Abstract
The hedgehog (Hh) pathway is a developmental signaling pathway that is essential to the proper embryonic development of many vertebrate systems. Dysregulation of Hh signaling has been implicated as a causative factor in the development and progression of several forms of human cancer. As such, the development of small molecule inhibitors of Hh signaling as potential anti-cancer chemotherapeutics has been a major area of research interest in both academics and industry over the past ten years. Through these efforts, synthetic small molecules that target multiple components of the Hh pathway have been identified and advanced to preclinical or clinical development. The goal of this review is to provide an update on the current status of several synthetic small molecule Hh pathway inhibitors and explore the potential of several recently disclosed inhibitory scaffolds.
Keywords: Basal cell carcinoma, gli, hedgehog signaling, medulloblastoma, smoothened, synthetic small molecules
INTRODUCTION
The Hedgehog (Hh) signaling pathway plays a crucial role in the embryonic development of many tissues and organs. In adult tissues, the pathway is significantly less active with the primary function of maintaining homeostasis of stem cell populations, primarily in the skin and central nervous system. Abnormal regulation of the pathway, specifically via its constitutive activation, has been linked to a variety of human cancers; most notably, basal cell carcinoma (BCC) and medulloblastoma (MB) [1, 2].
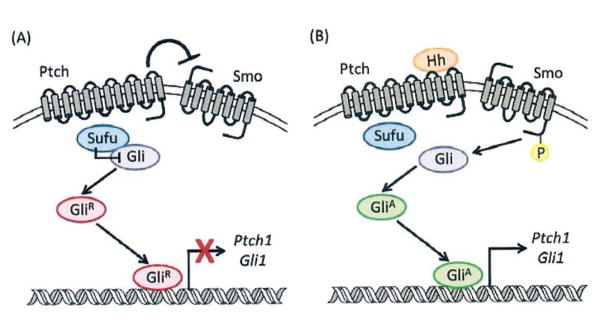
The pathway is regulated through a complex cascade involving the following key signaling proteins: Hh ligands [Sonic (SHh), Indian (IHh), or Desert (DHh)]; Patched (Ptch) a 12 trans-membrane (TM) domain cell-surface receptor; Smoothened (Smo), a 7-TM G-protein coupled receptor (GPCR)-like receptor; Suppressor of Fused (Sufu); and the glioma-associated oncogene (Gli) family of zinc-finger transcription factors. Hh ligands undergo multiple biochemical modifications prior to initiating pathway signaling. Ligands are made as precursors in secreting cells, which undergo autolytic cleavage, followed by the sequential covalent addition of cholesterol to the C-terminus and a palmitoyl moiety to the N-terminus to yield the fully activated Hh ligand. Mature ligands are then secreted to receiving cells via Dispatched (Disp), a transporter protein. The released mature Hh proteins reach the receiving cells through numerous mechanisms including both active and passive transport [3, 4]. When Hh ligands are absent, Ptch prevents the trafficking and localization of Smo to the primary cilia, in turn preventing activation of the signaling cascade (Fig. 1A) [5]. Smo is believed to inhibit various protein kinases (PKA, GSK-3b, and CK1) and cannot do so in the presense of Ptch. In this unliganded state, Gli and Sufu form a heteroprotein complex in the cytosol while Smo remains at the base of the primary cilium. This allows for PKA and Kif7 to promote the proteolytic processing of Gli3 to the repressor form (GliR), while Sufu stabilizes the unprocessed Gli proteins and inhibits Gli2 transcriptional activity [7]. In addition, PKA prevents the accumulation of full-length Gli2 in the cilium; therefore, only the truncated form of Gli3 (GliR) translocates to the nucleus and serves as a direct re pressor of Hh signaling by inhibiting the transcription of key pathway target genes, which include Ptch1 and Gli1.
Fig. (1).
The Hh signaling cascade in the absence (A) or presence (B) of Hh ligand.
When an Hh ligand binds to Ptch, the Hh/Ptch complex is internalized, relieving its repression of Smo, which results in accumulation of activated Smo in the primary cilia [5]. Smo is then phosphorylated, which prevents PKA function and promotes the movement and accumulation of Sufu and Gli at the ciliary tips [6, 7]. Subsequent phosphorylation of the intracellular C-terminus of Smo disrupts the Gli-Sufu complex and full-length Gli (GliA) translocates to the nucleus, where it serves to activate Hh target gene expression [2].
Aberrant or constitutive activation of the Hh pathway in human cancer can evolve via either ligand-independent or ligand-dependent mechanisms. Mutations in Ptch, Smo, or Sufu can promote oncogenic signaling irrespective of canonical Hh ligand activation and cancers that rely on this form of pathway activation are termed ligand-independent. BCCs are the most prominent ligand-independent forms of cancer reliant on aberrant Hh signaling, with a majority of sporadic BCCs exhibiting detectable genetic mutations in Ptch1 (~75%), Smo (10%) or Sufu (~5%) [8, 9]. In addition, Sufu and Ptch mutations have been identified in multiple forms of MB and rhabdomyosarcoma [10–13]. Gli2 amplification has been observed in MB, albeit at low frequency, and it remains to be seen if this amplification is correlative with an increased incidence of acquired resistance to Smo antagonists [14, 15]. Studies have identified multiple mechanisms that contribute to ligand-dependent forms of aberrant Hh signaling in a variety of human cancers; however, it is unclear whether these forms of cancer are dependent on a constitutively active Hh signaling cascade. More detailed descriptions of Hh signaling within the context of both normal development and cancer can be found in several recent review articles [1, 2,16–18].
1. SYNTHETIC SMALL MOLECULE SMO ANTAGONISTS
1.1. Established Smo Antagonists
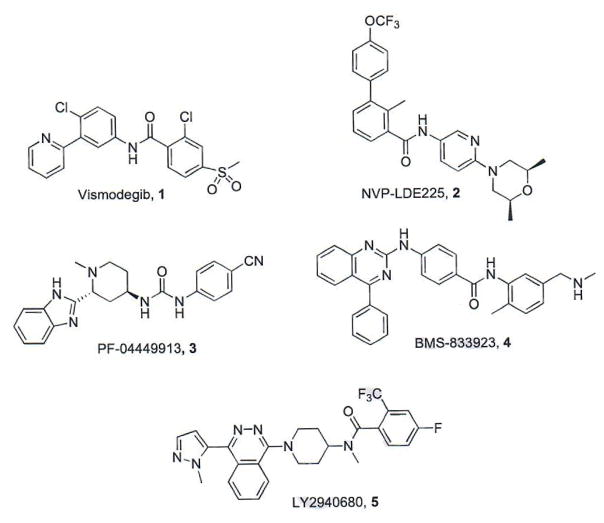
To date, Smo has been the most druggable target for the development of small molecule Hh pathway inhibitors and each of the pathway inhibitors that have advanced into clinical trials is a direct Smo antagonist (Fig. 2, Table 1). The first small molecule Smo antagonist to enter clinical trials and ultimately gain FDA approval for the treatment of advanced BCC was vismodegib (GDC-0449/Erivedge™), which was co-developed by Curis and Genentech [19–21]. Several other pharmaceutical companies have also advanced small molecule pathway inhibitors to clinical trials. NVP-LDE225 (erismodegib/sonidegib) was originally identified and developed at the Novartis Institute for Biomedical Research and has performed well in phase I/II Trials for the treatment of basal cell carcinoma, acute leukemia, and relapsed medulloblastoma [21, 22]. A medicinal chemistry program at Pfizer resulted in the identification of PF-04449913 (glasdegib) as a potent Smo antagonist with favorable pharmacological and pharmacokinetic (PK) properties in vitro and in vivo [23]. PF-04449913 is currently being evaluated in multiple phase I and phase II studies for the treatment of acute myeloid leukemia and myelodysplastic syndrome [21]. Exelixis and Bristol-Myers Squibb collaborated to develop XL-139/BMS-833923, which has been evaluated in phase I and II trials as both a single agent and in combination regimens for the treatment of a variety of human cancers [21, 24]. Eli Lilly has also advanced a small molecule Smo antagonist, LY2940680 (taladegib) into phase I and II trials for pediatric medulloblastoma/rhabdomyosarcoma and small cell lung cancer [21, 25]. Due to the well-established nature of these compounds as Hh pathway inhibitors, each of them has been extensively reviewed elsewhere and further discussions of their path to clinical candidacy will not be detailed herein [3, 4, 26, 27].
Fig. (2).
Structures of established Smo antagonists.
Table 1.
Clinically relevant synthetic Smo antagonists.
| Compound | IC50 Wild-Type Smo | IC50 D473H Smo | Development Status | References |
|---|---|---|---|---|
|
| ||||
| GDC-0449 | 3–22 nMa | Loss of activity | Clinically approved | [19–21] |
| NVP-LDE225 | 2.5 nM | Loss of activity | Phase III | [21,22] |
| PF-04449913 | 5 nM | ND | Phase II | [21,23] |
| BMS-833923 | 21 nM | ND | Phase II | [21,24] |
| LY2940680 | 2.4 nM | Activeb | Phase II | [21,25] |
ND = Not determined or not reported
Dependent on cellular assay
Specific IC50 values were not provided
A major hurdle for the continued advancement of small molecule Hh pathway inhibitors towards clinical efficacy and a driving force behind current drug discovery programs targeting the pathway is the emergence of multiple mechanisms of resistance to these Smo antagonists. An unsuccessful clinical trial for vismodegib in MB resulted in the isolation of a clinically relevant Smo mutant (D473H) that rendered the patient insensitive to further treatment with vismodegib [28–30]. More recently, multiple research groups have identified additional point mutations in Smo that confer resistance to patients receiving vismodegib for the treatment of advanced BCC [31–33]. Smo mutations that prevent ligand binding or conferred constitutive reactivation of the pathway were identified in over 50% of patients that experienced a BCC relapse while receiving vismodegib [33]. Similar reactivating mutations in Smo were identified in a preclinical murine model of Hh-dependent MB following treatment with NVP-LDE225 [14]. Chromosomal amplification of Gli2, induced expression of P-glycoprotein, and direct activation of Gli downstream of Smo have all been identified as additional mechanisms through which Hh-dependent cancers overcome treatment with a Smo antagonist [14, 32–36]. Taken together, these findings clearly highlight the need to evaluate the activity of small molecule pathway inhibitors against resistant forms of Hh signaling at an early stage to provide a better understanding of their potential for clinical efficacy.
1.2. Recently Disclosed Smo Antagonists with Novel Scaffolds
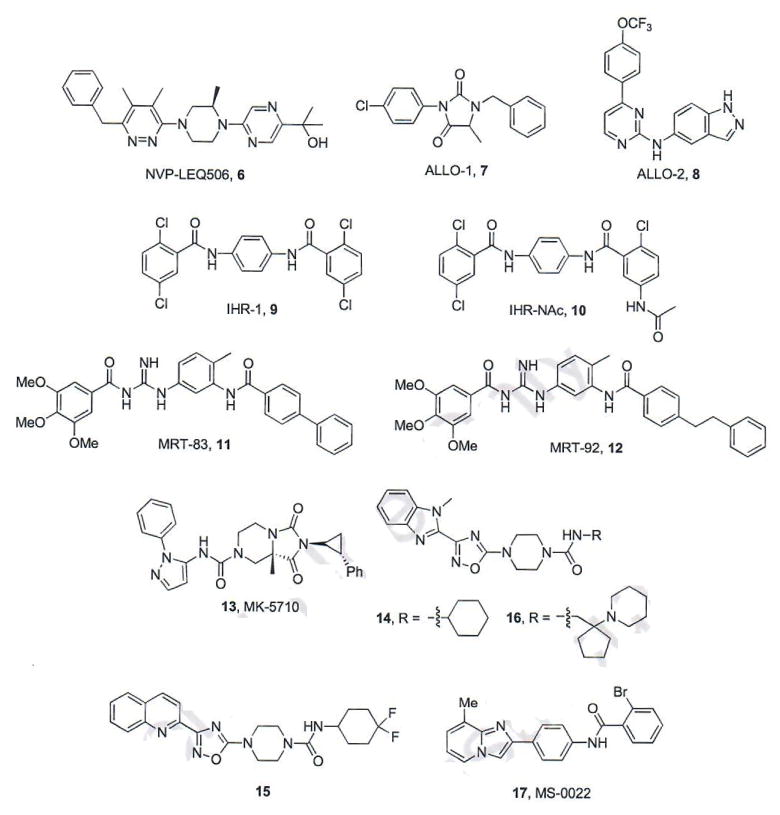
The development of resistance towards vismodegib influenced Novartis to optimize a series of pyridazines as second-generation Hh inhibitors that remain potent in the presence of D473H. The most active compound identified through this process, NVP-LEQ506 (6, Fig. 3), inhibits Hh signaling in vitro against both wild-type and D473H Smo (IC50 values = 1 and 96 nM, respectively) [37]. NVP-LEQ506 reduced Gli mRNA expression and promoted tumor regression in a murine allograft of Hh-dependent MB following oral administration (10–40 mg/kg, q.d. dosing) [37]. Preclinical in vivo PK studies for NVP-LEQ506 established a good bioavailability profile, low clearance, and the ability to cross the blood-brain barrier [37].
Fig. (3).
Recently characterized Smo antagonists.
The Scripps Research Institute, Novartis, and Harvard University collaborated on efforts to identify Hh inhibitors that are effective in the presence of wild-type and mutant Smo [38]. Both ALLO-1 (7) and ALLO-2 (8) were discovered via a high-throughput screen of ~50,000 compounds from Maybridge and ChemDiv. Both compounds inhibit the Hh pathway in a variety of in vitro assays including Gli-luciferase reporter assays (TM3-Gli-Luc cells) and Gli1 expression assays with IC50 values between 5.6 and 410 nM. Because of its structural similarity to several kinase inhibitors, ALLO-2 was screened for inhibitory activity against a panel of kinases; however, it was not active against any of the 99 kinases evaluated. The mechanisms that govern inhibition of Hh signaling by the two compounds were explored in a variety of in vitro assays. Neither compound inhibited the Hh pathway following treatment with Sufu-specific siRNA indicating that ALLO-1 and ALLO-2 act upstream within the Hh signaling cascade. Both compounds successfully competed with BODIPY-cyclopamine (BODIPY-Cyc), a fluorescently-labeled natural product probe commonly utilized to identify direct Smo binding interactions, for binding to mouse Smo over-expressed in CHO-K1 cells, indicating that they form direct binding interactions with Smo (ALLO-1, IC50 = 140 nM and ALLO-2, IC50 = 2.8 nM) [38]. Interestingly, while ALLO-2 demonstrated competitive characteristics with tritiated cyclopamine, [3H]Cyc, at concentrations comparable to its displacement of BODIPY-Cyc in reporter assays, ALLO-1 was inactive in this assay. These results suggest that the binding site of ALLO-1 might reside in a pocket adjacent to the Cyc binding site where the BODIPY interacts with Smo. Finally, both ALLO-1 (IC50 = 1 μM) and ALLO-2 (IC50 = 83 nM) were capable of inhibiting the mouse homolog of Smo D473H (D477G) overexpressed in the TM3-Gli-Luc cell line with only a modest reduction in potency compared to wild-type Smo [38].
