Abstract
There is much debate about the interaction between helminths and allergic disease. The “Hygiene Hypothesis,” a very popular concept among scientists and the lay public, states that infections, especially during childhood, can protect against allergic diseases. Indeed, helminth infections are known to induce regulatory responses in the host that can help the control of inflammation (including allergic inflammation). However, these infections also induce type-2-associated immune responses including helminth-specific IgE that can cross-react against environmental allergens and mediate IgE-driven effector responses. Thus, it is the delicate balance between the parasites' anti- and pro-allergenic effects that define the helminth/allergy interface.
The immune system has evolved, in large part, through its interaction with microbes and parasites, an interaction that drives specific or specialized immune responses to deal with the widely varying groups of microorganisms. For example, parasite-derived induction of interleukin (IL)-4, IL-5, and IL-13 coordinate the prototypical responses to metazoan helminth pathogens,1 whereas viral- and bacterial-specific induction of Type 1 and Type 2 interferons are required for control of these types of infections.1 Interestingly, these responses (broadly inflammatory in nature) themselves, if uncontrolled, can harm the host by causing allergic diseases (Th2-associated inflammation) or autoimmune/inflammatory disorders (typically Th1- and/or Th17-associated). Typically, on the heels of such inflammation come anti-inflammatory networks that are required to prevent long-standing tissue damage.2
These regulatory (or anti-inflammatory) processes triggered during infection underlies the “Hygiene Hypothesis”3 that states that infections, especially during childhood when immune responses are being “educated” and the T- and B-cell memory pool is being created, protect against inflammation-associated disorders4 because they modulate or limit immune-mediated effector responses. Indeed, the presence of helminth infections has been associated (to a small degree) with modulation of the severity of inflammatory bowel disease,5 diabetes,6 and arthritis7–9 to cite just a few examples.
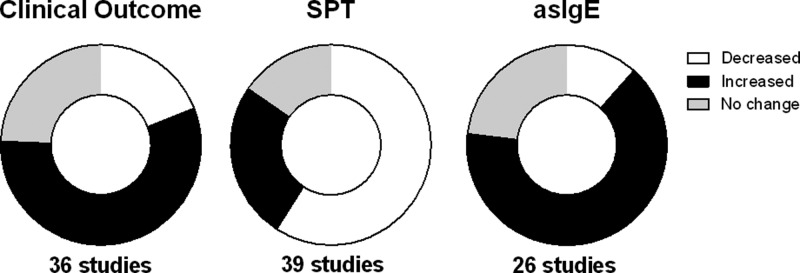
There is little consensus among the many studies that have examined the interaction between helminth infection and atopy (Table 1). This lack of consensus is most likely related to the differences in outcome measures/definitions used in the many studies that have used a variety of outcomes including: 1) the severity or frequency of asthma, rhinitis, or eczema; 2) the frequency of allergen sensitization by skin prick tests (SPTs); or 3) the levels of allergen-specific IgE (asIgE) levels in the blood. Other causes for the disparate results relate to variation among the species of infecting helminths and differences in the age of the populations being studied. To attempt to reconcile these differences, meta-analyses have been performed; these, too, have not been conclusive. For example, while Ascaris lumbricoides was found to be a risk factor for the development of asthma, hookworm infection was associated with a protective effect.60 Infection with other parasites such as Trichuris trichiura, Enterobius vermicularis, and Strongyloides stercoralis had no effect on the outcome of asthma.60 Conversely, the presence of A. lumbricoides was found to lower the frequency of atopy (measured by SPT) to at least one environmental allergen in most studies61 (Table 1), but not to the perennial allergens, cockroach or house dust mite (HDM).61 Hookworm infection has also been associated with protection from atopy to some allergens, but not to HDM or to cockroach extract.61 Interestingly, the majority of the published studies demonstrate that while helminth infection decreases the frequency of SPT positivity, these infections are associated with increased allergen-specific IgE (asIgE) (Figure 1 ).
Table 1.
Detailed list of human studies examining the influence of helminth infection on atopy
| First author, year | Country | N | Helminth | Allergen(s) | Clinical evaluation |
SPT‡ | asIgE§ | |
|---|---|---|---|---|---|---|---|---|
| Parasites* | Assessed† | Outcome | Effect | Prevalence | ||||
| Authors concluded that helminths offer protection against allergies | ||||||||
| Hagel, 199310 | Venezuela | 89 | Al | Not informed | ND | ↓ | ↓ | |
| van den Biggelaar, 200011 | Gabon | 520 | Sh | HDM | ND | ↓ | ND | |
| Araujo, 200012 | Brazil | 175 | Sm | HDM, mold | ND | ↓ | Trend ↓ | |
| van den Biggelaar, 200113 | Gabon | 520 | Sh | HDM | ND | ↓ | ↑ | |
| Nyan, 200114 | Gambia | 429 | Al | Various | Wheeze | No change | ↓ | ND |
| Scrivener, 200115 | Ethiopia | 604 | Hkw, Al | HDM | Wheeze | ↓ | No change | No change |
| Huang, 200216 | Taiwan | 3,107 | Ev | Not informed | Asthma, rhinitis | ↓ | ND | ND |
| Cooper, 2003a17 | Ecuador | 4,433 | Al, Tt | Various | Wheeze, rhinitis, rash | No change | ↓ | ND |
| Cooper, 2003b18 | Ecuador | 2,865 | Various | Various | ND | ↓ | ||
| Dagoye, 200319 | Ethiopia | 563 | Al | HDM, CR | Wheeze | ↓ | No change | |
| Wahyuni, 200520 | Indonesia | 466 | Bm | HDM | ND | Trend ↓ | ↑ | |
| Flohr, 200621 | Vietnam | 1,601 | Hkw, Al | HDM, CR | ND | ↓ | ND | |
| Rodrigues, 200822 | Brazil | 1,055 | Al, Tt | Various | ND | ↓ | ||
| Endara, 201023 | Ecuador | 3,901 | Al, Tt | Not informed | Eczema | ↓ | ↓ | ND |
| Supali, 201024 | Indonesia | 441 | Bm | CR | ND | ↓ | ||
| Rujeni, 201225 | Zimbabwe | 672 | Sh | HDM | ND | ↓ | ↓ | |
| Kanobana, 201226 | Cuba | 958 | Tc, Al, Hkw | Not informed | Asthma | ↑ (Tc) | ND | ND |
| ↓ (Hkw) | ||||||||
| Alcantara-Neves, 201227 | Brazil | 1,445 | Various | Various | Asthma/wheeze | No change | ↓ | No change |
| Mendonça, 201228 | Brazil | 1,148 | Tc | HDM, CR | ND | ↓ | ↑ | |
| Manuel, 201229 | Malaysia | 170 | Tc | Not informed | Rhinitis | ↓ | ||
| Oliveira, 201430 | Brazil | 91 | Sm | HDM, CR, mold | ND | ↓ | ND | |
| Authors found mixed or no association or took no conclusions | ||||||||
| Davey, 200531 | Ethiopia | 7,649 | Various | HDM, CR | Wheeze | No change | ↓ | |
| Ponte, 200632 | Brazil | 113 | Al | HDM | Asthma | No change | No change | No change |
| Calvert, 201033 | South Africa | 3,322 | Al | HDM | EIB | ↑ | ↓ | No change |
| Vereecken, 201234 | Cuba | 1,285 | Al, Tc, Hkw | HDM, CR | Asthma | No change | ↓ | ↑ |
| Souza, 201435 | Brazil | 20 | Al | HDM | ND | ND | No change | |
| Alcantara-Neves, 201436 | Brazil | 1,271 | Al, Tt, Tc | Various | ND | ↓ | No change | |
| Hamid, 201537 | Indonesia | 623 | Al, Tc | HDM, CR | Wheeze, eczema | No change | ↓ | ↑ (Tc) |
| Wordemann, 200838 | Cuba | 1,320 | Ev, Hkw, Al | Various | Asthma, rhinitis, AD | ↑ (Ev, Hkw) | No change | ND |
| ↓ (Al) | ||||||||
| Obeng, 201439 | Ghana | 1,385 | Sh, Al, Tt | Various | Asthma/wheeze | No change | ↓ (Sh) | NE |
| ↑ (Tt) | ||||||||
| Authors concluded that helminths are a risk factor for allergies | ||||||||
| Kayhan, 197840 | Turkey | 100 | Al | Not informed | Asthma | ↑ | ND | ND |
| Joubert, 198041 | South Africa | 259 | Al | Various | ND | ↑ | ↑ | ↑ |
| Alshishtawy, 199142 | Egypt | 105 | Sm | HDM, pollen | Asthma | ↑ | ND | ↑ |
| Buijs, 199743 | Netherlands | 1,379 | Tc | HDM, cat, dog | Asthma | ↑ | ↑ | |
| Lynch, 199744 | Venezuela | 89 | Al | HDM | Asthma | ↑ | ↑ | ↑ |
