Abstract
An intraluminal duodenal diverticulum (IDD) is a rare congenital anomaly that is the result of incomplete recanalization of the embryologic foregut leaving a fenestrated membrane within the lumen of the duodenum. Years of peristalsis acting on the membrane result in the formation of a diverticulum. Most patients are asymptomatic, while some may have abdominal pain, bloating, or fullness. Rare complications include gastrointestinal bleeding, obstruction, pancreatitis, and cholangitis. We present 2 cases with endoscopic findings consistent with partially obstructing symptomatic IDD.
Introduction
An intraluminal duodenal diverticulum (IDD) is a true diverticulum, as it is formed from all 3 layers of the mucosa. The pathogenesis involves incomplete recanalization of the embryologic foregut in the 8th week of gestation.1 In normal development, there is initially hyperplasia of the epithelial cells of the duodenal mucosa that results in occlusion of the lumen. As a result of years of peristaltic forces, there is progressive ballooning of the tissue to form a pulsion-type diverticulum. For this reason, the median time of presentation is the fourth decade of life. The site of attachment is almost always in the second part of the duodenum, just distal to the ampulla of Vater.2 The diaphragm of the IDD may partially occlude the duodenal lumen or encompass its entire circumference.3 Size can vary, with lengths of upwards of 10 cm being reported.
Case Report
Case 1: A 22-year-old female with no significant medical history presented with several weeks of abdominal cramping, low appetite, and frequent episodes of diarrhea. She admitted to a few pounds of weight loss. Her symptoms only partially responded to a trial of proton pump inhibitors. She had numerous visits to the emergency room for complaints of abdominal pain, nausea, and vomiting. Further diagnostic evaluation, including laboratory data and a noncontrast CT of the abdomen, was unrevealing. Due to persistent symptoms and the stated weight loss, she underwent an upper endoscopy that revealed nonerosive gastritis and a single IDD in the second part of the duodenum. An upper GI series confirmed the finding of a partially obstructing diverticulum, which, when distended, occupied greater than half the diameter of the duodenum (Figures 1 and 2). The patient was offered surgical diverticulectomy, but she preferred conservative management and was lost to follow-up.
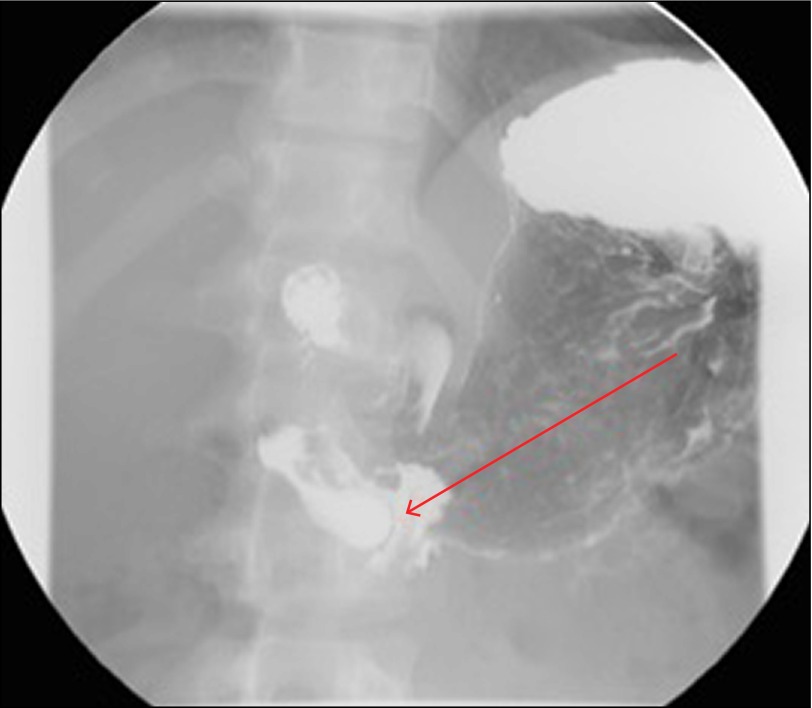
Figure 1.