Researchers at the University of Texas Southwestern Medical Center identified a series of small molecule Hh pathway inhibitors from a cell-based screen [39]. Several compounds were identified as potent inhibitors of pathway signaling (IHR-1 – IHR-7) with a range of IC50 values from 7.6 – 200 nM. Compounds IHR-1 – IHR-7 (1 μM) successfully competed with BODIPY-Cyc for binding to Smo over-expressed in Cos-7 cells. The most potent of these compounds, IHR-1 (9, Fig. 3, IC50 = 8.9 nM), also demonstrated the ability to prevent translocation of Smo to the primary cilia and down-regulate endogenous expression of Gli mRNA in Ptch1−/− cells. Interestingly, IHR-1 was significantly less active when the Hh pathway was stimulated with SAG, a small molecule Smo agonist (IC50 > 10 μM). Follow-up studies demonstrated that IHR-1 is cell impermeable and unable to compete with SAG for binding to Smo when the receptor is within the cell. An acetylated version of IHR-1, IHR-NAc (10), demonstrated enhanced cellular permeability and was significantly more active against pathway signaling in the presence of SAG (IC50 = 19 nM). These results strongly suggest that Smo antagonists may not be exhibiting maximum results due to their inability to cross the cell membrane and reach all cellular Smo populations [39].
Using a combined molecular modeling and virtual screening approach, researchers at the Centre National de la Recherche Scientifique have designed, synthesized, and evaluated multiple generations of acylureas, acylthioureas, and acylguanidines as potent Smo antagonists [40–43]. A comprehensive medicinal chemistry approach on these molecular scaffolds identified acylguanidine MRT-83 (11) as a potent Hh pathway inhibitor (IC50 value = 15 nM), with the ability to displace BODIPY-Cyc from Smo (IC50 = 5 nM) [41]. Molecular docking studies of MRT-83 with several conformations of Smo suggested that MRT-83 bound to a cleft-opened conformation of Smo and that further extension of the biaryl moiety would provide enhanced interactions between the scaffold and binding pocket [42]. Based on these results, a series of elongated MRT-83 derivatives was synthesized and evaluated. The most potent of these, MRT-92 (12), inhibits Hh signaling (IC50 = 6 nM), prevents Smo accumulation in the primary cilia, and displaces BODIPY-Cyc from human Smo in vitro. Binding studies with a tritiated analogue of MRT-92 ([3H]MRT-92) demonstrated that the compound binds comparably to both WT and D473H Smo (Ki values for both forms of Smo = 0.7 nM). Extensive binding studies between several Smo antagonists (including MRT-92) and various Smo mutants, suggests specific binding interactions which account for the ability of these compounds to retain binding affinity to Smo D473H and potentially provides a new binding mode for the development of Smo antagonists [42].
Researchers at Merck have disclosed the preclinical development of multiple small molecule scaffolds as Hh pathway inhibitors, including MK-5710, [44–47] which is based on the bicyclic hydantoin class of Smo antagonists previously reported [48]. Optimization of the hydantoin scaffold resulted in the identification of homochiral unsubstituted bicyclic tetrahydroimidazo [1,5-a]pyrazine-l,3(2H,5H)-diones as Hh pathway inhibitors [44, 45]. The most potent of these was MK-5710 (13, Fig. 3), which inhibited pathway signaling with an IC50 of 17 nM and displaced BODIPY-Cyc from Smo with high affinity (IC50 = 13 nM). The in vivo pre-clinical assessment of MK-5710 revealed favorable pharmacokinetic properties (oral bioavailability and low clearance) and potent Hh inhibitory activity (40–160 mg/kg, b.i.d) against a murine allograft model of Hh-dependent MB [45]. In addition to MK-5710, a class of piperazinyl ureas were also identified as potent inhibitors of Hh signaling by Merck researchers [46]. Several compounds (14–15, Fig. 3) demonstrated potent down-regulation of Gli1 expression (IC50 values = 5 nM) and displacement of BODIPY-cyc from Smo (IC50 values = 3 and 13 nM, respectively). Continued development of the urea scaffold culminated in compound 16 (Fig. 3), which inhibits Hh signaling with an IC50 of 4 nM and binds with high affinity to Smo (IC50 for BODIPY-Cyc displacement = 5 nM). Further in vitro and in vivo characterization of the anti-cancer activity of 16 has not been reported.
A screen of 12K compounds identified MS-0022 (17, Fig. 3) as an inhibitor of Hh-dependent differentiation of C3H10T1/2 mouse embryonic fibroblasts [49]. MS-0022 demonstrated an IC50 of 100 nM against pathway signaling and modest anti-proliferative effects across several cancer cell lines including pancreatic adenocarcinoma, prostate carcinoma and melanoma. IP administration of MS-0022 in a xenograft model of pancreatic adenocarcinoma (50 mg/kg, q.i.d) decreased tumor volume by 38%. Mechanism of action studies for MS-0022 suggested a dual inhibition through direct binding to Smo (IC50 = 259 nM for competitive displacement of BODIPY-Cyc) as well as micromolar activity against downstream components of the signaling cascade [49].
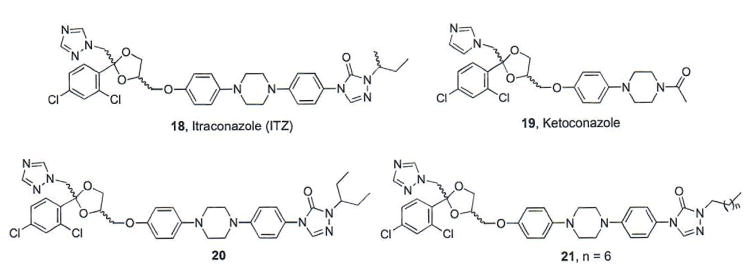
1.3. Itraconazole
Itraconazole (ITZ, 18, Fig. 4) is a member of the triazole class of antifungal agents that is FDA-approved for use in a variety of fungal infections. ITZ exhibits its anti-fungal activity through inhibition of a crucial enzyme in ergosterol biosynthesis, 14α-lanosterol demethylase (14LDM) [50]. A cell-based screen of approximately 2400 FDA-approved or post-phase I compounds identified ITZ as a potent inhibitor of the Hh signaling pathway (initial IC50 = 800 nM) [51]. Based on these results, several structurally related azoles were screened for their ability to inhibit Hh signaling; however, only ketoconazole was identified as a modest Hh pathway inhibitor (IC50 value = 9 μM) while other azole antifungals were completely inactive. These results suggest that the anti-Hh activity of ITZ is via a mechanism distinct from its ability to interact with 14LDM[51].
Fig. (4).
Structures of azole anti-fungals that inhibit Hh signaling.
Multiple lines of evidence support Smo as the cellular target that mediates the anti-Hh properties of ITZ. Cellular activities of ITZ closely mimic the known Smo antagonist Cyc; however, ITZ failed to displace BODIPY-Cyc from human Smo overexpressed in cell culture, suggesting it binds at a distinct site on Smo [51]. Hh pathway activation by a small molecule Smo agonist (SAG) was attenuated with increasing concentrations of ITZ, suggesting that ITZ acts as a non-competitive inhibitor to the SAG binding site [51]. More recently, ITZ demonstrated the ability to inhibit the known Smo antagonist [3H]MRT-92 from binding to human Smo with high potency (Ki = 28 nM), providing strong evidence that ITZ directly antagonizes Smo [42]; however, the exact binding interactions between ITZ and Smo have not been definitively established. ITZ has also demonstrated the ability to inhibit pathway signaling in vitro and in vivo in the presence of multiple Smo mutants (including D477G) [51, 52]. ITZ inhibits Smo D477G-mediated Hh signaling in cell-based assays at levels comparable to its inhibition of Smo WT (IC50 values = 500 nM and 270–690 nM, respectively) [51, 52]. Treatment with ITZ significantly decreased tumor volume and increased survival in Smo D477G MB allografts and oral administration of ITZ (75 mg/kg, bid) significantly increased survival in an orthotopic model of MB expressing Smo D477G, highlighting the ability of ITZ to be centrally active against intracranial tumors. A phase II trial evaluating oral ITZ against BCC demonstrated initial positive results, providing the basis for expanding its use in larger, longer duration trials for the treatment of Hh-dependent BCC [53].
To date, structure-activity relationships (SAR) for ITZ-mediated inhibition of Hh signaling have focused solely on modifications to the alkyl side chain appended to the triazolone [54]. ITZ analogues were evaluated for their ability to inhibit Gli1 expression and proliferation of Hh-dependent MB cells. Interestingly, down-regulation of Gli1 expression did not generally correlate with anti-proliferative activity, suggesting a complicated mechanism of action in the cellular model in which these analogues were evaluated. With respect to down-regulation of Gli1 expression, generally considered the more appropriate model of Hh inhibition, results from these studies suggest that this region is amenable to minor modifications that incorporate linear or branched aliphatic side chains, such as in compounds 20 and 21. Analogues that incorporate cyclic or bulky side chain moieties were significantly less active in their ability to inhibit Hh-dependent MB proliferation and down-regulate Gli1 expression in vitro [54].
Interestingly, ITZ has also been identified as a potent inhibitor of angiogenesis and its anti-cancer activity through this mechanism is also under investigation [54–57]. Preliminary SAR for the ITZ scaffold indicates that inhibition of angiogenesis and Hh signaling do not correlate, suggesting additional cellular targets that might contribute to the anti-cancer properties for this well-established antifungal.
1.4. Pathway Inhibitors that Prevent Smo Translocation/Accumulation to the Primary Cilia
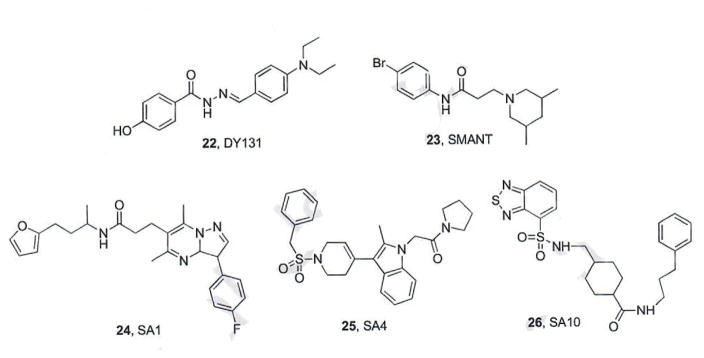
A high content screen of FDA approved drugs, clinical candidates, and small molecules with known biological activity to identify compounds that inhibit Smo accumulation in the primary cilia was recently conducted by researchers at Harvard University [58]. Two small molecules, DY131 (22, Fig. 5) and SMANT (23, Fig. 5), were identified as modest inhibitors of Hh pathway signaling (IC50 values = 0.8 – 2 and 1.1 – 3 μM, respectively). DY131, originally prepared as an Estrogen Related Receptor (ERR) agonist, demonstrated the ability to displace BODIPY-Cyc from Smo. By contrast, SMANT did not compete for Smo binding with Cyc, suggesting a novel mechanism of action for this scaffold [58].
Fig. (5).
Hh Pathway inhibitors that disrupt Smo ciliary distribution.
A similar high content screen performed at the University of California at San Francisco to select small molecules that inhibit Smo translocation to the cilia and/or ciliogenesis identified ten compounds as modest inhibitors of Hh signaling (represented by SA1 and SA4, IC50 value range 0.92 – 19 μM) [59]. All ten compounds reduced Gli1 mRNA expression in Ptch1−/− MEFs and BCC cells, but only compounds SA1-9 displaced BODIPY-Cyc from Smo, demonstrating they function via direct binding to Smo. While SA10 (26, Fig. 5) did not displace BODIPY-Cyc, it was inactive in Sufu−/− cells indicating it also functions at the level of Smo, albeit through an undefined mechanism. Finally, this screen also identified two other small molecules that inhibited ciliogenesis; however, neither of these compounds inhibited Hh signaling in cell culture [59].
1.5. Benzamide-based Smo Antagonists
1.5.1. N-(2-pyrimidinylamino)benzamides
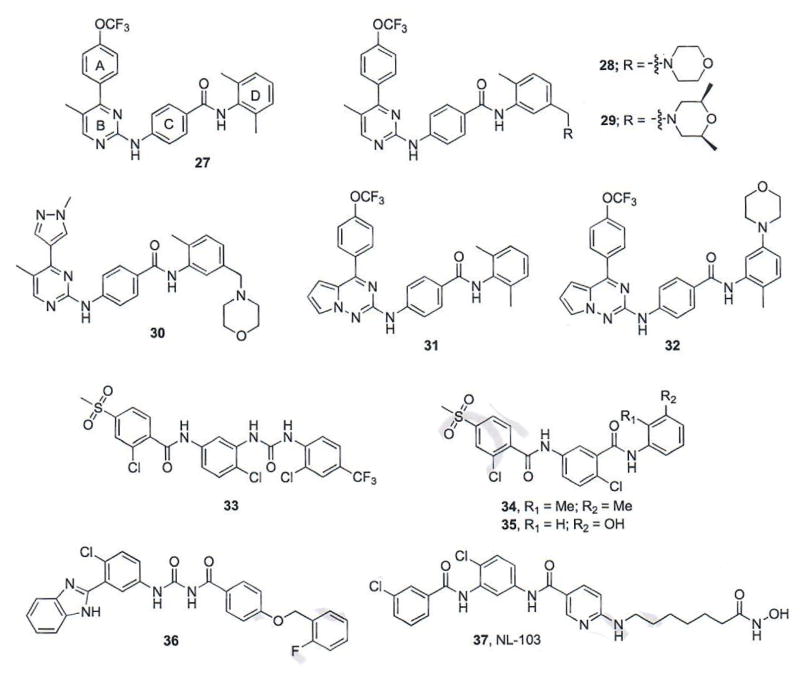
Researchers at Jiangsu Simcere Pharmaceutical have identified a series of N-(2-pyrimidinylamino) benzamides designed by combining structural features of ALLO-2, LDE-225, and BMS-833923 as potent Smo antagonists (Fig. 6) [60, 61]. Overall compound design focused on incorporating the 4-(trifluoromethoxy)phenyl moiety of LDE-225 and ALLO-2 (A), the pyrimidinylamino moiety of ALLO-2 (B) and the biaryl amide functionality of BMS-833923 (C and D) into a single scaffold. The results from an initial screen of three novel hybrid compounds identified compound 27 (Fig. 6) as an Hh pathway inhibitor equipotent to vismodegib (IC50 values = 1.3 and 7.2 nM, respectively) [60].
Fig. (6).
Benzamide-based Smo antagonists.