| Dold, 199845 | Germany | ∼2,300 | Al | Various | Asthma, rhinitis | ↑ | ↑ | ↑ |
| Palmer, 200246 | China | 2,164 | Al | Various | Asthma | ↑ | ↑ | ND |
| Benicio, 200447 | Brazil | 1,132 | Al, Tt | Not informed | Wheeze | ↑ | ND | ND |
| Daschner, 200548 | Spain | 135 | As | AsE | Chronic urticaria | ↑ | ND | ND |
| Obihara, 200649 | South Africa | 359 | Al(IgE) | HDM | Asthma | ↑ | ↑ | ↑ |
| Bahceciler, 200750 | Istanbul | 1,018 | Ev | Various | Wheeze, rhinitis | ↑ | No change | ND |
| Pereira, 200751 | Brazil | 1,011 | Al | Various | Wheeze/asthma | ↑ | ↓ | |
| Hagel, 200752 | Venezuela | 470 | Al | AlE, HDM | Asthma | ↑ | ↑ (Al-IgE) | ND |
| Hunninghake, 200753 | Costa Rica | 439 | Al | HDM, CR | Asthma | ↑ | ↑ | ↑ |
| Alcantara-Neves, 201054 | Brazil | 682 | (IgE)Tt, Al | Various | Wheeze | ↑ | ND | ↑ |
| Walsh, 201155 | United States | 12,174 | Tc | NE | Asthma | ↑ | ND | ND |
| Choi, 201156 | Korea | 1,116 | Cs | Various | Asthma, rhinitis | No change | ↑ | ↑ |
| Moncayo, 201257 | Ecuador | 376 | Al | AlE, HDM, CR | Wheeze | ↑ | No change | ↑ |
| Buendia, 201558 | Colombia | 313 | Al | HDM | Asthma | ↑ | ↑ | ↑ |
| Webb, 201659 | Uganda | 2,316 | Various | Various | Wheeze | ↑ | ↑ | ↑ |
Al = Ascaris lumbricoides; As = Anisakis simplex; Bm = Brugia malayi; Cs = Clonorchis sinensis; Ev = Enterobius vermicularis; Hkw = hookworm; Sh = Schistosoma haematobium; Sm = Schistosoma mansoni; Tc = Toxocara canis; Tt = Trichuris trichiura.
AlE = Ascaris lumbricoides extract; AsE = Anisakis simplex extract; CR = cockroach; HDM = house dust mite.
SPT = skin prick test.
asIgE = aeroallergen-specific IgE; AD = allergic dermatitis; EIB = exercise-induced bronchorreactivity; ND = not determined.
Figure 1.
Aggregated overview of multiple studies on the helminth/allergy interface. The areas shaded black indicate increased prevalence of allergic reactivity in the presence of helminth infection, the areas shaded white indicate decreased prevalence, and those in gray indicate no change in prevalence on clinical outcome (left circle), skin prick test (SPT; middle circle), and aeroallergen-specific IgE (asIgE; right circle).
The concept that helminth infection modulates allergic diseases emerged in the 1970s62–64 and has been debated ever since.40,65–70 As depicted in Table 1, it has often been observed that helminth infections commonly reduce the frequency of SPT reactivity and increase the levels of asIgE (Table 1 and Figure 1). This apparent dichotomy was felt to reflect the expansion of polyclonal IgE-secreting B cells, an expansion that would lead to high levels of IgE with multiple specificities leading to an inability to trigger a mast cell or basophil response. It was thought that allergens, in such conditions, could not physically cross-link the asIgE bound to the high-affinity Fc epsilon (FcϵRI) because of the multiple differing IgE antibody specificities on proximal FcϵRIs. Although theoretically possible, this concept has largely been discarded based on studies that suggest that the ratio of polyclonal to antigen-specific IgE needed to prevent basophil or mast cell degranulation rarely is achieved in vivo since it requires only cross-linking a few hundred FcϵRIs on cell membranes to trigger activation.71,72 More recent data allow us to propose other mechanisms at work in helminth infection that drive asIgE in helminth-infected populations (e.g., cross-reactive IgE) or modulate SPT positivity (e.g., IL-10) as discussed below.
Because chronic helminth infections often induce both IgE and IgG isotypes, especially IgG4 antibodies, it has been proposed that IgG can also contribute to the modulation of type I hypersensitivity responses. IgGs are usually induced in quantities 1,000- to 10,000-fold greater than those of IgE, and as such, the IgG antibodies bind antigen prior to the antigen being available to trigger an IgE-mediated effector response. This so-called “blocking phenomenon” has been explored, and two mechanisms have been identified: physical73,74 and inhibition of target cells by FcγRIIb activation by IgG complexed to antigens.74–76 Although other IgG isotypes have been implicated in physical IgE blocking, IgG4 seems to play a major role.77 It has been demonstrated that IgG can intercept allergen before it binds to IgE present on membranes of mast cells and basophils,74 and that allergen-IgG complexes can deactivate target cell by activation of the inhibitory Fc receptor (FcγRIIb), that in turn activates phosphatases in the molecular cross-linking regions of IgE shutting down FcϵRI signaling.78,79
A more unifying explanation suggests that IL-10, a primarily T-cell–derived cytokine commonly induced in chronic helminth infection,80–82 may underlie the protection from SPT positivity.11,83 Indeed, it is believed that the IL-10 modified Th2 response may be responsible for the parasite-antigen-specific tolerance imprinted on the host by helminth infection.80,84,85 Parasite-induced IL-10 or other regulatory mechanisms—that can involve cell populations such as Tregs81,86 and Bregs87–89—can increase the IgE-induced activation threshold of basophils,90 regulate T-cell91,92 and B-cell activation,92 promote IgG class switch,93 and IgG4-producing B-cell differentiation and proliferation.94 Moreover, IL-10 has been shown to drive down the production of IgE while at the same time induce isotype switching to IgG4.95,96
Despite the regulatory responses that helminth infections can induce in the host, these may not be sufficient to counteract the Th2-associated processes that mediate many allergic diseases. This puts into a framework the concept that a very delicate balance between pro- and anti-allergenic effector responses is required to maintain homeostasis. It is widely known that helminth parasites are associated with antigen-driven expansion of Th2 cells97–99 along with polyclonal T-cell activation.97–99 Interestingly, allergen extracts and helminths excretory/secretory products often share similar properties that can lead to Type 2-associated responses. For example, both are rich in proteases100 that can promote Th2 differentiation through protease-activated receptor 2101–103 directly on T cells102 or indirectly by inducing IL-33 or thymic stromal lymphopoietin production by tissue cells104,105 or IL-13 production by macrophages.106 In addition, both allergens and helminths are known to increase numbers and activity of type 2 innate lymphoid cells107–110 that license dendritic cells to promote Th2 priming in lymph nodes.111
Along with this strong, specific, and polyclonal Th2 activation induced by helminth infection, these infections also induce both polyclonal- and antigen-specific IgE production.112 Whether a result of this Th2-associated response or of some other type of response (e.g., Treg, IL-10, transforming growth factor-β), it has been observed that helminth infection causes decreases in IgG responses to parenterally-administered vaccines113 and increases in IgE responses to bystander antigens.114–117 This IgE bystander effect has been suggested to be one of the reasons that helminth parasites can promote allergic reactivity through mechanisms that include: B-cell IgE polyclonal activation; IgE potentiation in which infection creates an environment that will favor IgE production to other nonparasite antigens; and IgE cross-reactivity among parasite antigens and environmental allergens.
