Abstract
A practical one-pot C–H activation/borylation/oxidation sequence for the generation of 3,5-disubstituted phenols is presented. Specifically, 3-bromo-5-methylphenol is prepared from 3-bromotoluene, without isolation of intermediates, on a multigram scale, and in high yield. The process proceeds under mild conditions and can be completed within one day.
Keywords: phenol, C–H activation, borylation, oxidation, boronic ester
Phenols are ubiquitous motifs in organic chemistry.1 Within the gamut of phenols known to the synthetic chemist, 3,5-disubstituted phenols constitute an interesting subclass that have garnered interest from the pharmaceutical and fine chemical industries.2 Such phenols, especially those with ortho-/para-directing groups, are often difficult to access through conventional, electronically governed, aromatic substitution chemistry. Illustrative of such difficulties is 3-bromo-5-chlorophenol, which was originally prepared by Hodgson and Wignall.3 Amazingly, for over seventy-five years, no additional syntheses of this molecule appeared in the peer-reviewed literature!
Earlier this year, a group of Hoffman-La Roche chemists lead by Davidson described the challenges of preparing contra-electronically substituted 3,5-disubstituted phenols on a large scale.2 They offered their own preparation of such phenols, which involved an initial ipso-substitution of a halide by an alkoxide followed by subsequent deprotection, as an elegant solution to the problem.2 In that same report, the authors noted that other recent approaches to such molecules suffered in terms of their operation at large or even multigram scale.
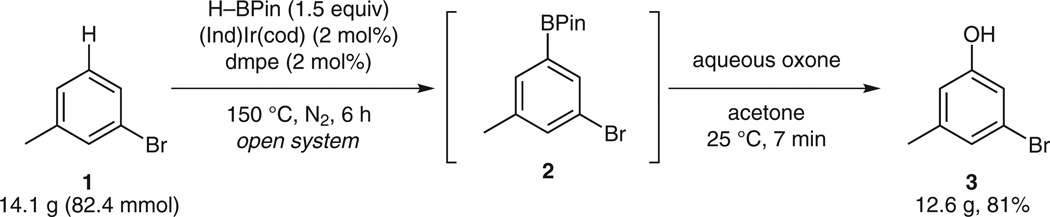
Such criticism certainly applied to our own iridium-catalyzed C–H activation/borylation4/oxidation sequence for the preparation of 3-bromo-5-chlorophenol and other meta-orientated di- and trisubstituted phenols.5 Since the regioselectivity in the direct borylation chemistry of arenes is sensitive to sterics,6 1,3-substituted benzenes are readily borylated at the meta position. Furthermore, these borylations are highly atom economical and clean with hydrogen gas being the only stoichiometric byproduct of the reaction. In lieu of this, we determined that the borylation event could be followed with an Oxone oxidation7 of the crude boronic ester intermediate to the corresponding phenol (Scheme 1). With this one-pot C–H activation/ borylation/oxidation sequence, we prepared a variety of 3,5-disubstituted phenols, including those represented in Scheme 2.5
Scheme 1.
One-pot C–H activation/borylation/oxidation for the preparation of 3 on a multigram scale
Scheme 2.
Representative products originally prepared at 1.0-mmol scale by the one-pot C–H activation/borylation/oxidation of substituted arenes5
Electron-poor and electron-neutral di- and trisubstituted arenes, including heterocyclic, proved amenable to the chemistry and a variety of functional groups, including esters, ethers, amines, and halides were well tolerated. Usefully, Botting and co-workers extended the scope of the methodology to include electron-rich bis-protected resorcinols.8 Thus, C–H activation/borylation/oxidation constitutes the most direct route to a wide variety of 3,5-disubstituted phenols, marking a valuable advance in making accessible otherwise difficult to obtain phenols.9
Nonetheless, as Davidson noted,2 the reactions had only been reported on 1 mmol of arene and the borylation step was performed in a sealed air-free flask. In such a closed system, both the applied heat and the hydrogen gas being generated resulted in pressure buildups that made multigram syntheses10 inherently hazardous and, thus, impractical.