To explore further SAR for this scaffold, each region (A–D) was modified to generate a series of 39 compounds that were evaluated for their ability to inhibit Hh signaling via a luciferase reporter in NIH3T3 cells stably transfected with a Gli-reporter construct [60]. SAR for region A suggested that a single electron-withdrawing substituent (-OCF3, -CN, -F) in either the ortho- or para-position was important for optimal Hh inhibition when this region was a phenyl ring. The incorporation of heteroaryl functionalities was also well-tolerated in this location. With respect to region B, SAR determined that small hydrophobic groups at either position 5 or 6 of the pyrimidinyl moiety retain potent activity. In addition, removal of the N-1 of the pyrimidinyl ring was detrimental to Hh inhibition. Modifications to the benzamide phenyl ring (D) demonstrated that a wide range of functionalities were tolerated in this region; however, a basic amine was required to improve the overall PK properties of the scaffold. From this series, compounds 28 and 29 demonstrated potent inhibition of Hh signaling (IC50 values = 0.53 and 0.73, respectively); unfortunately, both compounds demonstrated unfavorable PK parameters, presumably due to higher lipophilicity values (28, AlogP = 7.07 and 29, AlogP = 7.37) [61]. Further structural modifications to decrease lipophilicity and improve the overall drug-like properties of the scaffold ultimately resulted in compound 30, which demonstrated potent inhibition of Hh signaling (IC50 =1.3 nM) and more favorable PK parameters (AlogP = 3.6) [61]. Preliminary in vivo PK studies for compounds 28–30 demonstrated that analogue 30 had enhanced oral bioavailability and the authors note that the results of its evaluation in in vivo models of Hh-dependent cancer is forthcoming.
1.5.2. Pyrrolo[2,1-f][1,2,4]triazine Benzamides
A parallel optimization of the N-(2-pyrimidinylamino)benzamide scaffold at Jiangsu was highlighted by substituting the pyrimidine ring (B) with a pyrrolo[2,1-f][1,2,4]triazine [62]. The initial compound in this series (compound 31, Fig. 6) demonstrated potent inhibition of Hh signaling with an IC50 value of 1.6 nM. A similar SAR strategy to that described above for this scaffold yielded a series of compounds that demonstrate enhanced inhibition of Hh signaling (IC50 values = 0.62 – 12.3 nM) and several of the most potent compounds were further analyzed in vivo to determine preliminary PK properties. Compound 32 (IC50 value = 0.83 nM), which contained the initial p-trifluoromethoxy aryl ring and a meta-morpholine moiety on ring D, demonstrated good serum concentration (Cmax = 4185 ng/mL), oral exposure (AUC = 1935 h ng/mL), and half-life (T1/2 = 8.3 h) [62]. The in vivo activity of compound 32 or other analogues in this series has not been disclosed.
1.5.3. Vismodegib-based Benzamides
A third series of benzamide-based Hh inhibitors developed at Jiangsu focused on replacing the pyridine ring functionality of vismodegib with an amide- or urea-linked phenyl ring [63]. It was proposed that the addition of this spacer would enhance hydrogen bonding interactions with Smo. The initial proof-of-concept structure prepared in this series (33, Fig. 6) demonstrated modest inhibition of Hh signaling (IC50 = 300 nM) and led to the preparation and evaluation of several related structures. The most active compounds identified, 34 (IC50 = 100 nM) and 35 (IC50 = 40 nM) displaced BODIPY-Cyc from human Smo overexpressed in HEK-293T cells at concentrations comparable to their anti-Hh IC50 values. Because of its enhanced water solubility, compound 35 was further evaluated in a variety of in vitro and in vivo model systems. Most notably, compound 35 significantly decreased fluorescence associated with Gli-dependent RFP in a transgenic zebra fish model of Hh signaling [63].
Researchers at Shenyang Pharmaceutical University have recently disclosed a series of bisamide derivatives based on a hybrid vismodegib/MRT-83/PF-04449913 scaffold as Smo antagonists [64]. The most potent compound to emerge from these SAR studies, 36 (Fig. 6), incorporates the benzimidazole moiety of PF-04449913, the central 2-chloro phenyl ring of vismodegib and a bisamide moiety as a corollary to the acylguanidine in MRT-83. Compound 36 inhibits Hh signaling with IC50 values between 25 and 63 nM, depending on the cellular assay utilized. Compound 36 also displaced BODIPY-Cyc from human Smo overexpressed in HEK293 cells, verifying its Smo antagonism (IC50 = 19 nM). Molecular modeling studies with 36 docked in the vismodegib binding site on Smo suggest that it maintains minimal interactions with the clinically mutated D473 residue; however, experimental data to verify it retains activity in the presence of SmoD473H has not been reported [64].
Following reports that inhibitors of histone deacetylase enzymes (HDACs) are capable of anti-Hh activity, a group of researchers at the Chinese Academy of Sciences designed and synthesized a chimeric compound, NL-103 (37), which consists of structural components of vismodegib and the FDA approved HDAC inhibitor vorinostat [65, 66]. NL-103 inhibited Hh signaling in a Gli-reporter cell line with an IC50 value of 19.7 nM. NL-103 also inhibited HDACs 1–3 at levels comparable to inhibition with vorinostat. NL-103 displaced BODIPY-Cyc from human Smo overexpressed in HEK293T cells (KD = 79.6 nM). To further explore the cellular effects of NL-103, its ability to regulate Gli1, Gli2, and Gli3 mRNA levels was evaluated in vitro in the absence and presence of Shh-N. Data obtained in the presence of Shh-N contradicted previous findings in the NIH3T3-Gli reporter cell line. Interestingly, in the absence of Shh-N, both NL-103 (10 μM) and vorinostat (3 μM) stimulated Gli1, but reduced Gli2 mRNA expression. By contrast, vismodegib demonstrated significant down-regulation of Gli1 at all concentrations evaluated, but had minimal effects on Gli2 [66]. Taken together, these results suggest that while NL-103 may interact with Smo, the scaffold acts in a fashion more similar to the HDAC inhibitor vorinostat than to Hh inhibitor vismodegib; HDAC inhibitors exhibit anti-Hh activity via the downregulation of Gli2 expression.
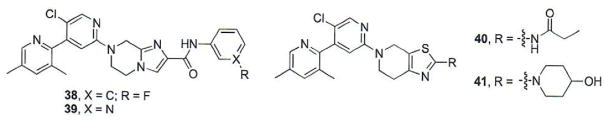
1.6. Additional Vismodegib-Based Smo Antagonists
Researchers at Soochow University have utilized a scaffold hopping approach to design and optimize a series of Smo antagonists based on the scaffold of several known Smo antagonists [67–69]. Previous reports suggested that the amide bonds of both vismodegib and NVP-LDE225 were important for linking the aryl regions of these two molecules, but did not exert specific binding interactions with Smo [68–71]. It was hypothesized that replacing the amide bond of vismodegib with an alkyl or heteroalkyl ring would reduce planarity and rotatable bonds, increase solubility, absorption, and metabolic stability [67]. This strategy led to the development of two related series of compounds based on either a tetrahydroimidazo[1,2-a]pyrazine or tetrohydrothiazolo[5,4-c]pyridine scaffold [67, 68]. An extensive medicinal chemistry effort in which a wide range of alkyl and aryl esters and amides were appended as the distal substituent of the imidazopyridine provided important SAR for this molecular scaffold. Short alkyl substituents, both straight chain and ring structures, were less active while phenyl amides that incorporated an electron withdrawing moiety at the meta- or para- position demonstrated the most potency [67]. The most active analogues in this series, 38 and 39 (Fig. 7), inhibited pathway signaling with IC50 values of 87 and 200 nM, respectively. Interestingly, for the closely related thiazolopyridine scaffold, the most potent compounds evaluated incorporate either an ethyl side chain (compound 40, IC50 = 80 nM) or substituted piperidine (compound 41, IC50 = 58 nM) as the distal substituent [68]. Both of these compounds displaced BODIPY-Cyc from full length human Smo over-expressed in U2OS cells at levels comparable to their inhibition of pathway signaling. Preliminary in vivo studies for 40 and 41 demonstrated promising PK properties similar to those exhibited by vismodegib.
Fig. (7).
Additional vismodegib-based Smo antagonists.
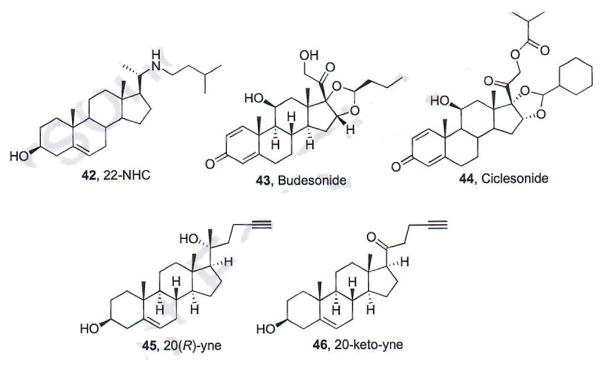
1.7. Sterol-Based Smo Antagonists
Multiple sterol-based Smo antagonists have been identified by several different academic research groups [72–74]. The azasterol 22-NHC (42, Fig. 8) inhibits Hh signaling with an IC50 of 3 μM and does not affect interactions between Smo and other known Smo antagonists acting on the Smo TM region [72]. The synthetic glucocorticoids Budesonide (43) and Ciclesonide (44) were initially identified as pathway inhibitors through their ability to inhibit Smo ciliary accumulation (Fig. 8) [73]. In a panel of in vitro assays, Budesonide demonstrated modest and comparable inhibition of pathway signaling against both wild-type and D473H Smo (IC50 values ~50 μM). 20(R)-yne (45) and 20-keto-yne (46), were also identified as modest Hh pathway inhibitors (IC50 values of approximately 5 – 10 μM) [74]. Both 45 and 46 retained the ability to inhibit Hh signaling mediated through Smo D477G (~75% inhibition of signaling at 25 μM). Follow-up studies for these sterols has demonstrated that, in contrast to the Smo antagonists described above, each of these function through direct binding to a recently identified binding site on the extracellular cysteine rich domain of Smo (CRD, described in more detail below). The anti-Hh activity for key Smo antagonists is summarized in Table 2.
Fig. (8).
Sterol-based smo antagonists.
Table 2.
Key synthetic Hh pathway inhibitors that function via Smo.
| Compound | IC50 Wild-Type Smo | IC50 D473H Smo | References |
|---|---|---|---|
|
| |||
| NVP-LEQ506 | 1.0 nM | 96.0 nM | [37] |
| ALLO-1 | 410 nM | 1 μM | [38] |
| ALLO-2 | 41 nM | 83 nM | [38] |
| IHR-1 | 0.01 μM | ND | [39] |
| IHR-NAc | 0.0031 μM | ND | [39] |
| MRT-92 | 0.7 ± 0.1 nM | 0.7 ± 0.2 nM | [42] |
| MK-5710 | 17 nM | ND | [41] |
| 16 | 4 nM | ND | [46] |
| MS-0022 | 100 nM | ND | [49] |
| ITZ | 270–690 nMa | 500 nM | [50–53] |
| 27 | 1.3 nM | ND | [60] |
| 30 | 1.3 nM | ND | [61] |
| 32 | 0.83 nM | ND | [62] |
| 35 | 40 nM | ND | [63] |
| 36 | 19 nM | ND | [64] |
| NL-103 | 19.7 nM | Activeb | [65, 66] |
| 38 | 87 nM | ND | [68] |
| 40 | 80 nM | ND | [68] |
| 41 | 58 nM | ND | [68] |
| 22-NHC | 3 μM | ND | [72] |
ND = Not determined or not reported.
Dependent on cellular assay.
Specific IC50 values were not reported.
1.8. Identification and Mapping of Small Molecule Binding Sites on Smo
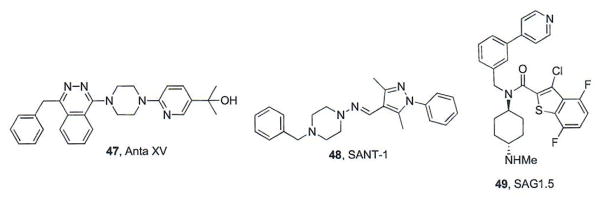
Detailed structural information about small molecule binding site(s) on the 7-TM domain of Smo has come from several recently published co-crystal structures describing the region in complex with either an antagonist (LY2940680, Cyc, SANT1 (48), or Anta VX (47), or agonist (SAG1.5, 49, [75–77] (Fig. 9). These studies have revealed a long, narrow cavity formed by the 7-TM helices, the extracellular domain (ECD) and three extracellular loops (ECLs 1, 2, and 3). The continuous nature of this cavity provides multiple binding sites or “sub-sites” suitable for a variety of small molecule scaffolds. SANT1 binds deep in the pocket and its entire structure is contained within the lipid bilayer [77] By contrast, the other antagonists and SAG1.5 orient closer to the extracellular surface of the cavity in a sub-site that primarily consists of the ECD linker domain and the ECLs [77]. Their proximity to the binding site opening provides a structural rationale for the attachment of bulky moieties or reporters (Biotin, BODIPY, etc.) to these scaffolds. In addition, these studies have provided insight as to potential structural mechanisms that govern the development of chemoresistance for certain Smo antagonists. Direct contact between LY2940680 and D473 is minimal (4.04 – 4.31 Å between the aspartic acid carboxylate and the Smo antagonist), presumably due to strong interactions between LY2940680 and ECL3, which shifts the phthalazine core to an axial conformation, ultimately decreasing interactions with D473H. By contrast, Anta XV does not form tight binding interactions with ECL3 and the piperazine ring is equatorial, resulting in a direct interaction with D473H (3.3 Å) [77]. Interestingly, binding of SAG1.5 in this binding site induced a remodeling of several polar interactions between R400, D473, and E518, which may be responsible for its ability to functionally activate Hh signaling.
Fig. (9).
Additional Smo modulators utilized for structural studies.
In addition to small molecule binding sites within the 7-TM domain of Smo, several independent research groups have explored the ability of Hh pathway modulators to exert their activity through direct binding interactions with the extracellular cysteine rich domain (SmoCRD) [72–74, 78–79]. These studies have all focused on exploring the role of the SmoCRD in binding Hh agonists and antagonists with a sterol or sterol-like scaffold. Initial assays utilizing Smo deletion and/or ligand affinity protocols demonstrated that the Hh agonist properties of oxysterols (OHCs) are mediated through direct binding to the SmoCRD and that small molecules that bind in this location can be agonists or antagonists, depending on structure [72–74, 78]. In addition, these experiments provided strong evidence that the OHC binding site is localized to “site 1” of the CRD, a hydrophobic pocket formed by side chain α-helices that is analogous to the lipid-binding grove previously identified in the Frizzled CRD. Two recent reports have provided additional detailed structural insights into this small molecule binding site via the crystal structure of the zebrafish SmoCRD (zSmoCRD, 2.3 Å resolution) [74] and the NMR solution structure of the Drosophila SmoCRD (dSmoCRD) [79]. Molecular docking studies between zSmoCRD and 20(S)-OKC, a well-characterized pathway agonist, suggested the tetracyclic core of the OHC scaffold lies in the base of the lipid-binding groove at site 1 lined by W87 and L90. This docking model predicts that the 3β-hydroxy of 20(S)-OHC orients towards helix 1 residues L90 and N92, while the 20(S)-hydroxy and side chain are in close proximity to residues P142 and F144.74 Mutagenesis studies on residues within the predicted binding site supported the structure-based model of the OHC binding pocket; however, specific molecular interactions between the OHC scaffold and these residues have not been detailed [74]. The solution structure of Budesonide in complex with dSmoCRD further supports the site 1 lipid-binding groove as the key binding pocket for sterol-based pathway modulators of Hh signaling within the CRD [79]. Chemical shift perturbations identified potential binding interactions between budesonide and R161, W109, G111, and L112, all of which reside within site 1 and were previously identified as key for 20(S)-OHC binding.