There has been increased interest in IgE cross-reactivity involving helminth parasites and allergens as well.116–128 We have demonstrated that helminth infection can be associated with increased IgE responses to many different allergens, especially those structurally related to parasite antigens.116 Individuals infected with filarial parasites were shown to have higher levels of IgE against HDM and cockroach extracts (that have several major allergens with homologues in filarial parasites) but not against timothy grass extract, an allergen extract with just a few minor allergen homologues in helminths.116
In more detailed studies, cross-reactivity among allergens and parasite molecules has been well described for tropomyosin,120,122,129 considered a pan-allergen.130 Tropomyosins are highly conserved across many species and cross-reactivity is not surprising from the structural point-of-view. This allergen has dominated discussions about helminth-allergen cross-reactivity, and many reviews have already discussed its implications in detail.131,132 However, studies on allergen and parasite protein sequences have found that huge numbers of allergens have both linear133 and conformational134 epitopes with significant similarity to homologous helminth proteins. The structural basis for this “allergenicity” has been inferred from bioinformatic analyses, in which it has been shown that the level of identity conservation between allergens and parasite proteins were negatively correlated with IgE prevalence to that allergen.133 Furthermore, most of the major allergens with homologues in helminth parasites show medium to low levels of conservation with helminth proteins (amino acid identities ranging from 20% to 40%),133 levels of identity deemed unlikely to be subject of antibody cross-reactivity. Nevertheless, evidences of cross-reactivity among less-conserved pairs of antigens have been demonstrated recently,116,117,120,123,133 suggesting that this may be a broader phenomenon than previously appreciated. It was noticed that extracts of the cockroach Blatella germanica (Bla g) can inhibit the binding of IgE to several Anisakis simplex allergens.118 Similarly, IgE binding to several Ascaris allergens can also be inhibited by Dermatophagoides pteronyssinus (Der p) or Blomia tropicalis (Blo t) extracts, including glutathione-S-transferase (GST).120
GSTs are major allergens of HDM,119 cockroach,135 mold,136,137 and parasites.138,139 Among the 13 classes of canonical GSTs, there are many that show very little amino acid conservation.140 Even with the small degree of sequence conservation, cross-reactivity among parasite and allergenic GST has been demonstrated formally.123 There was found to be a significant positive correlation of antiallergenic (Bla g 5, Der p 8, and Blo t 8) and antiparasite (Ascaris and filarial) GST-specific IgE levels in humans.123,138 In addition, experimental models have corroborated these findings. For example, Heligmosomoides polygyrus infection induced IgE anti-cockroach GST,123 and immunization studies with Ascaris antigens induced IgE and Th2 cells against HDM extract.117 Despite helminth protein and allergen cross-reactions leading to cross-sensitization in humans are possible,58,141–144 formal proof has not, to date, been demonstrated, especially for poorly conserved allergen-helminth proteins pairs.
Thus, the interface between helminth infection and allergic disorders reflects the delicate balance between the regulatory responses and the pro-allergenic effects of the parasites. Despite the relatively consistent finding that the presence of an active helminth infection results in lower prevalence of SPT to common environmental allergens, the fact that these same helminth infections markedly increase levels of antigen-specific IgE suggest that the complex interplay among this antigen- and allergen-specific IgE, the high affinity FcϵRI on effector cells, the regulatory and effector cytokines, and the cells at barrier sites must be studied in concert to truly understand this very complex interaction.
Footnotes
Financial support: This work was supported, in part, by the Division of Intramural Research (DIR), National Institute of Allergy and Infectious Diseases (NIAID), the Conselho Nacional de Pesquisa (CNPq), and the Fundação de Amparo a Pesquisa de Minas Gerais (Fapemig).
Authors' addresses: Helton C. Santiago, Departamento de Bioquímica e Imunologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil, E-mail: heltonsantiago@icb.ufmg.br. Thomas B. Nutman, Laboratory of Parasitic Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, E-mail: tnutman@niaid.nih.gov.
References
- 1.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 5.Elliott DE, Weinstock JV. Where are we on worms? Curr Opin Gastroenterol. 2012;28:551–556. doi: 10.1097/MOG.0b013e3283572f73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aravindhan V, Mohan V, Surendar J, Rao MM, Ranjani H, Kumaraswami V, Nutman TB, Babu S. Decreased prevalence of lymphatic filariasis among subjects with type-1 diabetes. Am J Trop Med Hyg. 2010;83:1336–1339. doi: 10.4269/ajtmh.2010.10-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panda AK, Ravindran B, Das BK. Rheumatoid arthritis patients are free of filarial infection in an area where filariasis is endemic: comment on the article by Pineda et al. Arthritis Rheum. 2013;65:1402–1403. doi: 10.1002/art.37883. [DOI] [PubMed] [Google Scholar]
- 8.Pineda MA, McGrath MA, Smith PC, Al-Riyami L, Rzepecka J, Gracie JA, Harnett W, Harnett MM. The parasitic helminth product ES-62 suppresses pathogenesis in collagen-induced arthritis by targeting the interleukin-17-producing cellular network at multiple sites. Arthritis Rheum. 2012;64:3168–3178. doi: 10.1002/art.34581. [DOI] [PubMed] [Google Scholar]
- 9.Rocha FA, Leite AK, Pompeu MM, Cunha TM, Verri WA, Jr, Soares FM, Castro RR, Cunha FQ. Protective effect of an extract from Ascaris suum in experimental arthritis models. Infect Immun. 2008;76:2736–2745. doi: 10.1128/IAI.01085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagel I, Lynch NR, Perez M, Di Prisco MC, Lopez R, Rojas E. Modulation of the allergic reactivity of slum children by helminthic infection. Parasite Immunol. 1993;15:311–315. doi: 10.1111/j.1365-3024.1993.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 11.van den Biggelaar AH, van Ree R, Rodrigues LC, Lell B, Deelder AM, Kremsner PG, Yazdanbakhsh M. Decreased atopy in children infected with Schistosoma haematobium: a role for parasite-induced interleukin-10. Lancet. 2000;356:1723–1727. doi: 10.1016/S0140-6736(00)03206-2. [DOI] [PubMed] [Google Scholar]
- 12.Araujo MI, Lopes AA, Medeiros M, Cruz AA, Sousa-Atta L, Sole D, Carvalho EM. Inverse association between skin response to aeroallergens and Schistosoma mansoni infection. Int Arch Allergy Immunol. 2000;123:145–148. doi: 10.1159/000024433. [DOI] [PubMed] [Google Scholar]