Herein, we report a revised C–H activation/borylation/oxidation protocol whereby the borylation step is conducted in a standard three-necked round-bottom flask that is fitted with a condenser and open to the atmosphere (albeit a nitrogen atmosphere). Of practical importance, the reagents and solvents employed in both steps were used as purchased and without additional purification,11 while the final product isolation consisted of extraction and short column chromatography with silica gel.
In selecting a substrate for scale-up, we chose 3-bromotoluene (1) as it represents a commercially available arene of midlevel reactivity. Thus following our modified protocol, 3-bromotoluene (1, 14 g, 82 mmol) was borylated neat in an open system. Once GC-FID indicated that the starting material was fully consumed, the volatiles were removed under reduced pressure, and then the corresponding crude boronic ester 2 (without any purification) was subjected to oxidation with Oxone. After liquid– liquid extraction, passing the crude phenol through a short silica gel column, evaporation, and filtration of the hexane slurry, pure phenol 3 (12.6 g, 81% isolated yield) was obtained. The yield and purity of the final product were identical to those observed on a 1-mmol scale. Therefore, the ability to produce multigram quantities of 3,5-disubstituted phenols via a practical one-pot catalytic C–H activation/ borylation/oxidation protocol has been demonstrated. We anticipate that this report will find immediate application for the straightforward generation and use of such phenols in academia as well as industry.
Pinacolborane (H–BPin) stabilized with 1% Et3N was obtained from BASF, acetone and CH2Cl2 were both obtained from Mallinckrodt Chemicals, and 1,2-bis(dimethylphosphino)ethane (dmpe) from Strem; they were all used as received. Oxone was purchased from Aldrich as 2 KHSO5·KHSO4·K2SO4 and used as received. (Ind)Ir(cod) was prepared according to a literature procedure.12 Silica gel was purchased from Silicycle (60 Å porosity and 230–400 mesh particle size). The borylation reaction was monitored by Varian CP-3800 GC-FID. 1H and 13C NMR spectra were recorded on a VXR-500 MHz instrument in CDCl3, with chemical shifts reported relative to the residue peak of solvent CHCl3 (δ = 7.24 for 1H and δ = 77.0 for 13C). Melting point was measured on a Thomas-Hoover capillary melting point apparatus and is uncorrected.
3-Bromo-5-methylphenol (3)
Borylation step
Under an N2 atmosphere,13 an oven-dried, 500-mL, three-neck round-bottom flask equipped with a magnetic stir bar was charged with (Ind)Ir(cod) (685 mg, 1.65 mmol) and H–BPin (18.0 mL, 124.0 mmol), followed by dmpe (275 µL, 1.65 mmol) and 3-bromotoluene11 (1, 10.0 mL, 82.4 mmol). The flask was fitted with a reflux condenser and the reaction (still under N2) was heated in an oil bath (150 °C) for 6 h. After being allowed to cool to r.t., the mixture was transferred with CH2Cl2 to a 1-L one-neck roundbottom flask and the volatiles were removed under reduced pressure with a rotary evaporator.
Oxidation step
To the 1-L one-neck round-bottom flask containing crude boronic ester 2, acetone (264 mL) was added. To the resulting solution, aq Oxone solution [50.7 g, 824 mmol dissolved in H2O (264 mL)] was added over 25 min.14 The resulting grey slurry was stirred, open to air, at r.t. for an additional 7 min, and then quenched with sat. aq NaHSO3 (100 mL).
Workup and purification
The reaction slurry was transferred to a separatory funnel and the layers were separated. The aqueous layer was extracted with CH2Cl2 (3 × 200 mL). The combined organic layers were washed with brine, followed by H2O, and concentrated with a rotary evaporator to afford 25.9 g of a dark orange oil. The oil was dissolved in CH2Cl2 and then passed through a short silica gel column [silica gel (300 mL), 100% CH2Cl2]. The product containing fractions were combined and evaporated to dryness. Hexanes (100 mL) were added to flask. The resulting slurry was stirred and then filtered through a Büchner funnel to provide pure 3-bromo-5-methylphenol (3) (12.6 g, 67.2 mmol, 81%) as an off-white solid; mp 56–57 °C (Lit.5 55–57 °C); TLC analysis: Rf = 0.39 (silica gel, CH2Cl2).