2. HEDGEHOG PATHWAY INHIBITORS FUNCTIONING UPSTREAM OF SMO
2.1. RU-SKI 43
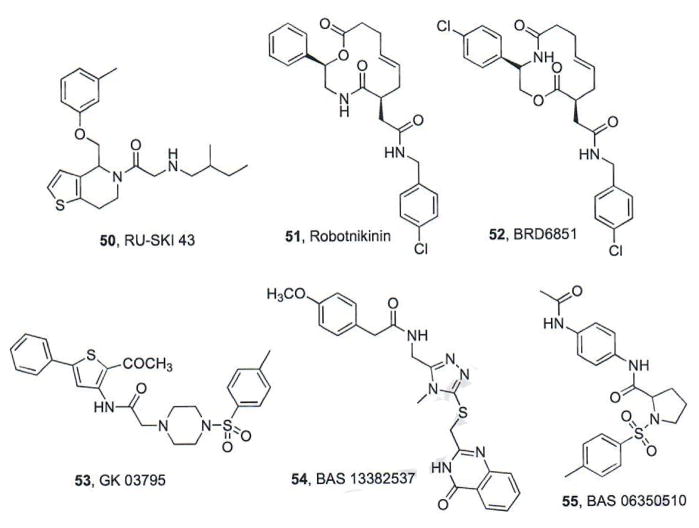
Hedgehog acyltransferase (Hhat) catalyzes the covalent attachment of a palmitoyl group to the N-terminal cysteine of SHh through an amide bond; a post-translational modification required for its full range of biological activity [80]. A high-throughput screen of approximately 64K compounds designed to identify inhibitors of Hhat-mediated SHh palmitoylation identified four structurally-related thiophenes with IC50 values ranging from 0.2–5 μM [81]. Of these, RU-SKI 43 (50, IC50 = 0.85 μM, Fig. 10) was chosen for further evaluation. RU-SKI 43 was identified as a non-competitive inhibitor (Ki = 6.9 μM) with respect to [I125] iodo-palmitoyl CoA and an uncompetitive inhibitor with respect to SHh. In addition, RU-SKI 43 was selective for Hhat and not generally active against other fatty acyltransferases; however, neither the in vitro nor in vivo anti-cancer activities of RU-SKI 43 have been reported [81].
Fig. (10).
Hh pathway inhibitors that function upstream of Smo in the signaling cascade.
2.2. Robotnikin and Related Compounds
Several macrolactones that inhibit Hh signaling through direct binding to SHh were identified in a microarray-based screen (10K compounds) by researchers at the Broad Institute [82]. Optimization of the lead scaffold resulted in robotnikinin (51, Fig. 10), which binds to SHhN (an active N-terminal fragment of SHh) with a Kd value of 3.1 μM and inhibits Gli-luciferase reporter activity in Hh-dependent cells (IC50 = 4 μM) [82]. Additional SAR studies produced a series of analogues, including BRD6851 (52, Fig. 10), as an Hh inhibitor with improved activity (IC50 = 0.4 μM) [83]. Interestingly, even though BRD6851 was derived from the robotnikin scaffold, preliminary studies into its mechanism of Hh inhibition suggested it functions at the level of Smo; however, these studies were indirect and displacement of a known small molecule Smo antagonist by BRD6851 has not been demonstrated. To date, in vivo data in Hh-dependent models of cancer have not been reported.
Computational approaches to design small molecules that perturb SHh/Ptch binding interactions have also been undertaken [84, 85]. Robotnikin and structurally-related analogues were utilized to develop a 3D-pharmacophore model that was used in a virtual screen of >100K compounds to identify structurally-related small molecules as possible Hh pathway inhibitors [84]. This screen and follow-up modeling studies suggested that GK 03795 (53, Fig. 10) should bind SHh in the same location as robotnikin. A second virtual screen of >200K utilizing a computational model of key SHh/Ptch binding interactions was performed to identify small molecules that disrupt this specific interaction [85]. Additional docking and computational analysis of two hit compounds, BAS 13382637 (54) and BAS 06350510 (55), suggest their potential to bind SHh with high affinity (Fig. 10) [85]. To date, experimental verification of these simulation results in in vitro or in vivo assays has not been reported.
3. PATHWAY INHIBITORS THAT FUNCTION DOWNSTREAM OF SMO
3.1. Gli Antagonists
As noted above, the development of resistance to vismodegib has resulted in multiple groups undertaking development strategies that target other Hh pathway components. In addition, the development of MB can result from Sufu mutations, loss of heterozygosity of the Sufu gene, and increased Gli effector function. Taken together, these findings highlight the need to develop small molecule inhibitors that function at a level downstream of Smo. Gli proteins play an integral role as transcriptional regulators that directly control Hh target gene expression. Thus, inhibitors of Gli protein function possess the potential to be broadly active in both Smo-dependent and Smo-independent forms of Hh-dependent cancer.
3.1.1. GANT58 and GANT61
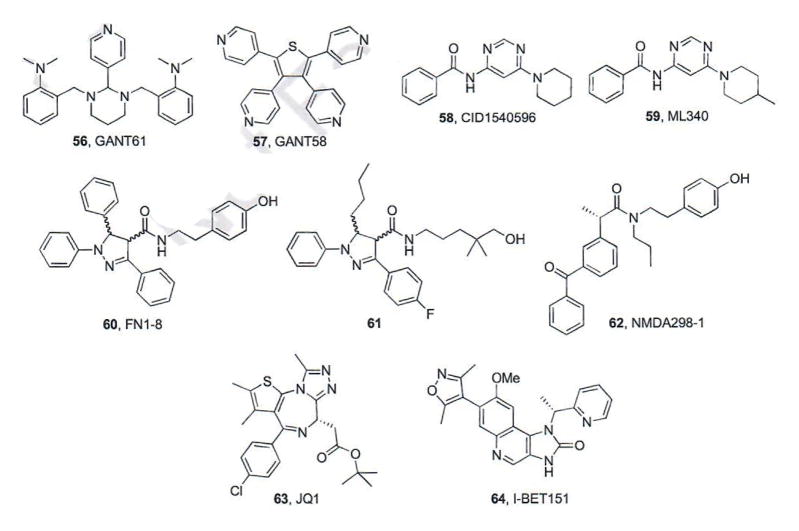
In 2007, the first Gli inhibitors, GANT61 (56) and GANT58 (57), were discovered during a screening assay searching for small molecules that inhibit Gli-mediated gene transcription at the Karolinska Institutet (Fig. 11) [86]. Both GANT compounds inhibited Gli1-and Gli2-mediated transcription in vitro in a dose-dependent manner with IC50 values of ~5 μM. In addition, both compounds reduced Gli1 and Hip1 mRNA expression in Sufu−/− mouse embryonic fibroblasts demonstrating that their molecular target is downstream of Smo. Treatment with GANT61 in HEK293 cells showed increased accumulation of transfected Gli1 in the nucleus suggesting inhibition of nuclear Gli, which was verified by electrophoretic mobility shift assays in which pretreatment with GANT61 reduced the Gli-DNA binding band [86]. Gli regulation has demonstrated the potential to play a broader role in tumor formation and growth than canonical Hh signaling; therefore, Gli antagonists may be more applicable to a wider range of cancers when compared to Smo antagonists. As such, both GANT58 and GANT61 have been evaluated in vitro and in vivo for their anti-cancer properties in a variety of human cancers, including colon, lung, pancreas, and leukemia [87–92].
Fig. (11).
Pathway inhibitors that function at the level of Gli.
Recently, a combined in vitro and in situ approach was utilized to determine the region in which GANT61 interacts with the Gli proteins [90, 93]. In general, Gli proteins possess five zinc finger (ZF) domains, of which ZF4 and ZF5 are responsible for binding to the target DNA while the other three are thought to contribute to the recruitment of other regulatory co-factors by binding to the phosphate backbone [90, 93]. GANT61 was shown to selectively bind between ZF2 and ZF3 of Gli1 at E119 and E167 sites. Sequence alignment of Gli1 and Gli2 confirmed that the amino acid residues within 3.5 Å of GANT61 were conserved [90]. In addition, this binding interaction was specific to Gli1 as GANT61 did not demonstrate binding affinity for several other zinc finger transcription factors [90, 93].
3.1.2. ML340
A screen for inhibitors of the Hh pathway that function downstream of Sufu was performed at Sanford-Burnham Medical Research Institute using the NIH Molecular Libraries-Small Molecule Repository Collection (~360K compounds) [94]. An initial screening hit (58, IC50 values 24 – 56 nM) underwent medicinal chemistry efforts in which modifications were made to the phenyl ring, pyrimidine, and piperidine. Interestingly, following these extensive modifications the compound chosen for further analysis, ML340 (59, IC50 = 34 nM), was strikingly similar to the initial hit. ML340 was shown to be selective for Hh signaling when compared to the Wnt pathway and demonstrated high permeability and plasma stability in vitro. By contrast, ML340 is poorly soluble in aqueous media, demonstrates reduced stability in both human and mouse liver microsomes and binds plasma proteins at a high level. Taken together, these data suggest that ML340 will be useful as a chemical probe of Hh activity downstream of Smo, but requires additional medicinal chemistry efforts to improve essential preclinical PK properties if the scaffold is to advance as an anti-cancer chemotherapeutic [94].
3.1.3. FN1-8/NMDA298-1
Recently, researchers at the University of California at San Francisco demonstrated that the conserved α-helix region of Gli, “FXXΦΦ” (X= any residue; Φ=hydrophobic residue) interacts with the transcriptional co-activator TBP-Associated Factor 9 (TAF9) [95–97]. Based on these observed interactions and the residues within this conserved domain, a small molecule inhibitor of this protein-protein interaction, FN1-8 (60, Fig. 11), was designed, synthesized, and evaluated as a racemic mixture. Cellular assays demonstrated that FN1-8 specifically disrupted the co-localization of Gli/TAF9 and did not affect TAF9 interactions with other transcription factors such as p53 [94, 95]. Follow-up studies in non-small cell lung cancer (NSCLC) and pancreatic cancer verified that FN1-8 functioned downstream of Smo at the level of Gli and treatment of these cell cultures with FN1-8 resulted in concentration-dependent inhibition of cellular proliferation [95]. A small SAR series for this scaffold ultimately resulted in the preparation of compound 61, which reduced Gli expression and tumor volume in xenograft models of NSCLC, melanoma, and mesothelioma [96]. Purification of the racemic mixture into its constituent enantiomers identified one enantiomer as significantly more potent; however, the structure of the more potent compound has not been disclosed [96].
Based on the initial activity of FN1-8, a group at St. Jude Children’s Research Hospital performed SAR studies by removing the pyrazoline while maintaining a tyramine amide moiety [97]. The studies ultimately provided NMDA298-1 (62), which possessed approximately 3-fold selectivity for inhibition of Gli1-mediated transcription compared to Gli2 (IC50 values = 6.9 and 23.9 μM, respectively). Cellular growth of multiple cultured cancer cell lines, including breast (MDA-MB321), rhabdomyosarcoma (Rh30), prostate (DU145), and medulloblastoma (DAOY), was inhibited by NMDA298-1; however, a correlative relationship between cell viability and Gli-mediated transcription was not identified, suggesting potential non-specific effects of NMDA298-1 that complicate understanding of its cellular effects [97].
3.2. Bromodomain Inhibitors
Bromodomains (BRD) are protein recognition elements that selectively bind acetylated lysine residues on a variety of proteins. Most commonly, bromodomains are associated with the ability to recognize and bind acetylated histones, thus regulating gene expression in an epigenetic fashion [98, 99]. A major class of bromodomain-containing proteins are the bromo- and extraterminal (BET) family, which consist of several related proteins that contain two highly conserved N-terminal BRDs and an extra C-terminal domain. Not only do BET proteins interact with acetylated N-lysine motifs on open chromatin, but they have also been shown to interact with a phosphorylated serine residue on RNA polymerase II and positive transcription elongation factor (P-TEFb) [100].
A recent study demonstrated that the BET protein BRD4 regulates GLI transcription downstream of Smo and Sufu by directly occupying the GLI1 and GLI2 promoters [101]. These results led researchers at Stanford University to explore whether inhibition of BRD4 could down-regulate Gli expression and inhibit Hh signaling. JQ1 (63, Fig. 11) is a known small molecule inhibitor of BRD4 that demonstrates high affinity for both bromo-domains in the protein (Kd values = 50–90 nM) [100]. JQ1 binds to the acetyl-lysine binding cavity of BRD4 and stabilizes the binding pocket by rigidifying the loop domains. JQ1 down-regulated Gli1 and Ptchl expression in WT MEFs at levels comparable to vismodegib and NVP-LDE225. In addition, JQ1 demonstrated potent down-regulation of both Gli1 and Gli2 in Sufu−/− MEFS, confirming that its Hh inhibitory properties were downstream of Smo/Sufu. JQ1 inhibited pathway signaling and decreased cell viability in vitro in several Hh-dependent models of MB, including an MB cell line containing the vismodegib-resistant Smo D477G mutant (IC50 values = 50–150 nM) [101]. Finally, JQ1 administration (50 mg/kg, i.p.) significantly reduced tumor growth in both flank and intracranial models of MB containing either Smo WT or Smo D477G and was moderately active against Hh-dependent BCC.
A cell-based screening effort at the University of Miami to identify epigenetic regulators that could inhibit Hh signaling identified I-BET151 (64), an inhibitor of several BET proteins (BRD2–4), as a potent Hh pathway inhibitor (IC50 value = 31 nM) [102]. I-BET151 maintained potent inhibition in Sufu−/− MEFs, verifying that its ability to inhibit pathway activity was at the level of Gli. Additional mechanism of action studies demonstrated that I-BET151 decreases the occupancy of BRD4 on the transcriptional start site of the Gli1 locus, suggesting its inhibition of Hh signaling is a result of its direct binding to BRD4. I-BET151 reduced Gli1 expression and cell viability in Ptch+/− MB cells in vitro (IC50 ~500 nM). In a similar subcutaneous model of Hh-dependent MB, I-BET151 administration (30 mg/kg, i.p.) reduced both tumor volume and tumor-associated Gli1 expression, highlighting its in vivo anti-Hh properties [102]. The anti-Hh activity for key pathway inhibitors that function upstream or downstream of Smo is summarized in Table 3.
Table 3.
Key Hh pathway inhibitors that affect signaling upstream or downstream of Smo.
| Compound | Target | IC50 Wild-Type Smo | IC50 D473H Smo | References |
|---|---|---|---|---|
| Robotnikin | SHh | [82] | ||
| BRD6851 | SHh | [83] | ||
| GANT58 | Gli | 5 μM | ND | [86] |
| GANT61 | Gli | 5 μM | ND | [86] |
| ML 340 | Gli | 34 nM | ND | [94] |
| FN1-8 | Gli | Activea | ND | [95] |
| 39 | Gli | 0.5–1.4 μM | ND | [96] |
| NMDA298-1 | Gli | 6.9 μM | ND | [97] |
| JQ1 | BRD4 | 50–150 nM | Activea | [101] |
| I-BET151 | BRD4 | 31 nM | ND | [102] |
ND = not determined or not reported.
