass® cholangioscopy and direct peroral cholangioscopy (DPC). SpyGlass® cholangioscopy is a 3.3-mm 4-lumen catheter with a 1.2-mm accessory channel. Its small size enables better access to biliary tree at the cost of image quality. In addition, it has limited endoscopic therapy secondary to small accessory channel.10,11 DPC is an ultraslim endoscope with an outer diameter of 5-6 mm. It has a larger working channel enabling the accommodation of argon plasma coagulation probes, larger biopsy forceps, and lithotripsy fibers. It also has superior image quality. However, DPC requires a larger sphincterotomy and dilated bile duct.12 It also lacks of access to proximal common bile duct. Therefore, the proximal biliary angiodysplasia in our patient would have not been identified had we used DPC.
Transarterial embolization is the first line of intervention to stop bleeding from hemobilia.9 Hayashi et al and Komaki et al reported that biliary angiodysplasia could be treated with coil embolization and arterial embolization, respectively.1,2 Literature revealed successful neodymium-doped yttrium aluminum garnet (Nd-YAG) laser coagulation and argon plasma coagulation to control bleeding from biliary angiodysplasia using DPC.2,3 The present patient was managed successfully with discontinuation of dual antiplatelet therapy. Given the unexpected location of the biliary angiodysplasia and the lack of available SpyGlass® cholangioscopy compatible therapeutic accessories, no endoscopic intervention for bleeding was performed. Some studies showed the association between antiplatelet therapy use and increased risks of morbidity and mortality from gastrointestinal bleeding.13 Cessation of antiplatelet therapy significantly lowered our patient’s risk of rebleeding.
Disclosures
Author contributions: KS Foong interpreted the data, wrote the manuscript, and is the article guarantor. A. Lee critically revised the manuscript. S. Kudakachira collected the data. H. Ramberan contributed to the intellectual content of this case and approved the final manuscript.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Hayashi S, Baba Y, Ueno K, et al. Small arteriovenous malformation of the common bile duct causing hemobilia in a patient with hereditary hemorrhagic telangiectasia. Cardiovasc Intervent Radiol. 2008;31(Suppl 2):S131–4. [DOI] [PubMed] [Google Scholar]
- 2.Komaki Y, Kanmura S, Funakawa K, et al. A case of hereditary hemorrhagic telangiectasia with repeated hemobilia arrested by argon plasma coagulation under direct peroral cholangioscopy. Gastrointest Endosc. 2014;80(3):528–9. [DOI] [PubMed] [Google Scholar]
- 3.Siddique I, Galati J, Ankoma-Sey V, et al. The role of choledochoscopy in the diagnosis and management of biliary tract diseases. Gastrointest Endosc. 1999;50(1):67–73. [DOI] [PubMed] [Google Scholar]
- 4.Yudt WM, Silverman ED, Kistler AM. Scintigraphic detection of hemobilia complicating angiodysplasia. J Nucl Med. 1994;35(5):870–1. [PubMed] [Google Scholar]
- 5.Kok KYY, Telisinghe PU. Angiodysplasia of the gallbladder. Int J Surg Case Rep. 2011;2(8):256–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boley SJ, Sammartano R, Adams A, et al. On the nature and etiology of vascular ectasias of the colon. Degenerative lesions of aging. Gastroenterology. 1977;72:650–60. [PubMed] [Google Scholar]
- 7.Aydinli M, Koruk I, Koruk S, et al. Intraoperative cholangioscopy with an ultrathin endoscope for hemobilia. Endoscopy. 2011;43(Suppl 2 UCTN):E410.. [DOI] [PubMed] [Google Scholar]
- 8.Moon JH, Choi HJ, Ko BM. Therapeutic role of direct peroral cholangioscopy using an ultra-slim upper endoscope. J Hepatobiliary Pancreat Sci. 2011;18(3):350–6. [DOI] [PubMed] [Google Scholar]
- 9.Green MH, Duell RM, Johnson CD, et al. Haemobilia. Br J Surg. 2001;88(6):773–86. [DOI] [PubMed] [Google Scholar]
- 10.Williamson JB, Draganov PV. The usefulness of SpyGlass™ choledochoscopy in the diagnosis and treatment of biliary disorders. Curr Gastroenterol Rep. 2012;14(6):534–41. [DOI] [PubMed] [Google Scholar]
- 11.Parsi MA. Peroral cholangioscopy in the new millennium. World J Gastroenterol. 2011;17(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monga A, Ramchandani M, Nageshwar Reddy D. Per-oral cholangioscopy. J Interv Gastroenterol. 2011;1(2):70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alli O, Smith C, Hoffman M, et al. Incidence, predictors, and outcomes of gastrointestinal bleeding in patients on dual antiplatelet therapy with aspirin and clopidogrel. J Clin Gastroenterol. 2011;45:410–4. [DOI] [PubMed] [Google Scholar]