Abstract
Visceral leishmaniasis (VL), or kala-azar, is mainly caused by two closely related Leishmania species, Leishmania infantum and Leishmania donovani. Leishmania infantum is responsible for zoonotic VL, with dogs as the main reservoir host in the Mediterranean, the Middle East, Asia, and South America. In the Indian subcontinent, VL is caused by L. donovani and is considered anthroponotic, although the only known vector, the sand fly, is zoophilic in nature. The role of domestic and stray dogs in VL transmission is still unclear in this area. We screened 50 stray dogs from VL-endemic areas of Bangladesh for serological and molecular evidence of Leishmania infection. We detected anti-Leishmania antibodies in six (12%) dog serum samples using rK39 immunochromatographic tests. We observed Leishmania kinetoplast DNA in 10 (20%) buffy coat DNA samples by real-time polymerase chain reaction (PCR), five of which were positive based on internal transcribed spacer 1-PCR. A sequencing analysis of the amplified products confirmed that the parasitic DNA was derived from L. donovani. Our findings support the hypothesis that stray dogs are an animal reservoir for L. donovani in this endemic region. Further studies are required to determine the precise role of dogs in the epidemiology of VL in Bangladesh.
Introduction
Visceral leishmaniasis (VL), or kala-azar, is a fatal vector-borne parasitic disease caused by the Leishmania donovani complex (Leishmania infantum and L. donovani) of intracellular protozoan parasites. VL is a serious public health problem in the Indian subcontinent; an estimated 200 million people are at risk, which represents approximately 67% of the global VL burden.1,2 In Bangladesh, the current prevalence is estimated to be 40,000–45,000 cases with more than 40.6 million people at risk of developing the disease.3,4 The disease is prevalent in 45 districts of Bangladesh, and most reported cases are from the Mymensingh District.3
VL has two epidemiological patterns. Anthroponotic VL is transmitted via infection from humans to humans and to a lesser extent from humans to animals. Zoonotic VL is transmitted from animals to humans and to a lesser extent from humans to humans. L. infantum is responsible for zoonotic VL, with dogs as the main reservoir hosts, in the Mediterranean, the Middle East, Asia, and South America. In areas where zoonotic VL is endemic, the prevalence of L. infantum infection in dogs is often high, although many infections are asymptomatic.5 The transmission of VL caused by L. donovani is thought to be anthroponotic in the Indian subcontinent and eastern Africa.6 The importance of animal reservoirs in these regions is not well studied.
There are several reports of L. donovani infection in leishmaniasis symptomatic dogs in Sudan7and in apparently healthy dogs in northwest Ethiopia.8 Infections in dogs with both L. donovani and L. infantum were reported in a village along the Albara River in eastern Sudan.9 Few studies have investigated the role of animal reservoirs in maintaining L. donovani in the Indian subcontinent. Recently, Leishmania amastigotes were detected in skin exudates of dogs in Sri Lanka10 and Leishmania DNA in cows, buffaloes, and goats in Nepal.11 In Himachal Pradesh, India, anti-Leishmania antibodies were detected in two of 31 dogs using the rK39 immunochromatographic test (ICT).12 Furthermore, Phlebotomus argentipes, the only known vector for L. donovani in the Indian subcontinent, is zoophilic, which supports the hypothesis of a zoonotic L. donovani transmission cycle.
In Bangladesh, the stray dog population is quite high, although the precise population size is unknown. These dogs typically live in or next to human houses, and thereby can contribute to the domestic transmission of major zoonotic diseases, including leishmaniasis. However, there is a lack of information about the importance of animals as a VL reservoir in Bangladesh. Recently, antibodies against the Leishmania parasite were detected in cattle from an endemic area of Bangladesh, but no parasitic DNA was detected by polymerase chain reaction (PCR).13 In our recent study, Leishmania DNA was detected in one stray dog from VL-endemic areas of Bangladesh.14 For further verification, we investigated additional stray dog samples from the same endemic areas and detected anti-Leishmania antibodies and Leishmania DNA, lending support to the hypothesis that dog is an animal reservoir for Leishmania parasites in the endemic area.
Materials and Methods
Sample collection and preparation.
In May 2012, 50 stray dogs (30 males and 20 females) were captured in Trishal and Fulbaria upazila (subdistricts) of the Mymensingh district in Bangladesh, which are two endemic areas for VL (Figure 1 ). Captured dogs had no obvious clinical signs of leishmaniasis, but most were emaciated, with slight skin lesions. From the saphenous/cephalic vein, 5 mL venous blood was collected in tubes containing disodium ethylenediaminetetraacetate (Na2EDTA). All tubes were immediately placed in a chilled ice box and stored until processing. The blood samples were centrifuged at 875 × g for 10 minutes at 4°C. The serum samples were stored at 4°C, and buffy coat samples were stored in lysis buffer for DNA extraction. Methods for stray dog capture and sample collection were approved by the Mymensingh Municipality Bureau and were described previously.14
Figure 1.
Map of Bangladesh. Dog samples were collected in the Mymensingh District.
rK39 dipstick test.
Of serum sample, 20 μL was used for the rK39 ICT (Kalazar Detect™ Rapid Test; In Bios International, Inc., Seattle, WA) according to the manufacturer's instructions. This test qualitatively detects anti-Leishmania circulating antibodies against a 39-amino-acid repeat that is conserved among viscerotropic Leishmania species (L. donovani, L. infantum, and Leishmania chagasi).12,15 The presence of a red line in the test area indicated a positive result according to the manufacturer's instructions. Sera of uninfected dogs (N = 3) from a nonendemic region were tested as negative controls for the dipstick test.
DNA extraction.
DNA was extracted from 20 μL of blood buffy coat using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA) in accordance with the manufacturer's instructions. Extracted DNA samples were kept at −20°C until further analysis.