Specific IC50 values were not reported.
4. INDIRECT INHIBITORS OF HEDGEHOG PATHWAY SIGNALING
4.1. Mebendazole
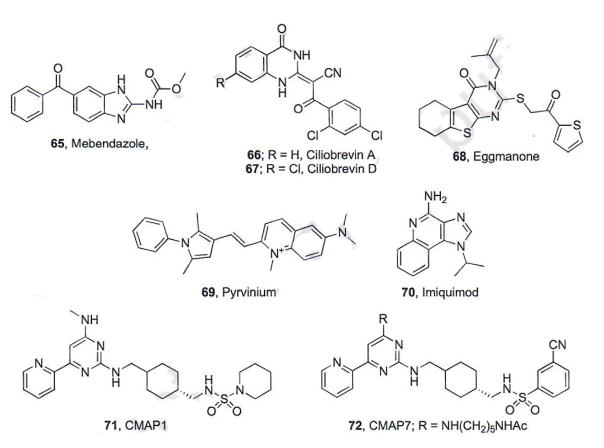
Mebendazole (MBZ, 65, Fig. 12) is a benzimidazole approved by the FDA for use as an antihelmintic agent [103]. MBZ exhibits its antihelmintic activity through direct binding to the colchicine site of tubulin and inhibition of microtubule polymerization. Inhibiting microtubule formation prevents the nematode from absorbing glucose and ultimately leads to nematode death. MBZ also interacts with human tubulin, but on an order of magnitude less than nematode tubulin, accounting for its general specificity for parasite infections. Because previous screens have identified microtubule-interacting drugs as modest Hh pathway inhibitors and due to the importance of microtubules in the formation of primary cilia, researchers at Johns Hopkins University evaluated MBZ for its ability to inhibit Hh signaling. MBZ inhibited Hh pathway signaling in vitro in Hh-dependent mouse embryonic fibroblasts (MEFs, IC50 ~1 μM) and DAOY medulloblastoma cells (IC50 = 0.5 μM) [103]. Other structurally related antihelmintic compounds were less effective or inactive in the MEFs, suggesting the specificity of MBZ. Oral administration of MBZ (50 mg/kg, once daily dosing) reduced Gli expression in an orthotopic xenograft model of medulloblastoma, which correlated with decreased tumor volume and increase survival. Finally, MBZ inhibited Hh signaling in the presence of multiple forms of vismodegib-resistant mutant Smo, including D477G, at levels comparable to wild-type Smo [103].
Fig. (12).
Hh inhibitors with indirect actions on pathway signaling.
4.2. Inhibitors of Cytoplasmic Dynein
A previous screening effort at Stanford University designed to identify Hh pathway inhibitors that function downstream of Smo identified HPI-4 (ciliobrevin A, 66, Fig. 12) as a modest inhibitor of Hh signaling (IC50 = 7 μM) [104]. Due to its promising Hh inhibitory properties and its ability to reduce the number and size of primary cilia in vitro, additional SAR and biochemical studies were performed on the scaffold to explore its mechanism of action [105]. Modifications to the 2,4-dichlorobenzoyl aromatic ring abolished inhibitory activity, while modest additions to the quinazolinone ring were well tolerated. Each active compound identified (ciliobrevins A–D) modestly inhibited Hh signaling (IC50 values = 7–14 μM). Each ciliobrevin inhibited Hh signaling in the presence of a constitutively active Smo mutant; however, only ciliobrevins A and D elevated ciliary Gli2 levels, providing evidence that these compounds target protein trafficking mechanisms. It has been previously shown that loss of the primary cilia-specific cytoplasmic dynein 2 heavy chain also increases ciliary Gli2 levels [105]. To study this effect, subcellular localization of intraflagellar transport 88 (IFT88), which requires cytoplasmic dynein 2 dependent retrograde-transport to the basal body, was measured. Within an hour of ciliobrevin D treatment, elevated levels of IFT88 were seen at the distal tip of primary cilia suggesting its ability to inhibit cytoplasmic dynein 2 function. Furthermore, this class of compounds prevented cytoplasmic dynein-dependent microtubule gliding and selectively inhibited the ATPase activity of cytoplasmin dynein, providing strong evidence that inhibitors of this enzyme can down-regulate Hh signaling by inhibiting proper Gli trafficking [105].
4.3. Inhibitors of Phosphodiesterase 4
A screen of ~30,000 small molecules designed to identify compounds with the ability to alter embryonic zebrafish patterning was performed at Vanderbilt University [106]. This screen identified a scaffold termed eggmanone (68) that selectively reproduced the Hh-null phenotype [106]. Eggmanone inhibited Gli-luciferase activity in a dose-dependent manner when stimulated with recombinant SHh ligand (IC50 ~ 1 μM); however, when Gli2 was transiently overexpressed, eggmanone treatment had no effect on Hh signaling, suggesting it targets a pathway component upstream of Gli. Further studies demonstrated that eggmanone did not displace BODIPY-Cyc from Smo in vitro and retained anti-Hh activity in Sufu−/− cells. Taken together these data indicated that eggmanone functions at a point in the Hh pathway between Sufu and fully active Gli. Due to the known role PKA plays with respect to regulating Gli activation/deactivation, eggmanone was evaluated for its ability to directly modulate PKA. While eggmanone was not shown to directly regulate PKA, immunostaining results did indicate that eggmanone treatment increased PKA activity at the base of the primary cilium, suggesting localized PKA activation by an indirect mechanism. PKA function in the basal body is activated in response to cyclic adenosine monophosphate (cAMP) and cAMP levels are tightly regulated through degradation by phosphodiesterase (PDEs) enzymes. Based on these control systems, eggmanone was evaluated for its ability to inhibit several PDEs and was identified as a potent, selective inhibitor of PDE4D3 (IC50 = 72 nM) [106]. Additional studies in Hh-dependent cell culture following exogenous addition of PDE4D3 or the pan-PDE inhibitor rolipram demonstrated that inhibition of Hh signaling by eggmanone is through direct antagonism of PDE4, which ultimately results in PKA-mediated degradation of Gli [106].
4.4. Activation of Negative Regulators of Hh Pathway Signaling
Antagonism of the Hh pathway is primarily achieved through the inhibition of Hh components, such as Smo or Gli; however there are multiple negative regulators of the signaling pathway, such as casein-kinase-1α (CK1α), adenosine receptor/protein kinase A (ADORA/PKA), and GPR39. Antagonism of Hh signaling can also be achieved via the activation of these negative regulators resulting in phosphorylation and destabilization of Gli proteins.
CK1α is a negative regulator of Hh signaling that plays a role in converting full-length Gli to GliR. Its activation could result in increased levels of GliR and ultimately inhibit Hh signaling [107]. Pyrvinium (69, Fig. 12), an anti-pinworm compound originally developed by Parke Davis (Povan), was recently identified as a potent allosteric agonist of CK1α and was subsequently evaluated for its ability to inhibit Hh signaling [108–110]. Pyrvinium demonstrated potent attenuation of pathway activity in Hh-dependent MEFs (IC50 ~10 nM) and down-regulated endogenous expression of Gli1 and Ptchl mRNA in a similar in vitro system [110] pyrvinium maintained its anti-Hh activity in Sufu−/− cells, indicating its ability to inhibit Hh signaling downstream of Sufu/Smo. Mechanistic studies demonstrated that pyrvinium-mediated inhibition of Hh signaling was dependent on active CK1α and that direct interactions between Gli1 and CK1α promote proteasome-mediated degradation of Gli1. In addition, pyrvinium maintains its anti-Hh activity in the presence of Smo D473H. Finally, in a Ptch+/− model of murine MB, subcutaneous administration of pyrvinium decreased Gli1 expression and tumor volume.
Adenosine receptors (ADORAs) are a group of G-protein-coupled receptors that are involved in a variety of cellular processes, all controlled by the concentration of their natural ligand, adenosine [111]. Previous studies in Drosophilia demonstrated that adenosine triggered the formation of CiR, the Drosophila homologue of GliR, suggesting adenosine and ADORAs serve as negative regulators of Hh signaling [112]. Imiquimod (IMQ, 70) is a nucleoside analogue developed by Medicis Pharmaceutical (Aldara®, Zylcara) that is FDA approved for the topical treatment of a variety of skin disorders, including superficial BCC. Several cellular mechanisms have been implicated as mediating the biological activity of IMQ, including toll-like receptors 7 and 8 (TLR7 and TLR8) and several of the ADORAs [113]. Based on the connections between its use as an anti-BCC agent and its ability to agonize ADORAs, researchers at the University of Salzburg sought to probe its ability to regulate Hh signaling as a downstream consequence of ADORA activation [114]. IMQ treatment (42 μM) decreased Gli1 protein levels in an Hh-dependent murine BCC cell line via a mechanism that was independent of TLR7 or TLR8. Follow-up studies demonstrated that the anti-Hh activity of IMQ is a result of ADORA activation, which initiates a cascade of phosphorylation events (through PKA) that ultimately enhances proteolytic processing of Gli to provide GliR. In addition, IMQ decreased Gli1 mRNA and protein levels in DAOY cells, while concomitantly increasing protein expression of GliR, highlighting the potential for activators of ADORAs to function as Hh pathway inhibitors [114].
In a search for Hh pathway inhibitors that function downstream of Smo, researchers at Novartis utilized a cell-based screen to identify a series of cyclohexyl-methyl aminopyrimidine-based compounds (CMAP) as inhibitors of Gli-mediated signaling [115]. The initial screening hit, CMAP1 (71, Fig. 12), was a modest inhibitor with IC50 values in the low micromolar range (1.1 – 2.3 μM). SAR studies for the scaffold demonstrated that the sulfonamide, pyridine, and pyrimidine regions were amenable to modifications. The most potent CMAP analogue identified through this medicinal chemistry program was compound CMAP7 (72), which exhibited potent inhibition of Hh signaling (IC50 = 20 nM). A series of chemical proteomic target identification studies identified an orphan GPCR, GRP39, as the cellular target of the CMAP scaffold [115]. Preliminary experiments suggest GPR39 activation may be a means to initiate crosstalk between MAP kinase and Gli signaling, which could ultimately result in Hh inhibition; however, further studies need to be performed to completely understand this mechanism of Hh inhibition.
4.5. Pathway Inhibitors With An Unknown Mechanism of Action
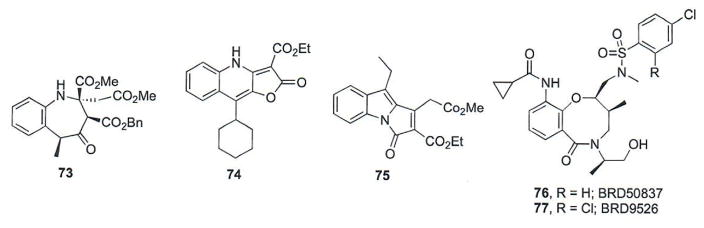
A diversity oriented approach to design small molecules that occupy a wide-range of chemical space as potential therapeutic drugs was undertaken at the Max-Planck-Institut für Molekulare Physiologie [116]. Compounds prepared through this synthetic route were evaluated in several biological assays, including for their ability to inhibit Hh signaling. Three compounds (73–75, Fig. 13) demonstrated pathway inhibition in Hh-dependent MEFs (IC50 values = 0.79, 0.84, and 0.16 μM, respectively). Initially, compound 73 was evaluated as a diastereomeric mixture. Complete purification of the mixture suggested the major diastereomer (shown in Fig. 13) is responsible for the anti-Hh activity of the scaffold (IC50 ~ 0.5 μM). None of the compounds displaced BODIPY-Cyc from Smo in HEK293T cells, suggesting their inhibitory activity is mediated through a different mechanism than direct Smo antagonism [116].
Fig. (13).
Small molecule pathway inhibitors that function through an undetermined mechanism.
A screen of approximately 20K small molecules and optimization of the resulting hit scaffold identified a series of small molecules that incorporate a central eight-membered heterocycle as inhibitors of Hh pathway signaling [117]. The most potent compounds, which also demonstrated promising drug-like solubility, BRD50837 (76) and BRD9526 (77), were chosen for additional studies to probe their mechanism of action (IC50 values = 90 and 60 nm, respectively). Neither compound displaced BODIPY-Cyc from Smo, suggesting they did not bind in the known Smo binding pocket. Interestingly, while neither compound inhibited pathway signaling in Ptch−/− cells, they retained partial activity in Sufu−/− cells, a seemingly contradictory result [117]. Further studies clarifying the cellular mechanism of action of these compounds and expanding on their anti-cancer properties have not been disclosed. The anti-Hh activity for key pathway inhibitors that function through an indirect mechanism(s) is summarized in Table 4.
Table 4.
Small molecules that inhibit Hh signaling through indirect or unknown mechanisms.
| Compound | Target | IC50 Wild-Type Smo | IC50 D473H Smo | References |
|---|---|---|---|---|
| Mebendazole | Tubulin | 0.5 μM | Activea | [103] |
| Ciliobrevin A | Cytoplasmic Dynein | 7 μM | ND | [105] |
| Ciliobrevin D | Cytoplasmic Dynein | 7 μM | ND | [105] |
| Eggmanone | Phosphodiesterase 4 | 72 nM | ND | [106] |
| Pyrvinium | Casein Kinase 1α | ~10 nM | Activea | [110] |
| Imiquimod | Adenosine Receptor | Activea | ND | [114] |
| CMAP 7 | GPR39 | 20 nM | ND | [115] |
Active = Specific IC50 values were not reported.
ND = not determined or not reported.
5. BIOLOGICS AS HEDGEHOG PATHWAY INHIBITORS
5.1. Antibodies that Bind Hh Ligands
Recently, the use of antibodies that target various Hh ligands has emerged as a means to inhibit Hh signaling with the development of 5E1 at Howard Hughes Medical Institute [118]. Antibody 5E1 demonstrated high affinity for both SHh and IHh with Kd values of 0.13–0.31 and 0.16–0.29 nM, respectively) along with reduced affinity for DHh (Kd = 1.7 nM) [119]. Antibody 5E1 attenuates tumor growth in colorectal and pancreatic cancer xenografts, as well as basal-like mouse mammary tumors [120–122]. Biochemical studies have shown that 5E1 binds to the pseudo-active site groove of SHh, but does not interact with zinc cations within this site [119].
In 2013, AstraZeneca reported the development of human monoclonal antibodies against SHh as Hh pathway inhibitors. In the report, two antibodies showed high affinity for SHh, 3H8 and 6D7 (MEDI-5304), with Kd values of 36 pM and 5 pM, respectively; however, only MEDI-5304 had affinity for IHh with a Kd of 34 pM, and neither had affinity for DHh [123]. Both antibodies were also shown to inhibit pathway signaling in Hh-dependent MEFs with IC50 values of 3.4 and 5.3 nM respectively. MEDI-5304 also decreased tumor volume in a compliant model of colorectal cancer, which mimics Hh-mediated paracrine cancer, in a dose-dependent manner with a modest overall growth reduction of 30–50% [123].