- 13.van den Biggelaar AH, Lopuhaa C, van Ree R, van der Zee JS, Jans J, Hoek A, Migombet B, Borrmann S, Luckner D, Kermsner PG, Yazdanbakhsh M. The prevalence of parasite infestation and house dust mite sensitization in Gabonese schoolchildren. Int Arch Allergy Immunol. 2001;126:321–338. doi: 10.1159/000049519. [DOI] [PubMed] [Google Scholar]
- 14.Nyan OA, Walraven GE, Banya WA, Milligan P, Van Der Sande M, Ceesay SM, Del Prete G, McAdam KP. Atopy, intestinal helminth infection and total serum IgE in rural and urban adult Gambian communities. Clin Exp Allergy. 2001;31:1672–1678. doi: 10.1046/j.1365-2222.2001.00987.x. [DOI] [PubMed] [Google Scholar]
- 15.Scrivener S, Yemaneberhan H, Zebenigus M, Tilahun D, Girma S, Ali S, McElroy P, Custovic A, Woodcock A, Pritchard D, Venn A, Britton J. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet. 2001;358:1493–1499. doi: 10.1016/S0140-6736(01)06579-5. [DOI] [PubMed] [Google Scholar]
- 16.Huang SL, Tsai PF, Yeh YF. Negative association of Enterobius infestation with asthma and rhinitis in primary school children in Taipei. Clin Exp Allergy. 2002;32:1029–1032. doi: 10.1046/j.1365-2745.2002.01424.x. [DOI] [PubMed] [Google Scholar]
- 17.Cooper PJ, Chico ME, Bland M, Griffin GE, Nutman TB. Allergic symptoms, atopy, and geohelminth infections in a rural area of Ecuador. Am J Respir Crit Care Med. 2003;168:313–317. doi: 10.1164/rccm.200211-1320OC. [DOI] [PubMed] [Google Scholar]
- 18.Cooper PJ, Chico ME, Rodrigues LC, Ordonez M, Strachan D, Griffin GE, Nutman TB. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003;111:995–1000. doi: 10.1067/mai.2003.1348. [DOI] [PubMed] [Google Scholar]
- 19.Dagoye D, Bekele Z, Woldemichael K, Nida H, Yimam M, Hall A, Venn AJ, Britton JR, Hubbard R, Lewis SA. Wheezing, allergy, and parasite infection in children in urban and rural Ethiopia. Am J Respir Crit Care Med. 2003;167:1369–1373. doi: 10.1164/rccm.200210-1204OC. [DOI] [PubMed] [Google Scholar]
- 20.Wahyuni S, Sartono E, Supali T, van der Zee JS, Mangali A, van Ree R, Houwing-Duistermaat JJ, Yazdanbakhsh M. Clustering of allergic outcomes within families and households in areas endemic for helminth infections. Int Arch Allergy Immunol. 2005;136:356–364. doi: 10.1159/000084255. [DOI] [PubMed] [Google Scholar]
- 21.Flohr C, Tuyen LN, Lewis S, Quinnell R, Minh TT, Liem HT, Campbell J, Pritchard D, Hien TT, Farrar J, Williams H, Britton J. Poor sanitation and helminth infection protect against skin sensitization in Vietnamese children: a cross-sectional study. J Allergy Clin Immunol. 2006;118:1305–1311. doi: 10.1016/j.jaci.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues LC, Newcombe PJ, Cunha SS, Alcantara-Neves NM, Genser B, Cruz AA, Simoes SM, Fiaccone R, Amorim L, Cooper PJ, Barreto ML. Early infection with Trichuris trichiura and allergen skin test reactivity in later childhood. Clin Exp Allergy. 2008;38:1769–1777. doi: 10.1111/j.1365-2222.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- 23.Endara P, Vaca M, Chico ME, Erazo S, Oviedo G, Quinzo I, Rodriguez A, Lovato R, Moncayo AL, Barreto ML, Rodrigues LC, Cooper PJ. Long-term periodic anthelmintic treatments are associated with increased allergen skin reactivity. Clin Exp Allergy. 2010;40:1669–1677. doi: 10.1111/j.1365-2222.2010.03559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Supali T, Djuardi Y, Wibowo H, van Ree R, Yazdanbakhsh M, Sartono E. Relationship between different species of helminths and atopy: a study in a population living in helminth-endemic area in Sulawesi, Indonesia. Int Arch Allergy Immunol. 2010;153:388–394. doi: 10.1159/000316350. [DOI] [PubMed] [Google Scholar]
- 25.Rujeni N, Nausch N, Bourke CD, Midzi N, Mduluza T, Taylor DW, Mutapi F. Atopy is inversely related to schistosome infection intensity: a comparative study in Zimbabwean villages with distinct levels of Schistosoma haematobium infection. Int Arch Allergy Immunol. 2012;158:288–298. doi: 10.1159/000332949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanobana K, Vereecken K, Junco Diaz R, Sariego I, Rojas L, Bonet Gorbea M, Polman K. Toxocara seropositivity, atopy and asthma: a study in Cuban schoolchildren. Trop Med Int Health. 2013;18:403–406. doi: 10.1111/tmi.12073. [DOI] [PubMed] [Google Scholar]
- 27.Alcantara-Neves NM, Veiga RV, Dattoli VC, Fiaccone RL, Esquivel R, Cruz AA, Cooper PJ, Rodrigues LC, Barreto ML. The effect of single and multiple infections on atopy and wheezing in children. J Allergy Clin Immunol. 2012;129:359–367. doi: 10.1016/j.jaci.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendonca LR, Veiga RV, Dattoli VC, Figueiredo CA, Fiaccone R, Santos J, Cruz AA, Rodrigues LC, Cooper PJ, Pontes-de-Carvalho LC, Barreto ML, Alcantara-Neves NM. Toxocara seropositivity, atopy and wheezing in children living in poor neighbourhoods in urban Latin American. PLoS Negl Trop Dis. 2012;6:e1886. doi: 10.1371/journal.pntd.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manuel AM, Kuljit S, Gopalakrishnan G, Suresh KG, Balraj P. The role of worm infestation in allergic rhinitis. Trop Biomed. 2012;29:360–365. [PubMed] [Google Scholar]
- 30.Oliveira SM, Bezerra FS, Carneiro TR, Pinheiro MC, Queiroz JA. Association between allergic responses and Schistosoma mansoni infection in residents in a low-endemic setting in Brazil. Rev Soc Bras Med Trop. 2014;47:770–774. doi: 10.1590/0037-8682-0249-2014. [DOI] [PubMed] [Google Scholar]
- 31.Davey G, Venn A, Belete H, Berhane Y, Britton J. Wheeze, allergic sensitization and geohelminth infection in Butajira, Ethiopia. Clin Exp Allergy. 2005;35:301–307. doi: 10.1111/j.1365-2222.2005.02181.x. [DOI] [PubMed] [Google Scholar]
- 32.Ponte EV, Rasella D, Souza-Machado C, Stelmach R, Barreto ML, Cruz AA. Reduced asthma morbidity in endemic areas for helminth infections: a longitudinal ecological study in Brazil. J Asthma. 2014;51:1022–1027. doi: 10.3109/02770903.2014.936454. [DOI] [PubMed] [Google Scholar]
- 33.Calvert J, Burney P. Ascaris, atopy, and exercise-induced bronchoconstriction in rural and urban South African children. J Allergy Clin Immunol. 2010;125:100–105. doi: 10.1016/j.jaci.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vereecken K, Kanobana K, Wordemann M, Junco Diaz R, Menocal Heredia L, Ruiz Espinosa A, Nunez FA, Rojas Rivero L, Bonet Gorbea M, Polman K. Associations between atopic markers in asthma and intestinal helminth infections in Cuban schoolchildren. Pediatr Allergy Immunol. 2012;23:332–338. doi: 10.1111/j.1399-3038.2011.01267.x. [DOI] [PubMed] [Google Scholar]