1H NMR (500 MHz, CDCl3): δ = 6.90 (m, 1 H), 6.80 (m, 1 H), 6.56 (m, 1 H), 4.78 (bs, 1 H), 2.26 (s, 3 H).
13C NMR (126 MHz, CDCl3): δ = 155.9, 141.4, 124.8, 122.4, 115.8, 115.0, 21.1.
Acknowledgments
We thank the Michigan Economic Development Corp. 21st Century Jobs Fund, the NIH (GM63188 to M.R.S.), the ACS/GCI Pharmaceutical Roundtable, Pfizer, and the Astellas USA Foundation for generous support.
References
- 1.Tyman JP. Synthetic and Natural Phenols. New York: Elsevier; 1996. [Google Scholar]
- 2.Davidson JP, Sarma K, Fishlock D, Welch MH, Sukhtankar S, Lee GM, Martin M, Cooper GF. Org. Process Res. Dev. 2010:477. [Google Scholar]
- 3. Hodgson HH, Wignall JS. J. Chem. Soc. 1926:2077. (b) Also see: Kohn M, Zandman A. Montsh. Chem. 1926;47:357.
- 4. Cho J-Y, Tse MK, Holmes D, Maleczka RE, Jr, Smith MR., III Science. 2002;295:305. doi: 10.1126/science.1067074. Boller TM, Murphy JM, Hapke M, Ishiyama T, Miyaura N, Hartwig JF. J. Am. Chem. Soc. 2005;127:14263. doi: 10.1021/ja053433g. For a review see: Mkhalid IAI, Barnard JH, Marder TB, Murphy JM, Hartwig JF. Chem. Rev. 2010;110:890. doi: 10.1021/cr900206p.
- 5.Maleczka RE, Jr, Shi F, Holmes D, Smith MR., III J. Am. Chem. Soc. 2003;125:7792. doi: 10.1021/ja0349857. [DOI] [PubMed] [Google Scholar]
- 6.C–H Acidities can also influence regiochemical outcomes, see: Vanchura BA, II, Preshlock SM, Roosen PC, Kallepalli VA, Staples RJ, Maleczka RE, Jr, Singleton DA, Smith MR., III Chem. Commun. 2010;46:7724. doi: 10.1039/c0cc02041a.
- 7.Webb KS, Levy D. Tetrahedron Lett. 1995;36:5117. [Google Scholar]
- 8.Marshall LJ, Cable KM, Botting NP. Tetrahedron Lett. 2010;51:2690. [Google Scholar]
- 9.For a Pd-catalyzed borylation/oxidation approach to similar phenols see: Lee Y, Kelly MJ. Tetrahedron Lett. 2006;47:4897.
- 10.For a multigram borylation of 1-chloro-3-iodobenzene with [Ir(OMe)(cod)]2-dtbpy and B2pin2 see: Ishiyama T, Takagi J, Nobuta Y, Miyaura N. Org. Synth. 2005;82:126.
- 11.So as to establish a benchmark yield herein, the starting 3-bromotoluene was distilled over CaH2 under reduced pressure and degassed before use. In most cases the starting arene can be used as received, but yields are dependent on starting material quality, which can vary by vendor and batch. A use test prior to scale up is recommended.
- 12.Merola JS, Kacmarcik RT. Organometallics. 1989;8:778. [Google Scholar]
- 13.(a) Whereas (Ind)Ir(cod) and H–BPin are relatively air stable, dmpe is ‘moderately sensitive to air’13b and should be handled under an inert atmosphere. Herein, the flask into which the initial set of reagents was charged was in a glovebox. Once charged, the flask was moved to a fume hood where the actual reaction was run Burt RJ, Chatt J, Hussain W, Leigh GJ. J. Organomet. Chem. 1979;182:203.
- 14.WARNING: As with any oxidation of this type, one should be watchful for exotherms and control the rate of addition accordingly.