5.2. miRNA Targeting
MicroRNAs (miRNAs) demonstrate the ability to regulate the expression of a variety of genes via direct interactions with mRNA. For this reason, miRNAs or corresponding synthetic RNA sequences are being explored as potential drugs against a variety of human disorders [124]. A qRT-PCR and western blot study at Harbin Medical University demonstrated that Smo was highly expressed in multiple glioma cell lines and this over-expression correlated with poor survival [125]. A previous study demonstrated that miR-326 serves as a negative regulator of the Hh pathway by targeting Gli2 and Smo [126]. The overexpression of miR-326 decreased expression of Smo and overall Hh activity, indicating an inverse correlation between miR-326 and Smo/Hh activity in Hh-dependent MEFs [126]. Further studies demonstrated that up-regulation of miR-326 reduced expression of Hh signaling components (Gli 1, N-myc, and Cyclin D1) as well as decreased the expression of stem-cell associated proteins in CD133+ glioma cells [125].
Overactive Hh signaling has been implicated as a potential causative factor for the initiation and progression of pancreatic ductal adenocarcinoma (PDAC), while miR-let7b is down-regulated in similar systems. A bifunctional micelle-based delivery system that directly incorporated vismodegib into the core and complexed with miR-let7b was utilized to evaluate whether this combination could demonstrate synergistic anticancer properties [127]. Co-delivery of vismodegib (10 μM) and miR-let7b (10 pM) using this system resulted in significant reductions in cell viability in a variety of cellular systems. Combination therapy (10 mg/kg vismodegib and 2 mg/kg miR-let7b) significantly reduced both Gli1 and SHh mRNA levels (50–70%) and tumor volume in an allograft model of PDAC to a greater extent than monotherapy with either treatment [127]. Multiple other Hedgehog specific microRNAs have been identified, such as miR-421, miR-let7i, and miR-125b; however, their potential therapeutic use as not been reported [128–129].
CONCLUSIONS
Although the Hh signaling pathway has been implicated as an anti-cancer target in a variety of human cancers, most recent evidence highlights BCC and MB as the only cancers truly dependent on the Hh signaling pathway for oncogenic transformation and tumor growth. These findings have reduced the potential scope of application for Hh pathway inhibitors; however, as BCC is the most common form of human cancer and no targeted therapies exist for MB, pathway inhibitors still exhibit the potential to treat a large number of patients. For these reasons, multiple research groups in academics and industry continue to develop small molecule pathway inhibitors that target multiple signaling components within the pathway. With the emergence of resistance to vismodegib and other Smo antagonists as well as the identification of oncogenic driver mutations in Sufu, a trend towards targeting pathway components downstream of Smo/Sufu has emerged. These strategies include directly targeting Gli proteins to prevent them from activating Hh gene transcription. In addition, multiple groups have identified receptors or enzymes that indirectly regulate GliR/GliA levels and these proteins also hold promise as potential targets for further exploration. The promise Hh pathway inhibitors have demonstrated in multiple preclinical and clinical settings, coupled with the pitfalls already identified for Smo antagonists, ensures the continued development of small molecule Hh inhibitors as anti-cancer agents.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the hedgehog pathway in cancer. Nat Med. 2013;19:1410–1422. doi: 10.1038/nm.3389. [DOI] [PubMed] [Google Scholar]
- 2.Briscoe J, Thérond PP. The mechanisms of hedgehog signaling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 3.Trinh TN, McLaughlin EA, Gordon CP, McCluskey A. Hedgehog signaling pathway inhibitors as cancer suppressing agents. Med Chem Commun. 2014;5:117–133. [Google Scholar]
- 4.Gupta S, Takebe N, LoRusso P. Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol. 2010;2:237–250. doi: 10.1177/1758834010366430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 6.Nozama YI, Lin C, Chuang P-T. Hedgehog Signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Current Opin Genetics Develop. 2013;23:429–437. doi: 10.1016/j.gde.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milenkovic L, Weiss LE, Yoon J, Roth TL, Su YS, Sahl SJ, Scott MP, Moerner WE. Single-molecule imaging of Hedgehog pathway protein Smoothened in primary cilia revels binding events regulated by Patched1. Proc Natl Acad Sci USA. 2015;112:8320–8325. doi: 10.1073/pnas.1510094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling G, Ahmadian A, Persson A, Undén AB, Afink G, Williams C, Uhlén M, Toftgård R, Lundenerg J, Pontén F. Patched and p53 gene alterations in sporadic and hereditary basal cell cancer. Oncogene. 2001;20:7770–7778. doi: 10.1038/sj.onc.1204946. [DOI] [PubMed] [Google Scholar]
- 9.Reifenberger J, Wolter M, Knobbe CB, Köhler B, Schönicke A, Scharwächter C, Kumar K, Blaschke B, Ruzicka T, Reifenberger G. Somatic mutations in the PTCH, SMOH, SUFUH, and TP53 genes in sporadic basal cell carcinoma. Br J Dermatol. 2005;152:43–51. doi: 10.1111/j.1365-2133.2005.06353.x. [DOI] [PubMed] [Google Scholar]
- 10.Raffel C, Jenkins RB, Frederick L, Hebrink D. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–845. [PubMed] [Google Scholar]
- 11.Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, Agatep R, Chiappa S, Gao L, Lowrance A, Hao A, Goldstein AM, Stavrou T, Scherer SW, Dura WT, Wainwright B, Squire JA, Rutka JT, Hogg D. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–310. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 12.Tostar U, Malm CJ, Meis-Kindblom JM, Kindblom LG, Toftgård R, Undén AB. Deregulation of the hedgehog signalling pathway: a possible role for the PTCH and SUFU genes in human rhabdomyoma and rhabdomyosarcoma development. J Pathol. 2006;208:17–25. doi: 10.1002/path.1882. [DOI] [PubMed] [Google Scholar]
- 13.Zibat A, Missiaglia E, Rosenberger A, Pritchard-Jones K, Shipley J, Hahn H, Fulda S. Activation of the hedgehog pathway confers a poor prognosis in embryonal and fusion gene-negative alveolar rhabdomyosarcoma. Oncogene. 2010;29:6323–6330. doi: 10.1038/onc.2010.368. [DOI] [PubMed] [Google Scholar]
- 14.Buonamici S, Williams J, Morrissey M, Wang A, Guo R, Vattay A, Hsiao K, Yuan J, Green J, Ospina B, Yu Q, Ostrom L, Fordjour P, Anderson DL, Monahan JE, Kelleher JF, Peukert S, Pan S, Wu X, Maira S-M, Garcia-Echeverria C, Briggs KJ, Watkins DN, Yao Y-M, Lengauer C, Warmuth M, Sellers WR, Dorsch M. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2:51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Northcott PA, Nakahara Y, Wu XC, Feuk L, Ellison DW, Croul S, Mack S, Kongkham PN, Peacock J, Dubuc A, Ra YS, Zilberberg K, Mcleod J, Scherer SW, Rao JS, Eberhart CG, Grajkowska W, Gillespie Y, Lach B, Grundy R, Pollack IF, Hamilton RL, Van Meter T, Carlotti CG, Boop F, Bigner D, Gilbertson RJ, Rutka JT, Taylor MD. Multiple recurrent genetic events converge on control of histone lysine methylation in medulloblastoma. Nat Genet. 2009;41:465–472. doi: 10.1038/ng.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Justilien V, Fields AP. Novel approaches for improved therapeutic targeting of hedgehog signaling in cancer stem cells. Clin Cancer Res. 2015;21:505–513. doi: 10.1158/1078-0432.CCR-14-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang L, Xie G, Fan Q, Xie J. Activation of the hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene. 2010;29:469–481. doi: 10.1038/onc.2009.392. [DOI] [PubMed] [Google Scholar]
- 18.Ruat M, Hoch L, Faure H, Rognan D. Targeting of smoothened for therapeutic gain. Trends Pharmacol Sci. 2014;34:237–246. doi: 10.1016/j.tips.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Dlugosz A, Agrawal S, Kirkpatrick P. Pipeline pioneers: vismodegib. Nat Rev Drug Discov. 2012;11:437–438. doi: 10.1038/nrd3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robarge KD, Brunton SA, Castanedo GM, Cui Y, Dina MS, Goldsmith R, Sould SE, Guichert O, Gunzner JL, Halladay J, Jia W, Khojasteh C, Koehler MF, Kotkow K, La H, Lalonde RL, Lau K, Lee L, Marshall D, Marsters JC, Murray LJ, Qian C, Rubin LL, Salphati L, Stanley MS, Stibbard JH, Sutherlin DP, Ubhayaker S, Wang S, Wong S, Xie M. GDC-0449-A potent inhibitor of the hedgehog pathway. Bioorg Med Chem Lett. 2009;19:5576–5581. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 21.ClinicalTrials.gov. [accessed May 8, 2015]; https://clinicaltrials.gov/ct2/home.
- 22.Pan S, Wu X, Jiang J, Gao W, Wan Y, Cheng D, Han D, Liu J, Englund NP, Wang Y, Peukert S, Miller-Moslin K, Yuan J, Guo R, Matsumoto M, Vattay A, Jiang Y, Tsao J, Sun F, Pferdekamper AC, Dodd S, Tuntland T, Maniara W, Kelleher JF, III, Yao Y, Warmuth M, Williams J, Dorsch M. Discovery of NVP-LDE225, a Potent and Selective Smoothened Antagonist. ACS Med Chem Lett. 2010;1:130–134. doi: 10.1021/ml1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munchhof MJ, Li Q, Shavna A, Borzillo GV, Boyden TL, Jones CS, LaGreca SD, Martinez-Alsina L, Patel N, Pelletier K, Reiter LA, Robbins MD, Tkalcevic GT. Discovery of PF-04449913, a potent and orally bioavailable inhibitor of smoothened. ACS Med Chem Lett. 2012;3:106–111. doi: 10.1021/ml2002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gendreau SB, Hawkins D, Ho C-H, Lewin A, Lin T, Merchant A, Rowley RB, Wang Q, Matsui W, Fargnoli J. Abstract B192: Preclinical characterization of BMS-833923 (XL139), a hedgehog (HH) pathway inhibitor in early clinical development. Mol Cancer Ther. 2009;8:B192. [Google Scholar]
- 25.Bender MH, Hipskind PA, Capen AR, Cockman M, Credille KM, Gao H, Bastian JA, Clay JM, Lobb KL, Sail DJ, Thompson ML, Wilson T, Wishart GN, Patel BKR. Abstract 2819: Identification and characterization of a novel smoothened antagonist for the treatment of cancer with deregulated hedgehog signaling. Cancer Res. 2011;71:2819. [Google Scholar]
- 26.Xin M. Hedgehog inhibitors: a patent review (2013-present) Expert Opin Ther Patents. 2015;25(5):549–565. doi: 10.1517/13543776.2015.1019864. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee U, Hadden MK. Recent advances in the design of Hedgehog pathway inhibitors for the treatment of malignancies. Expert Opin Drug Discov. 2014;9:751–771. doi: 10.1517/17460441.2014.920817. [DOI] [PubMed] [Google Scholar]
- 28.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, Holcomb T, Stinson J, Gould SE, Coleman B, LoRusso PM, Von Hoff DD, de Sauvage FJ, Low JA. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yauch RL, Dijkgraaf GJ, Alicke B, Januario T, Ahn CP, Holcomb T, Pujara K, Stinson J, Callahan CA, Tang T, Bazan JF, Kan Z, Seshagiiri S, Hann CL, Gould SE, Low JA, Rudin CM, de Sauvage FJ. Smoothened mutation confers resistance to a hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dijkgraaf GJP, Alicke B, Weinmann L, Januario T, West K, Modrusan Z, Burdick D, Goldsmith R, Robarge K, Sutherlin D, Scales SJ, Gould SE, Yauch RL, de Sauvage FJ. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71:435–444. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 31.Pricl S, Cartelazzi B, Col VD, Marson D, Laurini E, Fermeglia M, Licitra L, Pilotti S, Bossi P, Perrone F. Smoothened (SMO) receptor mutations dictate resistance to vismodegib in basal cell carcinoma. Mol Oncol. 2015;9:389–397. doi: 10.1016/j.molonc.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharpe HJ, Pau G, Dijkgraaf GJ, Basses-Seguin N, Modrusan Z, Januario T, Tsui V, Durham AB, Dlugosz AA, Haverty PM, Bourgon R, Tang JY, Sarin KY, Dirix L, Fisher DC, Rudin CM, Sofen H, Migden MR, Yauch RL, de Sauvage FJ. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell. 2015;27:327–341. doi: 10.1016/j.ccell.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atwood SX, Sarin KY, Whitson RJ, Li JR, Kim G, Rezaee M, Ally MS, Kim J, Yao C, Chang ALS, Oro AE, Tang JY. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 2015;27:342–353. doi: 10.1016/j.ccell.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee MJ, Hatton BA, Villavicencio EH, Khanna PC, Friedman SD, Ditzler S, Pullar B, Robison K, White KF, Tunkey C, LeBlanc M, Randolph-Habecker J, Knoblaugh SE, Hansen S, Richards A, Wainwright BJ, McGovern K, Olson JM. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc Natl Acad Sci USA. 2012;109:7859–7864. doi: 10.1073/pnas.1114718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Ding Q, Yen CJ, Xia W, Izzo JG, Lang JY, Li CW, Hsu JL, Miller SA, Wang X, Lee DF, Hsu JM, Huo L, Labaff AM, Liu D, Huang TH, Lai CC, Tsai FJ, Chang WC, Chen CH, Wu TT, Buttar NS, Wang KK, Wu Y, Wang H, Ajani J, Hung MC. The crosstalk of mTOR/S6K1 and hedgehog pathways. Cancer Cell. 2012;21:374–387. doi: 10.1016/j.ccr.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das S, Samant RS, Shevde LA. Nonclassical activation of hedgehog signaling enhances multidrug resistance and makes cancer cells refractory to smoothened-targeting hedgehog inhibition. J Biol Chem. 2013;288:11824–11833. doi: 10.1074/jbc.M112.432302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peukert S, He F, Dai M, Zhang R, Sun Y, Miller-Moslin K, McEwan M, Lagu B, Wang K, Yusuff N, Bourret A, Ramamurthy A, Maniara W, Amaral A, Vattay A, Wang A, Guo R, Yuan J, Green J, Williams J, Buonamici S, Kelleher JF, Dorsch M. Discovery of NVP-LEQ506, a second-generation inhibitor of smoothened. ChemMedChem. 2013;8:1261–1265. doi: 10.1002/cmdc.201300217. [DOI] [PubMed] [Google Scholar]