- 35.Souza V, Medeiros D, Sales I, Costa V, Silva A, Rizzo J, Sole D, Sarinho E. Ascaris lumbricoides infection in urban schoolchildren: specific IgE and IL-10 production. Allergol Immunopathol (Madr) 2014;42:206–211. doi: 10.1016/j.aller.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Alcantara-Neves NM, Britto GDSG, Veiga RV, Figueiredo CA, Fiaccone RL, da Conceicao JS, Cruz AA, Rodrigues LC, Cooper PJ, Pontes-de-Carvalho LC, Barreto ML. Effects of helminth co-infections on atopy, asthma and cytokine production in children living in a poor urban area in Latin America. BMC Res Notes. 2014;7:817–827. doi: 10.1186/1756-0500-7-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamid F, Wahyuni S, van Leeuwen A, van Ree R, Yazdanbakhsh M, Sartono E. Allergic disorders and socio-economic status: a study of schoolchildren in an urban area of Makassar, Indonesia. Clin Exp Allergy. 2015;45:1226–1236. doi: 10.1111/cea.12517. [DOI] [PubMed] [Google Scholar]
- 38.Wordemann M, Diaz RJ, Heredia LM, Collado Madurga AM, Ruiz Espinosa A, Prado RC, Millan IA, Escobedo A, Rojas Rivero L, Gryseels B, Gorbea MB, Polman K. Association of atopy, asthma, allergic rhinoconjunctivitis, atopic dermatitis and intestinal helminth infections in Cuban children. Trop Med Int Health. 2008;13:180–186. doi: 10.1111/j.1365-3156.2007.01988.x. [DOI] [PubMed] [Google Scholar]
- 39.Obeng BB, Amoah AS, Larbi IA, de Souza DK, Uh HW, Fernandez-Rivas M, van Ree R, Rodrigues LC, Boakye DA, Yazdanbakhsh M, Hartgers FC. Schistosome infection is negatively associated with mite atopy, but not wheeze and asthma in Ghanaian schoolchildren. Clin Exp Allergy. 2014;44:965–975. doi: 10.1111/cea.12307. [DOI] [PubMed] [Google Scholar]
- 40.Kayhan B, Telatar H, Karacadag S. Bronchial asthma associated with intestinal parasites. Am J Gastroenterol. 1978;69:605–606. [PubMed] [Google Scholar]
- 41.Joubert JR, van Schalkwyk DJ, Turner KJ. Ascaris lumbricoides and the human immunogenic response: enhanced IgE-mediated reactivity to common inhaled allergens. Bull World Health Organ. 1980;57:409–412. [PubMed] [Google Scholar]
- 42.Alshishtawy MM, Abdella AM, Gelber LE, Chapman MD. Asthma in Tanta, Egypt: serologic analysis of total and specific IgE antibody levels and their relationship to parasite infection. Int Arch Allergy Appl Immunol. 1991;96:348–354. doi: 10.1159/000235520. [DOI] [PubMed] [Google Scholar]
- 43.Buijs J, Borsboom G, Renting M, Hilgersom WJ, van Wieringen JC, Jansen G, Neijens J. Relationship between allergic manifestations and Toxocara seropositivity: a cross-sectional study among elementary school children. Eur Respir J. 1997;10:1467–1475. doi: 10.1183/09031936.97.10071467. [DOI] [PubMed] [Google Scholar]
- 44.Lynch NR, Palenque M, Hagel I, DiPrisco MC. Clinical improvement of asthma after anthelminthic treatment in a tropical situation. Am J Respir Crit Care Med. 1997;156:50–54. doi: 10.1164/ajrccm.156.1.9606081. [DOI] [PubMed] [Google Scholar]
- 45.Dold S, Heinrich J, Wichmann HE, Wjst M. Ascaris-specific IgE and allergic sensitization in a cohort of school children in the former East Germany. J Allergy Clin Immunol. 1998;102:414–420. doi: 10.1016/s0091-6749(98)70129-0. [DOI] [PubMed] [Google Scholar]
- 46.Palmer LJ, Celedon JC, Weiss ST, Wang B, Fang Z, Xu X. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med. 2002;165:1489–1493. doi: 10.1164/rccm.2107020. [DOI] [PubMed] [Google Scholar]
- 47.Benicio MH, Ferreira MU, Cardoso MR, Konno SC, Monteiro CA. Wheezing conditions in early childhood: prevalence and risk factors in the city of Sao Paulo, Brazil. Bull World Health Organ. 2004;82:516–522. [PMC free article] [PubMed] [Google Scholar]
- 48.Daschner A, Vega de la Osada F, Pascual CY. Allergy and parasites reevaluated: wide-scale induction of chronic urticaria by the ubiquitous fish-nematode Anisakis simplex in an endemic region. Allergol Immunopathol (Madr) 2005;33:31–37. doi: 10.1157/13070606. [DOI] [PubMed] [Google Scholar]
- 49.Obihara CC, Beyers N, Gie RP, Hoekstra MO, Fincham JE, Marais BJ, Lombard CJ, Dini LA, Kimpen JL. Respiratory atopic disease, Ascaris-immunoglobulin E and tuberculin testing in urban South African children. Clin Exp Allergy. 2006;36:640–648. doi: 10.1111/j.1365-2222.2006.02479.x. [DOI] [PubMed] [Google Scholar]
- 50.Bahceciler NN, Ozdemir C, Kucukosmanoglu E, Arikan C, Over U, Karavelioglu S, Akkoc T, Yazi D, Yesil O, Soysal A, Bakir M, Barlan IB. Association between previous enterobiasis and current wheezing: evaluation of 1018 children. Allergy Asthma Proc. 2007;28:174–182. doi: 10.2500/aap.2007.27.2904. [DOI] [PubMed] [Google Scholar]
- 51.Pereira MU, Sly PD, Pitrez PM, Jones MH, Escouto D, Dias AC, Weiland SK, Stein RT. Nonatopic asthma is associated with helminth infections and bronchiolitis in poor children. Eur Respir J. 2007;29:1154–1160. doi: 10.1183/09031936.00127606. [DOI] [PubMed] [Google Scholar]
- 52.Hagel I, Cabrera M, Hurtado MA, Sanchez P, Puccio F, Di Prisco MC, Palenque M. Infection by Ascaris lumbricoides and bronchial hyper reactivity: an outstanding association in Venezuelan school children from endemic areas. Acta Trop. 2007;103:231–241. doi: 10.1016/j.actatropica.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Hunninghake GM, Soto-Quiros ME, Avila L, Ly NP, Liang C, Sylvia JS, Klanderman BJ, Silverman EK, Celedon JC. Sensitization to Ascaris lumbricoides and severity of childhood asthma in Costa Rica. J Allergy Clin Immunol. 2007;119:654–661. doi: 10.1016/j.jaci.2006.12.609. [DOI] [PubMed] [Google Scholar]
- 54.Alcantara-Neves NM, Badaro SJ, dos Santos MC, Pontes-de-Carvalho L, Barreto ML. The presence of serum anti-Ascaris lumbricoides IgE antibodies and of Trichuris trichiura infection are risk factors for wheezing and/or atopy in preschool-aged Brazilian children. Respir Res. 2010;11:114. doi: 10.1186/1465-9921-11-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh MG. Toxocara infection and diminished lung function in a nationally representative sample from the United States population. Int J Parasitol. 2011;41:243–247. doi: 10.1016/j.ijpara.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 56.Choi MH, Chang YS, Lim MK, Bae YM, Hong ST, Oh JK, Yun EH, Bae MJ, Kwon HS, Lee SM, Park HW, Min KU, Kim YY, Cho SH. Clonorchis sinensis infection is positively associated with atopy in endemic area. Clin Exp Allergy. 2011;41:697–705. doi: 10.1111/j.1365-2222.2011.03746.x. [DOI] [PubMed] [Google Scholar]
- 57.Moncayo AL, Vaca M, Oviedo G, Workman LJ, Chico ME, Platts-Mills TA, Rodrigues LC, Barreto ML, Cooper PJ. Effects of geohelminth infection and age on the associations between allergen-specific IgE, skin test reactivity and wheeze: a case-control study. Clin Exp Allergy. 2013;43:60–72. doi: 10.1111/cea.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buendia E, Zakzuk J, Mercado D, Alvarez A, Caraballo L. The IgE response to Ascaris molecular components is associated with clinical indicators of asthma severity. World Allergy Organ J. 2015;8:8. doi: 10.1186/s40413-015-0058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Webb EL, Nampijja M, Kaweesa J, Kizindo R, Namutebi M, Nakazibwe E, Oduru G, Kabubi P, Kabagenyi J, Nkurunungi G, Kizito D, Muhangi L, Akello M, Verweij JJ, Nerima B, Tukahebwa E, Elliott AM. LaVIISWA Helminths are positively associated with atopy and wheeze in Ugandan fishing communities: results from a cross-sectional survey. Allergy. 2016;71:1156–1169. doi: 10.1111/all.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leonardi-Bee J, Pritchard D, Britton J. Asthma and current intestinal parasite infection: systematic review and meta-analysis. Am J Respir Crit Care Med. 2006;174:514–523. doi: 10.1164/rccm.200603-331OC. [DOI] [PubMed] [Google Scholar]