Upper GI series showing contrast within the lumen of IDD with a rim of lucency surrounding it (arrow). The "wind sock" sign as seen here, classically identifies an IDD.
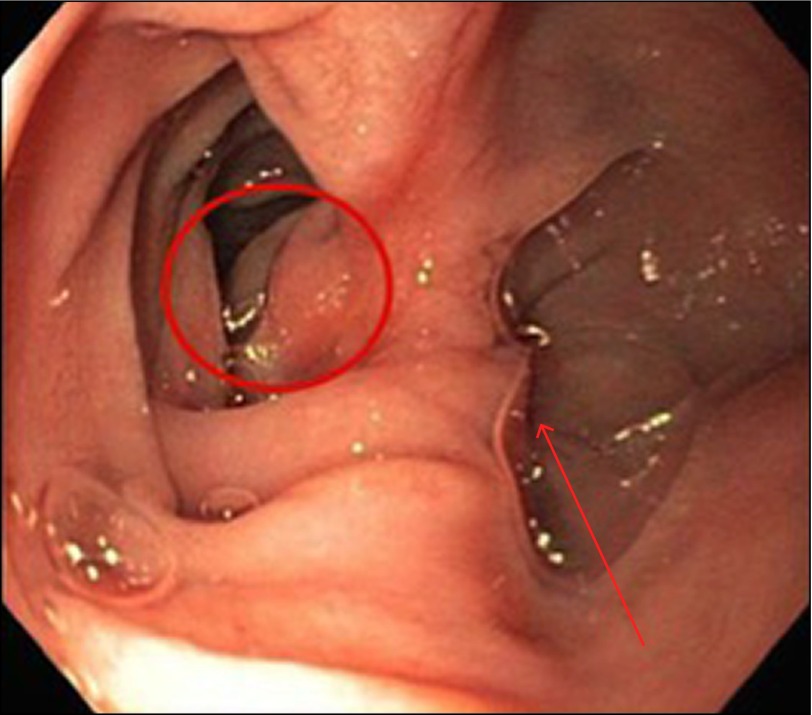
Figure 2.
False lumen, indicated by the arrow, as seen on upper endoscopy. The circle marks the sac of the IDD.
Case 2: A 58-year-old male with a past medical history significant for hyperlipidemia and diabetes presented with 6 months of nonexertional chest pain and abdominal bloating. He also reported 35 pounds of intentional weight loss. Diagnostic workup, including laboratory data, was unremarkable. A stress test was normal. Due to the persistence of his symptoms, the patient underwent an upper endoscopy that revealed short segment Barrett’s esophagus and an intraluminal diverticulum in the second part of duodenum (Figures 3 and 4). The patient underwent an upper GI series at another institution that reportedly confirmed the latter finding. He was recommended surgical diverticulectomy but elected for conservative management. At 1-year follow-up, he reported improvement of his chest pain and abdominal bloating.
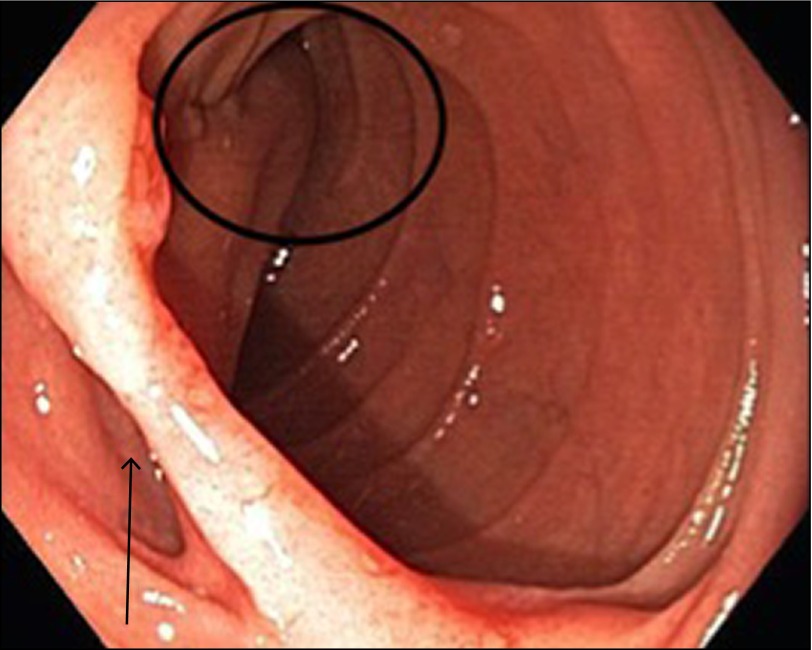
Figure 3.