- 38.Tao H, Jin Q, Koo D, Liao X, Englund NP, Wang Y, Ramamurthy A, Schultz PG, Dorsch M, Kelleher J, Wu X. Small molecule antagonists in distinct binding modes inhibit drug-resistant mutant of smoothened. Chem Biol. 2011;18:432–437. doi: 10.1016/j.chembiol.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Fan C, Chen B, Franco I, Lu J, Shi H, Wei S, Wang C, Wu X, Tang W, Roth MG, Williams NS, Hirsch E, Chen C, Lum L. The hedgehog pathway effector of smoothened exhibits signaling competency in the absence of ciliary accumulation. Chem Biol. 2014;21:1680–1689. doi: 10.1016/j.chembiol.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manetti F, Faure H, Roudaut H, Gorojankina T, Traiffort E, Schoenfelder A, Mann A, Solinas A, Taddei M, Ruat M. Virtual screening-based discovery and mechanistic characterization of the acylthiourea MRT-10 family as smoothened antagonists. Mol Pharmacol. 2010;78:658–665. doi: 10.1124/mol.110.065102. [DOI] [PubMed] [Google Scholar]
- 41.Solinas A, Faure H, Roudaut H, Traiffort E, Schoenfelder A, Mann A, Manetti F, Taddei M, Ruat M. Acylthiourea, acylurea, and acylguanidine derivatives with potent hedgehog inhibiting activity. J Med Chem. 2012;55:1559–1571. doi: 10.1021/jm2013369. [DOI] [PubMed] [Google Scholar]
- 42.Hoch L, Faure H, Roudaut H, Schoenfelder A, Mann A, Girard N, Bihannic L, Ayrault O, Petricci E, Taddei M, Rognan D, Ruat M. MRT-92 inhibits Hedgehog signaling by blocking overlapping binding sites in the transmembrane domain of the Smoothened receptor. FASEB J. 2015;29:1–13. doi: 10.1096/fj.14-267849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roudaut H, Traiffort E, Gorojankina T, Vincent L, Faure H, Schoenfelder A, Mann A, Manetti F, Solinas A, Taddei M, Ruat M. Identification and mechanism of action of the acylguanidine MRT-83, a novel potent smoothened antagonist. Mol Pharmacol. 2011;79:452–460. doi: 10.1124/mol.110.069708. [DOI] [PubMed] [Google Scholar]
- 44.Malancona S, Altamura S, Filocamo G, Kinzel O, Hernando JI, Rowley M, Scarpelli R, Steinkühler C, Jones P. Identification of MK-5710 ((8aS)-8a-methyl-1,3-dioxo-2-[(1S,2R)-2-phenylcyclopropyl]-N-(1-phenyl-1H-pyrazol-5-yl)hexahydroimidazo[1,5-a]pyrazine-7(1H)-carboxamide), a potent smoothened antagonist for use in hedgehog pathway dependent malignancies, part 1. Bioorg Med Chem Lett. 2011;21:4422–4428. doi: 10.1016/j.bmcl.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 45.Kinzel O, Alfieri A, Altamura S, Brunetti M, Bufali S, Colaceci F, Ferrigno F, Filocamo G, Fonsi M, Malancona S, Hernando JI, Monteagudo E, Orsale MV, Palumbi MC, Pucci V, Rowley M, Sasso R, Scarpelli R, Steinkühler C, Jones P. Identification of MK-5710 ((8aS)-8a-methyl-1,3-dioxo-2-[(1S,2R)-2-phenylcyclopropyl]-N-(1-phenyl-1H-pyrazol-5-yl)hexahydroimidazo[1,5-a]pyrazine-7(1H)-carboxamide), a potent smoothened antagonist for use in hedgehog pathway dependent malignancies, part 2. Bioorg Med Chem Lett. 2011;21:4429–4435. doi: 10.1016/j.bmcl.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 46.Ontoria JM, Bufi LL, Torrisi C, Bresciani A, Giomini C, Rowley M, Serafini S, Bin H, Hao W, Steinkühler C, Jones P. Identification of a series of 4-[3-(quinolin-2-yl)-1,2,4-oxadiazol-5-yl]piperazinyl ureas as potent smoothened antagonist hedgehog pathway inhibitors. Bioorg Med Chem Lett. 2011;21:5274–5282. doi: 10.1016/j.bmcl.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 47.Muraglia E, Ontoria JM, Branca D, Dessole G, Bresciani A, Fonsi M, Giuliano C, Llauger Bufi L, Monteagudo E, Palumbi MC, Torrisi C, Rowley M, Steinkühler C, Jones P. N-(2-alkylaminoethyl)-4-(1,2,4-oxadiazol-5-yl)piperazine-1-carboxamides as highly potent smoothened antagonists. Bioorg Med Chem Lett. 2011;21:5283–5288. doi: 10.1016/j.bmcl.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 48.Ishii T, Shimizu Y, Nakashima K, Kondo S, Ogawa K, Sasaki S, Matsui H. Inhibition mechanism exploration of investigational drug TAK-441 as inhibitor against vis-modegib-resistant smoothened mutant. Eur J Pharmacol. 2014;723:305–313. doi: 10.1016/j.ejphar.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Strand MF, Wilson SR, Dembinski JL, Holsworth DD, Khvat A, Okun I, Petersen D, Krauss S. A novel synthetic smoothened antagonist transiently inhibits pancreatic adenocarcinoma xenografts in a mouse model. PLoS One. 2011;6:e19904. doi: 10.1371/journal.pone.0019904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghannoum MA, Rice LB. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12:501–517. doi: 10.1128/cmr.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Tang JY, Gong R, Kim J, Lee JJ, Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, Reya T, Liu JO, Epstein EH, Stevens DA, Beachy PA. Itraconazole, a commonly used antifungal that inhibits hedgehog pathway activity and cancer growth. Cancer Cell. 2010;17:388–399. doi: 10.1016/j.ccr.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim J, Aftab BT, Tang JY, Kim D, Lee AH, Rezaee M, Kim J, Chen B, King EM, Borodovsky A, Riggins GJ, Epstein EH, Beachy PA, Rudin CM. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim DJ, Kim J, Spaunhurst K, Montoya J, Khodosh R, Chandra K, Fu T, Gilliam A, Molgo M, Beachy PA, Tang JY. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745–751. doi: 10.1200/JCO.2013.49.9525. [DOI] [PubMed] [Google Scholar]
- 54.Shi W, Nacev BA, Aftab BT, Head S, Rudin CM, Liu JO. Itraconazole side chain analogues: structure-activity relationship studies for inhibition of endothelial cell proliferation, vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, and hedgehog signaling. J Med Chem. 2011;54:7363–7374. doi: 10.1021/jm200944b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ, Liu JO. Inhibition of angiogenesis by the antifungal drug itraconazole. ACS Chem Biol. 2007;2:263–270. doi: 10.1021/cb600362d. [DOI] [PubMed] [Google Scholar]
- 56.Shi W, Nacev BA, Bhat S, Liu JO. Impact of absolute stereochemistry on the antiangiogenic and antifungal activities of traconazole. ACS Med Chem Lett. 2010;1:155–159. doi: 10.1021/ml1000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nacev BA, Grassi P, Dell A, Haslam SM, Liu JO. The antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cells. J Biol Chem. 2011;286:44045–44056. doi: 10.1074/jbc.M111.278754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, Arvanites AC, Davidow L, Blanchard J, Lam K, Yoo JW, Coy S, Rubin LL, McMahon AP. Selective identification of hedgehog pathway antagonists by direct analysis of smoothened ciliary translocation. ACS Chem Biol. 2012;7:1040–1048. doi: 10.1021/cb300028a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu VM, Chen SC, Arkin MR, Reiter JF. Small molecule inhibitors of smoothened ciliary localization and ciliogenesis. Proc Natl Acad Sci USA. 2012;109:13644–13649. doi: 10.1073/pnas.1207170109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xin M, Wen J, Tang F, Tu C, Shen H, Zhao X. The discovery of novel N-(2-pyrimidinylamino)benzamide derivatives as potent hedgehog signaling pathway inhibitors. Bioorg Med Chem Lett. 2013;23:6777–6783. doi: 10.1016/j.bmcl.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 61.Xin M, Wen J, Tang F, Tu C, Huang W, Shen H, Zhao X, Cheng L, Wang M, Zhang L. Synthesis and evaluation of 4-(2-pyrimidinylamino)benzamides inhibitors of hedgehog signaling pathway. Bioorg Med Chem Lett. 2014;24:983–988. doi: 10.1016/j.bmcl.2013.12.050. [DOI] [PubMed] [Google Scholar]
- 62.Xin M, Zhang L, Tang F, Tu C, Wen J, Zhao X, Liu Z, Cheng L, Shen H. Design, synthesis, and evaluation of pyrrolo[2,1-f][1,2,4]triazine derivatives as novel hedgehog signaling pathway inhibitors. Bioorg Med Chem. 2014;22:1429–1440. doi: 10.1016/j.bmc.2013.12.055. [DOI] [PubMed] [Google Scholar]
- 63.Wu T, Wang D, Xiang P, Zhang J, Sang Y, Lin H, Chen J, Xie G, Song H, Zhao Y, Xie Y. Synthesis and biological evaluation of novel benzamide derivatives as potent smoothened antagonists. Bioorg Med Chem. 2014;24:1426–1431. doi: 10.1016/j.bmcl.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Sun C, Li Y, Shi A, Zhang J, Li Y, Zhao M, Zhang L, Zheng H, Meng Y, Ding H, Song H. Synthesis and evaluation of novel N-3-benzimidazolephenylbisamide derivatives for antiproliferative and hedgehog pathway inhibitor activity. Med Chem Commun. 2015 In Press. [Google Scholar]
- 65.Canettieri G, Marcotullio LD, Greco A, Coni S, Antonucci L, Infante P, Pietrosanti L, De Smaele E, Ferretti E, Miele E, Pelloni M, Simone GD, Pedone EM, Gallinari P, Giorgi A, Steinkühler C, Vitagliano L, Pedone C, Schinin ME, Screpanti I, Gulino A. Histone deacetylase and cullin3-RENKCTD11 ubiquitin ligase interplay regulates hedgehog signaling through Gli acetylation. Nat Cell Biol. 2010;12:132–142. doi: 10.1038/ncb2013. [DOI] [PubMed] [Google Scholar]
- 66.Zhao J, Quan H, Xie C, Lou L. NL-103, a novel dual-targeted inhibitor of histone deacetylases and hedgehog pathway, effectively overcomes vismodegib resistance conferred by Smo mutations. Pharm Res Per. 2014;2:1–13. doi: 10.1002/prp2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lu W, Geng D, Sun Z, Yang Z, Ma H, Zheng J, Zhang X. Scaffold hopping approach to a new series of smoothened antagonists. Bioorg Med Chem Lett. 2014;24:2300–2304. doi: 10.1016/j.bmcl.2014.03.079. [DOI] [PubMed] [Google Scholar]
- 68.Ma H, Lu W, Sun Z, Luo L, Geng D, Yang Z, Li E, Zheng J, Wang M, Zhang H, Yang S, Zhang X. Design, synthesis, and structure-activity-relationship of tetrahydrothiazolopyridine derivatives as potent smoothened antagonists. Eur J Med Chem. 2015;89:721–732. doi: 10.1016/j.ejmech.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 69.Betti M, Castagnoli G, Panico A, Sanna Coccone S, Wiedenau P. Development of a scalable route to the SMO receptor antagonist SEN794. Org Process Res Dev. 2012;16:1739–1745. [Google Scholar]
- 70.Nair SK, Planken SP. Pyridine-2-derivatives as Smoothened Receptor Modulators. 2012052948. WO. 2012
- 71.Rohner A, Spilker ME, Lam JL, Pascual B, Bartkowski D, Li QJ, Yang AH, Stevens G, Xu M, Wells PA, Planken S, Nair S, Sun S. Effective targeting of hedgehog signaling in a medulloblastoma model with PF-527457, a potent and selective smoothened antagonist that penetrates the blood-brain barrier. Mol Cancer Ther. 2012;11:57–65. doi: 10.1158/1535-7163.MCT-11-0691. [DOI] [PubMed] [Google Scholar]
- 72.Nedelcu D, Liu J, Xu Y, Jao C, Salic A. Oxysterol binding to the extracellular domain of smoothened in hedgehog signaling. Nat Chem Biol. 2013;9:557–64. doi: 10.1038/nchembio.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang Y, Davidow L, Arvanites AC, Blanchard J, Lam K, Xu K, Oza A, Yoo JS, Ng JM, Curran T, Rubin LL, McMahon AP. Glucocorticoid compounds modify smoothened localization and hedgehog pathway activity. Chem Biol. 2012;19:972–82. doi: 10.1016/j.chembiol.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nachtergaele S, Whalen DM, Mydock LK, Zhao Z, Malinauskas T, Krishnan K, Ingham PW, Covey DF, Siebold C, Rohatgi R. Structure and function of the smoothened extracellular domain in vertebrate hedgehog signaling. Elife. 2013;2:e01340. doi: 10.7554/eLife.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C, Wu H, Katritch V, Han GW, Huang XP, Liu W, Siu FY, Roth BL, Cherezov V, Stevens RC. Structure of the human smoothened receptor bound to an antitumour agent. Nature. 2013;497:338–343. doi: 10.1038/nature12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weierstall U, James D, Wang C, White TA, Wang D, Liu W, spence JC, bruce doak R, Nelson G, Fromme P, Fromme R, Grotkohann I, Kupitz C, Zat-sepin NA, Liu H, Basu S, Wacker D, Han GW, Katritch V, Boutet S, Messerschmidt M, Williams GJ, Koglin JE, Marvin Seibert M, Klinker M, Gati C, Shoeman RL, Barty A, Chapman HN, Kirian RA, Beyerlein KR, Stevens RC, Li D, Shah ST, Howe N, Caffrey M, Cherezov V. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat Commun. 2014;5:3309. doi: 10.1038/ncomms4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang C, Wu H, Evron T, Vardy E, Han GW, Huang X-P, Hufeisen SJ, Mangano TJ, Urban DJ, Katritch V, Cherezov V, Caron MG, Roth BL, Stevens RC. Structural basis for smoothened receptor modulation and chemoresistance to anticancer drugs. Nature Commun. 2014;5:4355. doi: 10.1038/ncomms5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Myers BR, Sever N, Chong YC, Kim J, Belani JD, Rychnovsky S, Bazan JF, Beachy PA. Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev Cell. 2013;26:346–57. doi: 10.1016/j.devcel.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rana R, Carroll CE, Lee H, Bao J, Marada S, Grace CR, guibao CD, Ogden SK, Zheng JJ. Structural insights into the role of the smoothened cysteine-rich domain in hedgehog signalling. Nat Commun. 2013;4:2965. doi: 10.1038/ncomms3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K. Skinny hedgehog, an acyltransferase required for palmitoylation and activity of the hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- 81.Petrova E, Rios-Esteves J, Ouerfelli O, Glickman JF, Resh MD. Inhibitors of hedgehog acyltransferase block sonic hedgehog signaling. Nat Chem Biol. 2013;9:247–249. doi: 10.1038/nchembio.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stanton BZ, Peng LF, Maloof N, Nakai K, Wang X, Duffner JL, Taveras KM, Hyman JM, Lee SW, Koehler AN, Chen JK, Fox JL, Mandinova A, Schreiber SL. A small molecule that binds hedgehog and blocks its signaling in human cells. Nat Chem Biol. 2009;5:154–156. doi: 10.1038/nchembio.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dockendorff C, Nagiec MM, Weiwer M, Buhrlage S, Ting A, Nag PP, Germain A, Kim HJ, Youngsaye W, Scherer C, Bennion M, Xue L, Stanton BZ, Lewis TA, Macpherson L, Palmer M, Foley MA, Perez JR, Schreiber SL. Macrocyclic hedgehog pathway inhibitors: Optimization of cellular activity and mode of action studies. ACS Med Chem Lett. 2012;3:808–813. doi: 10.1021/ml300172p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hwang S, Thangapandian S, Lee Y, Sakkiah S, John S, Lee KW. Discovery and evaluation of potential sonic hedgehog signaling pathway inhibitors using pharmacophore modeling and molecular dynamics simulations. J Bioinf Comput Biol. 2011;9(Suppl 1):15–35. doi: 10.1142/s0219720011005732. [DOI] [PubMed] [Google Scholar]