- 61.Feary J, Britton J, Leonardi-Bee J. Atopy and current intestinal parasite infection: a systematic review and meta-analysis. Allergy. 2011;66:569–578. doi: 10.1111/j.1398-9995.2010.02512.x. [DOI] [PubMed] [Google Scholar]
- 62.Alcasid ML, Chiaramonte LT, Kim HJ, Zohn B, Bongiorno JR, Mullin W. Bronchial asthma and intestinal parasites. N Y State J Med. 1973;73:1786–1788. [PubMed] [Google Scholar]
- 63.Turton JA. Letter: IgE, parasites, and allergy. Lancet. 1976;2:686. doi: 10.1016/s0140-6736(76)92492-2. [DOI] [PubMed] [Google Scholar]
- 64.Turner KJ, Quinn EH, Anderson HR. Regulation of asthma by intestinal parasites. Investigation of possible mechanisms. Immunology. 1978;35:281–288. [PMC free article] [PubMed] [Google Scholar]
- 65.Tullis DC. Bronchial asthma associated with intestinal parasites. N Engl J Med. 1970;282:370–372. doi: 10.1056/NEJM197002122820706. [DOI] [PubMed] [Google Scholar]
- 66.Tullis DC. Ascaris and asthma. N Engl J Med. 1971;285:806. doi: 10.1056/NEJM197109302851424. [DOI] [PubMed] [Google Scholar]
- 67.Van Dellen RG, Thompson JH., Jr Absence of intestinal parasites in asthma. N Engl J Med. 1971;285:146–148. doi: 10.1056/NEJM197107152850304. [DOI] [PubMed] [Google Scholar]
- 68.Jarrett EE, Kerr JW. Threadworms and IgE in allergic asthma. Clin Allergy. 1973;3:203–207. doi: 10.1111/j.1365-2222.1973.tb01322.x. [DOI] [PubMed] [Google Scholar]
- 69.Carswell F, Meakins RH, Harland PS. Parasites and asthma in Tanzanian children. Lancet. 1976;2:706–707. doi: 10.1016/s0140-6736(76)90004-0. [DOI] [PubMed] [Google Scholar]
- 70.Macfarlane JT, Bachelor M, Ridyard JB, Ball PA. Asthma, IgE and environment in northern Nigeria. Clin Allergy. 1979;9:333–337. doi: 10.1111/j.1365-2222.1979.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 71.Mitre E, Norwood S, Nutman TB. Saturation of immunoglobulin E (IgE) binding sites by polyclonal IgE does not explain the protective effect of helminth infections against atopy. Infect Immun. 2005;73:4106–4111. doi: 10.1128/IAI.73.7.4106-4111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pritchard DI, Hooi DS, Brown A, Bockarie MJ, Caddick R, Quinnell RJ. Basophil competence during hookworm (Necator americanus) infection. Am J Trop Med Hyg. 2007;77:860–865. [PubMed] [Google Scholar]
- 73.Ejrnaes AM, Bodtger U, Larsen JN, Svenson M. The blocking activity of birch pollen-specific immunotherapy-induced IgG4 is not qualitatively superior to that of other IgG subclasses. Mol Immunol. 2004;41:471–478. doi: 10.1016/j.molimm.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 74.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J Clin Invest. 2006;116:833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saxon A, Kepley C, Zhang K. “Accentuate the negative, eliminate the positive”: engineering allergy therapeutics to block allergic reactivity through negative signaling. J Allergy Clin Immunol. 2008;121:320–325. doi: 10.1016/j.jaci.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 76.Zhu D, Kepley CL, Zhang M, Zhang K, Saxon A. A novel human immunoglobulin Fc gamma Fc epsilon bifunctional fusion protein inhibits Fc epsilon RI-mediated degranulation. Nat Med. 2002;8:518–521. doi: 10.1038/nm0502-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hussain R, Poindexter RW, Ottesen EA. Control of allergic reactivity in human filariasis. Predominant localization of blocking antibody to the IgG4 subclass. J Immunol. 1992;148:2731–2737. [PubMed] [Google Scholar]
- 78.Kepley CL, Cambier JC, Morel PA, Lujan D, Ortega E, Wilson BS, Oliver JM. Negative regulation of FcepsilonRI signaling by FcgammaRII costimulation in human blood basophils. J Allergy Clin Immunol. 2000;106:337–348. doi: 10.1067/mai.2000.107931. [DOI] [PubMed] [Google Scholar]
- 79.Mertsching E, Bafetti L, Hess H, Perper S, Giza K, Allen LC, Negrou E, Hathaway K, Hopp J, Chung J, Perret D, Shields M, Saxon A, Kehry MR. A mouse Fcgamma-Fcepsilon protein that inhibits mast cells through activation of FcgammaRIIB, SH2 domain-containing inositol phosphatase 1, and SH2 domain-containing protein tyrosine phosphatases. J Allergy Clin Immunol. 2008;121:441–447. doi: 10.1016/j.jaci.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 80.King CL, Medhat A, Malhotra I, Nafeh M, Helmy A, Khaudary J, Ibrahim S, ElSherbiny M, Zaky S, Stupi RJ, Brustoski K, Shehata M, Shata MT. Cytokine control of parasite-specific anergy in human urinary schistosomiasis: IL-10 modulates lymphocyte reactivity. J Immunol. 1996;156:4715–4721. [PubMed] [Google Scholar]
- 81.Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, Diallo AA, Soumaoro L, Coulibaly ME, Sanogo D, Doumbia SS, Traore SF, Mahanty S, Klion A, Nutman TB. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol. 2010;184:5375–5382. doi: 10.4049/jimmunol.0904067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steel C, Guinea A, Ottesen EA. Evidence for protective immunity to bancroftian filariasis in the Cook Islands. J Infect Dis. 1996;174:598–605. doi: 10.1093/infdis/174.3.598. [DOI] [PubMed] [Google Scholar]
- 83.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 84.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 85.Taylor JJ, Mohrs M, Pearce EJ. Regulatory T cell responses develop in parallel to Th responses and control the magnitude and phenotype of the Th effector population. J Immunol. 2006;176:5839–5847. doi: 10.4049/jimmunol.176.10.5839. [DOI] [PubMed] [Google Scholar]
- 86.Metenou S, Coulibaly YI, Sturdevant D, Dolo H, Diallo AA, Soumaoro L, Coulibaly ME, Kanakabandi K, Porcella SF, Klion AD, Nutman TB. Highly heterogeneous, activated, and short-lived regulatory T cells during chronic filarial infection. Eur J Immunol. 2014;44:2036–2047. doi: 10.1002/eji.201444452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilson MS, Taylor MD, O'Gorman MT, Balic A, Barr TA, Filbey K, Anderton SM, Maizels RM. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010;40:1682–1696. doi: 10.1002/eji.200939721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khan AR, Amu S, Saunders SP, Fallon PG. The generation of regulatory B cells by helminth parasites. Methods Mol Biol. 2014;1190:143–162. doi: 10.1007/978-1-4939-1161-5_11. [DOI] [PubMed] [Google Scholar]
- 89.van der Vlugt LE, Zinsou JF, Ozir-Fazalalikhan A, Kremsner PG, Yazdanbakhsh M, Adegnika AA, Smits HH. Interleukin 10 (IL-10)-producing CD1dhi regulatory B cells from Schistosoma haematobium-infected individuals induce IL-10-positive T cells and suppress effector T-cell cytokines. J Infect Dis. 2014;210:1207–1216. doi: 10.1093/infdis/jiu257. [DOI] [PubMed] [Google Scholar]