The arrow indicates the entry site of the false lumen of the IDD on endoscopy. The circle marks the sac of the IDD.
Figure 4.
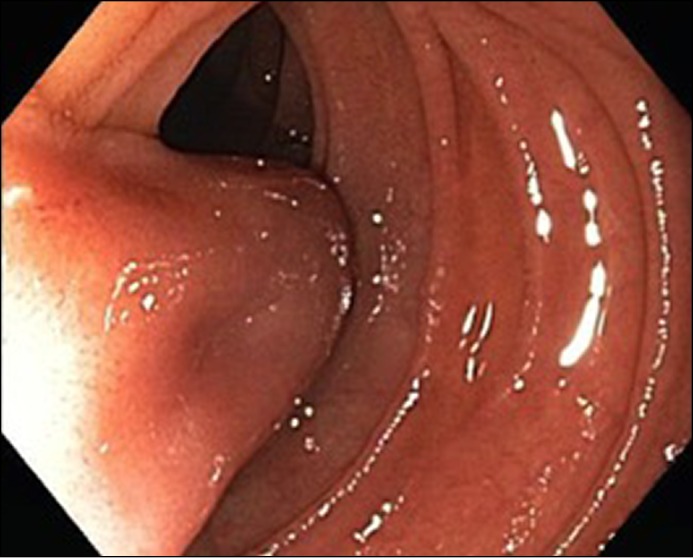
View of the body of the IDD extending into the true lumen of the duodenum with partial obstruction.
Discussion
While some IDD lesions give rise to complaints such as bloating, nausea, vomiting, and abdominal pain, most cases are asymptomatic.2,4,5 Typically, patients may experience weight loss due to discomfort associated with eating.4 Common complications of IDD are partial bowel obstruction, bleeding secondary to ulceration, and, rarely, pancreatitis due to intermittent blocking of the ampulla of Vater.3,6-8 In evaluating patients with such symptoms, the differential diagnosis includes a distal choledochocele, periampullary cystic mass, and duodenal duplication cyst.4,6 Unlike an IDD that is structurally continuous with the duodenal lumen, a duplication cyst is attached to the GI tract and is formed by only 2 layers of the duodenal mucosa.9
Endoscopic exam of the duodenum will reveal 2 lumens: one is the opening of the diverticulum, while the other is the true duodenal lumen. Peristaltic motion and size may limit views of the entirety of the IDD or its attachment site. Diagnosis is often confirmed by an upper GI series that typically shows contrast filling of the diverticulum. Classically, there is a distal radiolucent area that surrounds the diverticulum, representing its diaphragm. This finding is referred to as “windsock” or “finger-of-glove.”1,2 Some centers use computed tomographic (CT) and magnetic resonance imaging for diagnosis, particularly multidimensional CT with pancreatic protocol to enhance these lesions.10,11 However, imaging studies can be limited due to the fluctuating character of these lesions as they intermittently expand and collapse. One case report described a small IDD that was mistaken for a periampullary neoplasm on both CT and endoscopy, only later to be diagnosed as an IDD when a barium upper GI series revealed the classic radiographic findings and confirmed the diagnosis.8
Surgical diverticulectomy has traditionally been the treatment of choice.1-3 However, based on multiple case reports, endoscopic diverticulectomy is an emerging alternative.12,13 The optimal endoscopic approach has yet to be determined, although various techniques using a needle-knife and snares have been described.7,12-14 The greatest potential benefit to patients is that endoscopic intervention affords a faster recovery time than standard surgery, although the paucity of outcomes data is of concern.7 Further evaluation of such endoscopic procedures is warranted, particularly in regards to long-term outcomes.