- 85.Hwang S, Thangapandian S, Lee KW. Molecular dynamics simulations of sonic hedgehog-receptor and inhibitor complexes and their applications for potential anticancer agent discovery. PLoS One. 2013;9:e68271. doi: 10.1371/journal.pone.0068271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lauth M, Bergström Å, Shimokawa T, Toftgárd R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci USA. 2007;104:8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shi T, Mazumdar T, Devecchio J, Duan Z-H, Agyeman A, Aziz M, Houghton JA. cDNA microarray gene expression profiling of hedgehog signaling pathway inhibition in human colon cancer cells. PLoS One. 2010;5:e13054. doi: 10.1371/journal.pone.0013054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang L, Walter V, Hayes DN, Onaitis M. Hedgehog-GLI signaling inhibition suppresses tumor growth in squamous lung cancer. Clin Cancer Res. 2014;20:1566–1575. doi: 10.1158/1078-0432.CCR-13-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fu J, Rodova M, Roy SK, Sharma J, Singh KP, Srivastava RK, Shankar S. GANT-61 inhibits pancreatic cancer stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice xenograft. Cancer Lett. 2013;330:22–32. doi: 10.1016/j.canlet.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agyeman A, Jha BK, Mazumdar T, Houghton JA. Mode and specificity of binding of the small molecule GANT61 to GLI determines inhibition of GLI-DNA binding. Oncotarget. 2014;5:4492–4503. doi: 10.18632/oncotarget.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stecca B, Ruiz IAA. Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals. J Mol Cell Biol. 2010;2:84–95. doi: 10.1093/jmcb/mjp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hou X, Chen X, Zhang P, Fan Y, Ma A, Pang T, Song Z, Jin Y, Hao W, Liu F, Wang W, Wang Y. Inhibition of hedgehog signaling by GANT58 induces apoptosis and shows synergistic antitumor activity with AKT inhibitor in acute T cell leukemia cells. Biochimie. 2014;101:50–59. doi: 10.1016/j.biochi.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 93.Infante P, Mori M, Alfonsi R, Ghirga F, Aiello F, Toscano S, Ingallina C, Siler M, Cucchi D, Po A, Miele E, D’Amico D, Canettieri G, De Smaele E, Ferretti E, Screpanti I, Barretta GU, Botta M, Botta B, Gulino A, Di Marcotullio L. Gli/DnA interaction is a druggable target for Hedgehog-dependent tumors. EMBO J. 2015;34:200–217. doi: 10.15252/embj.201489213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ardecky R, Magnuson GK, Zou J, Ganji SR, Brown B, Ngo T, Lee J, Zeng F-Y, Sun Q, Stonich D, Salaniwal S, Sakata T, Rack PG, Casabar JKT, Mangravita-Novo A, Smith LH, Sergienko E, Chung TDY, Pinkerton AB, Pass I, Chen JK. Probe Reports from the NIH Molecular Libraries Program (Internet) Bethesda (MD): National Center for Biotechnology Information (US); 2010–2012. Dec 17, Small-molecule antagonists of Gli function. [PubMed] [Google Scholar]
- 95.Bosco-Clément G, Zhang F, Chen Z, Zhou HM, Li H, Mikami I, Yagui-Beltran A, Lui N, Do HT, cheng T, Tseng HH, Choi H, Fang LT, Kim IJ, Yue D, Wang C, Zheng Q, Fujii N, Mann M, Jablons DM, He B. Targeting Gli transcription activation by small molecule suppresses tumor growth. Oncogene. 2014;33:2087–2097. doi: 10.1038/onc.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.The Regents of the University of California. Targeting Gli proteins in human cancer by small molecules. 2014116651. WO. 2014
- 97.Mahindroo N, Connelly MC, Punchihewa C, Kimura H, Smeltzer MP, Wu S, Fujii N. Structure-activity relationships and cancer-cell selective toxicity of novel inhibitors of glioma associated oncogene homologue 1 (Gli1) mediated transcription. J Med Chem. 2009;52:4277–4287. doi: 10.1021/jm900106f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Müller S, Knapp S. Discovery of BET bromodomain inhibitors and their role in target validation. Med Chem Commun. 2014;5:288–296. [Google Scholar]
- 99.Gallenkamp D, Gelato KA, Haendler B, Weinmann H. Bromodomains and their pharmacological inhibitors. ChemMedChem. 2014;9:438–464. doi: 10.1002/cmdc.201300434. [DOI] [PubMed] [Google Scholar]
- 100.Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE. Selective inhibition of BET bromodomains. Nature. 2010;468:1067–1073. doi: 10.1038/nature09504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang Y, Gholamin S, Schubert S, Willardson MI, Lee A, Bandopadhayay P, Bergthold G, Masoud S, Nguyen B, Vue N, Balansay B, Yu F, Oh S, Woo P, Chen S, Ponnuswami A, Monje M, Atwood SX, Whitson RJ, Mitra S, Chesier SH, Qi J, Beroukhim R, Tang JY, Wechsler-Reya R, Oro AE, Link BA, Bradner JE, Cho Y-J. Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibition. Nat Med. 2014;20:732–740. doi: 10.1038/nm.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Long J, Li B, Rodriguez-Blanco J, Pastori C, Volmar C-H, Wahlstedt C, Capobianco A, Bai F, Pei X-H, Ayad NG, Robbins DJ. The BET bromodomain inhibitor I-BET151 acts downstream of smoothened to abrogate the growth of Hedgehog driven cancers. J Biol Chem. 2014;289:35494–35502. doi: 10.1074/jbc.M114.595348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Larsen AR, Bai R, Chung JH, Borodovsky A, Rudin CM, Riggins GJ, Bunz F. Repurposing the antihelmintic mebendazole as a hedgehog Inhibitor. Mol Cancer Ther. 2015;14:3–13. doi: 10.1158/1535-7163.MCT-14-0755-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hyman JM, Firestone AJ, Heine VM, Zhao Y, Ocasio CA, Han K, Sun M, Rack PG, Sinha S, Wu JJ, Solow-Cordero DE, Jiang J, Rowitch DH, Chen JK. Small-molecule inhibitors reveal multiple strategies for hedgehog pathway blockade. Proc Natl Acad Sci USA. 2009;106:14132–14137. doi: 10.1073/pnas.0907134106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Firestone AJ, Weinger JS, Maldonado M, Barlan K, Langston LD, O’Donnell M, Gelfand VI, Kapoor TM, Chen JK. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature. 2012;484:125–129. doi: 10.1038/nature10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Williams CH, Hempel JE, Hao J, Frist AY, Williams MM, Fleming JT, Sulikowski GA, Cooper MK, Chiang C, Hong CC. An in vivo chemical genetic screen identifies phosphodiesterase 4 as a pharmacological target for hedgehog signaling inhibition. Cell Rep. 2015;11:43–50. doi: 10.1016/j.celrep.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Price MA. CKI, there’s more than one: casein kinase I family members in Wnt and hedgehog signaling. Genes Devlop. 2006;20:399–410. doi: 10.1101/gad.1394306. [DOI] [PubMed] [Google Scholar]
- 108.Thorne CA, Hanson AJ, Schneider J, Tahinci E, Orton D, Cselenyi CS, Jernigan KK, Meyers KC, Hang BI, Waterson AG, Kim K, Melancon B, Ghidu VP, Sulikowski GA, LaFleur B, Salic A, Lee LA, Miller DM, Lee E. Small-molecule inhibition of Wnt signaling through activation of casein kinase 1α. Nat Chem Biol. 2010;6:829–836. doi: 10.1038/nchembio.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lum L, Yao S, Mozer B, Rovescalli A, Von Kessler D, Nirenberg M, Beachy PA. Identification of Hedgehog pathway components by RNAi in Drosophila cultured cells. Science. 2003;299:2039–2045. doi: 10.1126/science.1081403. [DOI] [PubMed] [Google Scholar]
- 110.Li B, Fei DL, Flaveny CA, Dahmane N, Baubet V, Wang Z, Bai F, Pei X-H, Rodriquez-Blanco J, Hang B, Orton D, Han L, Wang B, Capobianco AJ, Lee E, Robbins DJ. Pyrvinium attenuates hedgehog signaling downstream of smoothened. Cancer Res. 2014;74:4811–4821. doi: 10.1158/0008-5472.CAN-14-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trincavelli ML, Daniele S, Martini C. Adenosine receptors: What we know and what we are learning. Curr Top Med Chem. 2010;10:860–877. doi: 10.2174/156802610791268756. [DOI] [PubMed] [Google Scholar]
- 112.Mondal BC, Mukherjee T, Mandal L, Evans CJ, Sinenko SA, Martinez-Agosto JA, Banerjee U. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–1600. doi: 10.1016/j.cell.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schön MP, Schön M, Klotz KN. The small antitumoral immune response modifier imiquimod interacts with adenosine receptor signaling in a TLR7- and TLR8- independent fashion. J Invest Dermatol. 2006;126:1338–1347. doi: 10.1038/sj.jid.5700286. [DOI] [PubMed] [Google Scholar]
- 114.Wolff F, Loipetzberger A, Gruber W, Esterbauer H, Aberger F, Frischauf AM. Imiquimod directly inhibits hedgehog signaling by stimulating adenosine receptor/protein kinase A-mediated GLI phosphorylation. Oncogene. 2013;32:5574–5581. doi: 10.1038/onc.2013.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bassilana F, Carlson A, DaSilva JA, Grosshans B, Vidal S, Beck V, Wilmeringwetter B, Llamas LA, Showalter TB, Rigollier P, Bourret A, Ramamurthy A, Wu X, Harbinski F, Plonsky S, Lee L, Ruffher H, Grandi P, Schirle M, Jenkins J, Sailer AW, Bouwmeester T, Porter JA, Myer V, Finan PM, Tallarico JA, Kelleher JF, III, Seuwen K, Jain RK, Luchansky SJ. Target identification for a hedgehog pathway inhibitor reveals the receptor GPR39. Nat Chem Biol. 2014;10:343–349. doi: 10.1038/nchembio.1481. [DOI] [PubMed] [Google Scholar]
- 116.Garcia-Castro M, Kremer L, Reinkemeier CD, Unkelbach C, Strohmann C, Ziegler S, Ostermann C, Kumar K. De novo branching cascades for structural and functional diversity in small molecules. Nature Commun. 2015:1–13. doi: 10.1038/ncomms7516. [DOI] [PubMed] [Google Scholar]
- 117.Schaefer GI, Perez JR, Duvall JR, Stanton BZ, Shamji AF, Schreiber SL. Discovery of small-molecule modulators of the sonic hedgehog pathway. J Am Chem Soc. 2013;135:9675–9680. doi: 10.1021/ja400034k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two critical periods of sonic hedgehog signaling required for the specification of motor neuron identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 119.Maun HR, Wen X, Lingel A, de Sauvage FJ, Lazarus RA, Scales SJ, Hymowitz SG. Hedgehog pathway antagonist 5E1 binds hedgehog at the pseudo-active site. J Biol Chem. 2010;285:26570–80. doi: 10.1074/jbc.M110.112284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 121.O’Toole SA, Machalek DA, Shearer RF, Millar EK, Nair R, Schofield P, McLeod D, Cooper CL, McNeil CM, McFarland A, Nguyen A, Ormandy CJ, Qiu MR, Rabinovich B, Martelotto LG, Vu D, Hannigan GE, Musgrove EA, Christ D, Sutherland RL, Watkins DN, Swarbrick A. Hedgehog overexpression is associated with stromal interactions and predicts for poor outcome in breast cancer. Cancer Res. 2011;71:4002–4014. doi: 10.1158/0008-5472.CAN-10-3738. [DOI] [PubMed] [Google Scholar]
- 122.Chang Q, Foltz WD, Chaudary N, Hill RP, Hedley DW. Tumor-stroma interaction in orthotopic primary pancreatic cancer xenografts during hedgehog pathway inhibition. Int J Cancer. 2013;133:225–234. doi: 10.1002/ijc.28006. [DOI] [PubMed] [Google Scholar]
- 123.Michaud NR, Wang Y, McEachern KA, Jordan JJ, Mazzola AM, Hernandz A, Jalla S, Chesebrough JW, Hynes MJ, Belmonte MA, Wang L, Kang JS, Jovanovic J, Laing N, Jenkins DW, Hurt E, Liang M, Frantz C, Hollingsworth RE, Simeone DM, Blakey DC, Bedian V. Novel neutralizing hedgehog antibody MEDI-5304 exhibits antitumor activity by inhibiting paracrine hedgehog signaling. Mol Cancer Ther. 2014;13:386–398. doi: 10.1158/1535-7163.MCT-13-0420. [DOI] [PubMed] [Google Scholar]
- 124.Schmidt MF. Drug target miRNAs: chances and challenges. Trends Biotech. 2014;32:578–585. doi: 10.1016/j.tibtech.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 125.Du W, Liu X, Chen L, Dou Z, Lei X, Chang L, Cai J, Cui Y, Yang D, Sun Y, Li Y, Jiang C. Targeting the SMO oncogene by miR-326 inhibits glioma biological behaviors and stemness. Neuro-Oncology. 2015;17:243–253. doi: 10.1093/neuonc/nou217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang Z, Cushing L, Ai X, Lu J. miR-326 is downstream of sonic hedgehog signaling and regulates the expression of Gli2 and smoothened. Am J Respir Cell Mol Biol. 2014;51:273–283. doi: 10.1165/rcmb.2013-0127OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kumar V, Mondal G, Slavik P, Rachagani S, Batra SK, Mahato RI. Codelivery of small molecule hedgehog inhibitor and miRNA for treating pancreatic cancer. Mol Pharm. 2015;12:1289–1298. doi: 10.1021/mp500847s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gu W, Shou J, Gu S, Sun B, Che X. Identifying hedgehog signaling specific MicroRNAs in glioblastomas. Int J Med Sci. 2014;11:488–493. doi: 10.7150/ijms.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ferretti E, De Smaele E, Miele E, Laneve P, Po A, Pelloni M, Paganelli A, Di Marcotullio L, Caffarelli E, Screpanti I, Bozzoni I, Gulino A. Concerted microRNA control of Hedgehog signaling in cerebellar neuronal progenitor and tumour cells. EMBO J. 2008;27:2616–2627. doi: 10.1038/emboj.2008.172. [DOI] [PMC free article] [PubMed] [Google Scholar]