- 90.Larson D, Hubner MP, Torrero MN, Morris CP, Brankin A, Swierczewski BE, Davies SJ, Vonakis BM, Mitre E. Chronic helminth infection reduces basophil responsiveness in an IL-10-dependent manner. J Immunol. 2012;188:4188–4199. doi: 10.4049/jimmunol.1101859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Araujo MI, Hoppe B, Medeiros M, Jr, Alcantara L, Almeida MC, Schriefer A, Oliveira RR, Kruschewsky R, Figueiredo JP, Cruz AA, Carvalho EM. Impaired T helper 2 response to aeroallergen in helminth-infected patients with asthma. J Infect Dis. 2004;190:1797–1803. doi: 10.1086/425017. [DOI] [PubMed] [Google Scholar]
- 92.Hartmann W, Haben I, Fleischer B, Breloer M. Pathogenic nematodes suppress humoral responses to third-party antigens in vivo by IL-10-mediated interference with Th cell function. J Immunol. 2011;187:4088–4099. doi: 10.4049/jimmunol.1004136. [DOI] [PubMed] [Google Scholar]
- 93.Malisan F, Briere F, Bridon JM, Harindranath N, Mills FC, Max EE, Banchereau J, Martinez-Valdez H. Interleukin-10 induces immunoglobulin G isotype switch recombination in human CD40-activated naive B lymphocytes. J Exp Med. 1996;183:937–947. doi: 10.1084/jem.183.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998;160:3555–3561. [PubMed] [Google Scholar]
- 95.Akdis CA, Blaser K. IL-10-induced anergy in peripheral T cell and reactivation by microenvironmental cytokines: two key steps in specific immunotherapy. FASEB J. 1999;13:603–609. doi: 10.1096/fasebj.13.6.603. [DOI] [PubMed] [Google Scholar]
- 96.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG, Ruckert B, Akdis CA, Akdis M. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204–1212. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 97.Wadee AA, Piessens WF. Microfilariae of Brugia malayi contain a T cell mitogen. Am J Trop Med Hyg. 1986;35:141–147. doi: 10.4269/ajtmh.1986.35.141. [DOI] [PubMed] [Google Scholar]
- 98.Urban JF, Jr, Katona IM, Paul WE, Finkelman FD. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc Natl Acad Sci USA. 1991;88:5513–5517. doi: 10.1073/pnas.88.13.5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liwski RS, Lee TD. Nematode infection enhances survival of activated T cells by modulating accessory cell function. J Immunol. 1999;163:5005–5012. [PubMed] [Google Scholar]
- 100.Donnelly S, Dalton JP, Loukas A. Proteases in helminth- and allergen-induced inflammatory responses. Chem Immunol Allergy. 2006;90:45–64. doi: 10.1159/000088880. [DOI] [PubMed] [Google Scholar]
- 101.Devlin MG, Gasser RB, Cocks TM. Initial support for the hypothesis that PAR2 is involved in the immune response to Nippostrongylus brasiliensis in mice. Parasitol Res. 2007;101:105–109. doi: 10.1007/s00436-007-0467-1. [DOI] [PubMed] [Google Scholar]
- 102.Liang G, Barker T, Xie Z, Charles N, Rivera J, Druey KM. Naive T cells sense the cysteine protease allergen papain through protease-activated receptor 2 and propel TH2 immunity. J Allergy Clin Immunol. 2012;129:1377–1386. doi: 10.1016/j.jaci.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park MK, Cho MK, Kang SA, Park HK, Kim YS, Kim KU, Ahn SC, Kim DH, Yu HS. Protease-activated receptor 2 is involved in Th2 responses against Trichinella spiralis infection. Korean J Parasitol. 2011;49:235–243. doi: 10.3347/kjp.2011.49.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. 2009;183:1427–1434. doi: 10.4049/jimmunol.0900904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Snelgrove RJ, Gregory LG, Peiro T, Akthar S, Campbell GA, Walker SA, Lloyd CM. Alternaria-derived serine protease activity drives IL-33-mediated asthma exacerbations. J Allergy Clin Immunol. 2014;134:583–592. doi: 10.1016/j.jaci.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yamaguchi R, Yamamoto T, Sakamoto A, Ishimaru Y, Narahara S, Sugiuchi H, Hirose E, Yamaguchi Y. Mechanism of interleukin-13 production by granulocyte-macrophage colony-stimulating factor-dependent macrophages via protease-activated receptor-2. Blood Cells Mol Dis. 2015;55:21–26. doi: 10.1016/j.bcmd.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 107.Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671–678. doi: 10.1016/j.jaci.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boyd A, Killoran K, Mitre E, Nutman TB. Pleural cavity type 2 innate lymphoid cells precede Th2 expansion in murine Litomosoides sigmodontis infection. Exp Parasitol. 2015;159:118–126. doi: 10.1016/j.exppara.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, Hendriks RW. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–1116. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 110.Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, Englezakis A, Barlow JL, Hams E, Scanlon ST, Ogg GS, Fallon PG, McKenzie AN. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4+ T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Halim TY, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG, McKenzie AN. Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol. 2016;17:57–64. doi: 10.1038/ni.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harris N, Gause WC. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 2010;32:80–88. doi: 10.1016/j.it.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cooper PJ, Espinel I, Wieseman M, Paredes W, Espinel M, Guderian RH, Nutman TB. Human onchocerciasis and tetanus vaccination: impact on the postvaccination antitetanus antibody response. Infect Immun. 1999;67:5951–5957. doi: 10.1128/iai.67.11.5951-5957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jarrett E, Bazin H. Elevation of total serum IgE in rats following helminth parasite infection. Nature. 1974;251:613–614. doi: 10.1038/251613a0. [DOI] [PubMed] [Google Scholar]
- 115.Orr TS, Blair AM. Potentiated reagin response to egg albumin and conalbumin in Nippostrongylus brasiliensis infected rats. Life Sci. 1969;8:1073–1077. doi: 10.1016/0024-3205(69)90459-7. [DOI] [PubMed] [Google Scholar]
- 116.Santiago HC, Ribeiro-Gomes FL, Bennuru S, Nutman TB. Helminth infection alters IgE responses to allergens structurally related to parasite proteins. J Immunol. 2015;194:93–100. doi: 10.4049/jimmunol.1401638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Suzuki M, Hara M, Ichikawa S, Kamijo S, Nakazawa T, Hatanaka H, Akiyama K, Ogawa H, Okumura K, Takai T. Presensitization to Ascaris antigens promotes induction of mite-specific IgE upon mite antigen inhalation in mice. Allergol Int. 2016;65:44–51. doi: 10.1016/j.alit.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 118.Pascual CY, Crespo JF, San Martin S, Ornia N, Ortega N, Caballero T, Munoz-Pereira M, Martin-Esteban M. Cross-reactivity between IgE-binding proteins from Anisakis, German cockroach, and chironomids. Allergy. 1997;52:514–520. doi: 10.1111/j.1398-9995.1997.tb02594.x. [DOI] [PubMed] [Google Scholar]
- 119.Huang CH, Liew LM, Mah KW, Kuo IC, Lee BW, Chua KY. Characterization of glutathione S-transferase from dust mite, Der p 8 and its immunoglobulin E cross-reactivity with cockroach glutathione S-transferase. Clin Exp Allergy. 2006;36:369–376. doi: 10.1111/j.1365-2222.2006.02447.x. [DOI] [PubMed] [Google Scholar]