Adults presenting with IDD in the Western world are less likely to have other congenital anomalies and more likely to present with vague symptoms, or sequelae of IDD, including obstruction, pancreatitis, and anemia. We therefore suggest that IDD be included in the differential diagnosis for persistent yet vague abdominal complaints, as well as for patients with obstruction or bleeding of unknown source.
Disclosures
Author contributions: V. Anand wrote the manuscript. All authors edited and reviewed the manuscript. C. Gruss is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.D'Alessio MJ. Surgical management of intraluminal duodenaldiverticulum and coexisting anomalies. Am J Surg. 2005;201(1):143–8. [DOI] [PubMed] [Google Scholar]
- 2.Mahajan SK, Kashyap R, Chandel UK, Mokta J, Minhas SS. Duodenal diverticulum: review of literature. Indian J Surg. 2004;66:140–5. [Google Scholar]
- 3.Huang FC, Chuang JH, Ko SF. Intraluminal duodenal diverticulum presenting as obstructive chronic pancreatitis. J Pediatr Gastroenterol Nutr. 1998;27:593–5. [DOI] [PubMed] [Google Scholar]
- 4.Reichert MC. Recurrent pancreatitis caused by a huge intraluminal duodenal diverticulum. J Gastrointest Liver Disease. 2012;21:126.. [PubMed] [Google Scholar]
- 5.Hartley RH, Barlow AP, Kilby JO. Intraluminal duodenal diverticulum: An unusual cause of acute pancreatitis. Br J Surg. 1993;80:488.. [DOI] [PubMed] [Google Scholar]
- 6.Willemer S, Hans D, Johann FB, Rudolf A. Recurrent acute pancreatitis and intraluminal duodenal diverticulum. Pancreas. 1992;7:257–61. [DOI] [PubMed] [Google Scholar]
- 7.Acosta-Merida MA, Marchena-Gomez J, Hemmersbach-Miller M, Formoso FJD. Upper intestinal obstruction due to inverted intraduodenal diverticulum. J Surg Case Rep. 2011;1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemente G, G Gerardo ADR, Nuzzo G. Intramural duodenal diverticulum mimicking a periampullary neoplasm. Am J Surg. 2008;196:31–2. [DOI] [PubMed] [Google Scholar]
- 9.Adler D, Roy L. Duplication cysts: Diagnosis, management, and the role of endoscopic ultrasound. Endosc Ultrasound. 2014;3(3):152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston P. MDCT of intraluminal “windsock” duodenal diverticulum with surgical correlation and multiplanar reconstruction. Am J Roentgenol. 2004;183:249–50. [DOI] [PubMed] [Google Scholar]
- 11.Lawler LP, Keith DL, Elliot KF. Multidetector row computed tomography and volume rendering of an adult duodenal intraluminal “wind sock” diverticulum. J Comput Assist Tomogr. 2003;27:619–21. [DOI] [PubMed] [Google Scholar]
- 12.Stevens T, Chand B, Winans C. Endoscopic duodenal “windsock” diverticulotomy. Surg Endosc. 2013;27(4):1406.. [DOI] [PubMed] [Google Scholar]
- 13.Law R, Topazian M, Baron TH. Endoscopic treatment of intraluminal duodenal (“windsock”) diverticulum: Varying techniques from five cases. Endoscopy. 2012;44(12):1161–4. [DOI] [PubMed] [Google Scholar]
- 14.Beeks A, Gosche J, Giles H, Nowicki M. Endoscopic dilation and partial resection of a duodenal web in an infant. J Pediatr Gastroenterol Nutr. 2009;48:378–81. [DOI] [PubMed] [Google Scholar]