- 120.Acevedo N, Sanchez J, Erler A, Mercado D, Briza P, Kennedy M, Fernandez A, Gutierrez M, Chua KY, Cheong N, Jimenez S, Puerta L, Caraballo L. IgE cross-reactivity between Ascaris and domestic mite allergens: the role of tropomyosin and the nematode polyprotein ABA-1. Allergy. 2009;64:1635–1643. doi: 10.1111/j.1398-9995.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 121.Caraballo L, Acevedo N. Allergy in the tropics: the impact of cross-reactivity between mites and Ascaris. Front Biosci. 2011;3:51–64. doi: 10.2741/e219. [DOI] [PubMed] [Google Scholar]
- 122.Santiago HC, Bennuru S, Boyd A, Eberhard M, Nutman TB. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: implications for the hygiene hypothesis. J Allergy Clin Immunol. 2011;127:479–486. doi: 10.1016/j.jaci.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Santiago HC, LeeVan E, Bennuru S, Ribeiro-Gomes F, Mueller E, Wilson M, Wynn T, Garboczi D, Urban J, Mitre E, Nutman TB. Molecular mimicry between cockroach and helminth glutathione S-transferases promotes cross-reactivity and cross-sensitization. J Allergy Clin Immunol. 2012;130:248–256. doi: 10.1016/j.jaci.2012.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Valmonte GR, Cauyan GA, Ramos JD. IgE cross-reactivity between house dust mite allergens and Ascaris lumbricoides antigens. Asia Pac Allergy. 2012;2:35–44. doi: 10.5415/apallergy.2012.2.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakazawa T, Khan AF, Yasueda H, Saito A, Fukutomi Y, Takai T, Zaman K, Yunus M, Takeuchi H, Iwata T, Akiyama K. Immunization of rabbits with nematode Ascaris lumbricoides antigens induces antibodies cross-reactive to house dust mite Dermatophagoides farinae antigens. Biosci Biotechnol Biochem. 2013;77:145–150. doi: 10.1271/bbb.120626. [DOI] [PubMed] [Google Scholar]
- 126.Fitzsimmons CM, Falcone FH, Dunne DW. Helminth allergens, parasite-specific IgE, and its protective role in human immunity. Front Immunol. 2014;5:61. doi: 10.3389/fimmu.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodriguez-Perez R, Monsalve RI, Galan A, Perez-Pinar T, Umpierrez A, Lluch-Bernal M, Polo F, Caballero ML. Cross-reactivity between Anisakis spp. and wasp venom allergens. Int Arch Allergy Immunol. 2014;163:179–184. doi: 10.1159/000358060. [DOI] [PubMed] [Google Scholar]
- 128.Mueller GA, Pedersen LC, Glesner J, Edwards LL, Zakzuk J, London RE, Arruda LK, Chapman MD, Caraballo L, Pomes A. Analysis of glutathione S-transferase allergen cross-reactivity in a North American population: relevance for molecular diagnosis. J Allergy Clin Immunol. 2015;136:1369–1377. doi: 10.1016/j.jaci.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Santos AB, Rocha GM, Oliver C, Ferriani VP, Lima RC, Palma MS, Sales VS, Aalberse RC, Chapman MD, Arruda LK. Cross-reactive IgE antibody responses to tropomyosins from Ascaris lumbricoides and cockroach. J Allergy Clin Immunol. 2008;121:1040–1046. doi: 10.1016/j.jaci.2007.12.1147. [DOI] [PubMed] [Google Scholar]
- 130.Reese G, Ayuso R, Lehrer SB. Tropomyosin: an invertebrate pan-allergen. Int Arch Allergy Immunol. 1999;119:247–258. doi: 10.1159/000024201. [DOI] [PubMed] [Google Scholar]
- 131.Acevedo N, Caraballo L. IgE cross-reactivity between Ascaris lumbricoides and mite allergens: possible influences on allergic sensitization and asthma. Parasite Immunol. 2011;33:309–321. doi: 10.1111/j.1365-3024.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- 132.Sereda MJ, Hartmann S, Lucius R. Helminths and allergy: the example of tropomyosin. Trends Parasitol. 2008;24:272–278. doi: 10.1016/j.pt.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 133.Santiago HC, Bennuru S, Ribeiro JM, Nutman TB. Structural differences between human proteins and aero- and microbial allergens define allergenicity. PLoS One. 2012;7:e40552. doi: 10.1371/journal.pone.0040552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tyagi N, Farnell EJ, Fitzsimmons CM, Ryan S, Tukahebwa E, Maizels RM, Dunne DW, Thornton JM, Furnham N. Comparisons of allergenic and metazoan parasite proteins: allergy the price of immunity. PLOS Comput Biol. 2015;11:e1004546. doi: 10.1371/journal.pcbi.1004546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jeong KY, Jeong KJ, Yi MH, Lee H, Hong CS, Yong TS. Allergenicity of sigma and delta class glutathione S-transferases from the German cockroach. Int Arch Allergy Immunol. 2009;148:59–64. doi: 10.1159/000151506. [DOI] [PubMed] [Google Scholar]
- 136.Shankar J, Gupta PD, Sridhara S, Singh BP, Gaur SN, Arora N. Immunobiochemical analysis of cross-reactive glutathione-S-transferase allergen from different fungal sources. Immunol Invest. 2005;34:37–51. [PubMed] [Google Scholar]
- 137.Shankar J, Singh BP, Gaur SN, Arora N. Recombinant glutathione-S-transferase a major allergen from Alternaria alternata for clinical use in allergy patients. Mol Immunol. 2006;43:1927–1932. doi: 10.1016/j.molimm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 138.Acevedo N, Mohr J, Zakzuk J, Samonig M, Briza P, Erler A, Pomes A, Huber CG, Ferreira F, Caraballo L. Proteomic and immunochemical characterization of glutathione transferase as a new allergen of the nematode Ascaris lumbricoides. PLoS One. 2013;8:e78353. doi: 10.1371/journal.pone.0078353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hong SJ, Yun Kim T, Gan XX, Shen LY, Sukontason K, Kang SY. Clonorchis sinensis: glutathione S-transferase as a serodiagnostic antigen for detecting IgG and IgE antibodies. Exp Parasitol. 2002;101:231–233. doi: 10.1016/s0014-4894(02)00112-1. [DOI] [PubMed] [Google Scholar]
- 140.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 141.Acevedo N, Erler A, Briza P, Puccio F, Ferreira F, Caraballo L. Allergenicity of Ascaris lumbricoides tropomyosin and IgE sensitization among asthmatic patients in a tropical environment. Int Arch Allergy Immunol. 2011;154:195–206. doi: 10.1159/000321106. [DOI] [PubMed] [Google Scholar]
- 142.Fernandes J, Reshef A, Patton L, Ayuso R, Reese G, Lehrer SB. Immunoglobulin E antibody reactivity to the major shrimp allergen, tropomyosin, in unexposed Orthodox Jews. Clin Exp Allergy. 2003;33:956–961. doi: 10.1046/j.1365-2222.2003.01722.x. [DOI] [PubMed] [Google Scholar]
- 143.Purohit A, Shao J, Degreef JM, van Leeuwen A, van Ree R, Pauli G, de Blay F. Role of tropomyosin as a cross-reacting allergen in sensitization to cockroach in patients from Martinique (French Caribbean island) with a respiratory allergy to mite and a food allergy to crab and shrimp. Eur Ann Allergy Clin Immunol. 2007;39:85–88. [PubMed] [Google Scholar]
- 144.Wang J, Calatroni A, Visness CM, Sampson HA. Correlation of specific IgE to shrimp with cockroach and dust mite exposure and sensitization in an inner-city population. J Allergy Clin Immunol. 2011;128:834–837. doi: 10.1016/j.jaci.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]