ITS1-PCR assay.
An internal transcribed spacer 1-PCR (ITS1-PCR) assay was performed to amplify the ribosomal ITS1 region using the primers LITSR (5′-CTGGATCATTTTCCGATG-3′) and L5.8S (5′-TGATACCACTTATCGCACTT-3′) as previously described.16 The amplification conditions were as follows: initial heating at 95°C for 2 minutes, followed by 35 cycles of denaturation at 95°C for 20 seconds, annealing at 53°C for 30 seconds, and extension at 72°C for 1 minute, with a final extension step at 72°C for 6 minutes. PCR products were resolved by 2% agarose gel electrophoresis in 1× Tris-Borate-EDTA buffer and visualized using ultraviolet light after staining with RedSafe Nucleic Acid Staining Solution (iNtRON Biotechnology Inc., Sungnum, Korea). A positive control with L. donovani (strain MHOM/BD/2006/BD25) genomic DNA at 10 ng/μL and negative controls with DNA extracted from uninfected dogs (N = 3) from a nonendemic region and no-DNA (water) were included.
Real-time PCR.
A quantitative real-time PCR assay based on the amplification of kinetoplast minicircle DNA (kDNA) was performed using the LightCycler® Nano system (Roche Diagnostics, Tokyo, Japan) with the primers RV1 (5′-CTTTTCTGGTCCTCCGGGTAGG-3′) and RV2 (5′-CCACCCGGCCCTATTTTACACCAA-3′).17 The 20 μL reaction mixture contained 1× FastStart Essential DNA Green Master (Roche, Mannheim, Germany), 0.25 μM of each primer, and 2 μL of buffy coat DNA. The reaction conditions were as follows: initial denaturation at 95°C for 2 minutes, followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds. The standard curve was established using L. donovani DNA extracted from 1.4 × 108 parasites from culture. Aliquots from serial dilutions (1 μL), ranging from 0.005 to 500 pg of parasite DNA, were added to the reaction tubes. The assay included negative controls with DNA of uninfected dogs from a nonendemic region (N = 3) and water.
Sequencing.
The PCR products from the agarose gel were excised with a sterile gel cutter and purified using the NucleoSpin Extract II Kit (Clontech Laboratories Inc., MACHEREY-NAGEL, Düren, Germany). Sequencing reactions were performed with the BigDye v3.1 Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Direct cycle sequencing was performed using the ABI 310 Genetic Analyzer (Applied Biosystems). After generating a multiple alignment with a program BioEdit,18 the consensus sequences were compared with those in the National Center for Biotechnology Information database using BLASTn. The obtained DDBJ/EMBL/GenBank accession no. was LC123922.
Statistical analyses.
A Fisher's exact test was used to determine statistical differences between the numbers of male and female dogs that were positive for Leishmania infection based on each of the three different diagnostic methods, that is, rK39 ICT, ITS1-PCR, and real-time PCR (Table 1). Analyses were conducted using an online Fisher's exact test calculator (http://www.socscistatistics.com/tests/fisher/Default2.aspx). The level of agreement between the diagnostic techniques was evaluated using kappa statistics with 95% confidence intervals (CIs; http://graphpad.com/quickcalcs/kappa1.cfm). Kappa values (κ) of 0.20–0.60 indicate fair to moderate agreement and values of 0.60–0.80 indicate substantial agreement between observations.19
Table 1.
Infection rates for male and female dogs based on the three diagnostic methods
| Diagnostic methods | Positive dog samples (%) | P value | |
|---|---|---|---|
| Male | Female | ||
| rK39 ICT | 5 (16.7) | 1 (5) | 0.38 |
| ITS1-PCR | 4 (13.3) | 1 (5) | 0.63 |
| Real-time PCR | 8 (26.7) | 2 (10) | 0.28 |
ICT = immunochromatographic test; ITS1 = internal transcribed spacer 1; PCR = polymerase chain reaction.
Results
rK39 dipstick test.
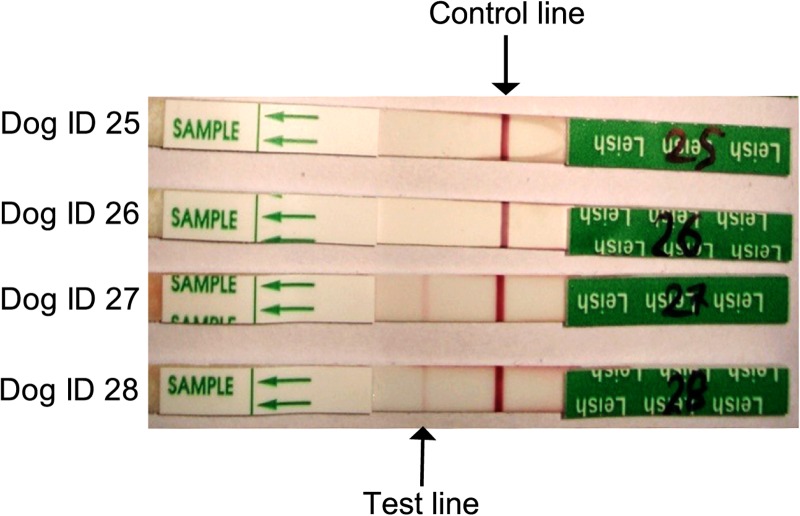
We detected anti-Leishmania antibodies in six of 50 (12%) dog serum samples (Figure 2 ). We observed moderately strong bands in the test line region for four samples (e.g., dog IDs 27 and 28 in Figure 2), but faint bands in the rK39 dipstick test for two samples (data not shown). The dipstick test showed negative results for the control dogs sera (N = 3) from a nonendemic region.
Figure 2.
rK39 immunochromatographic strip test results. Strips with only the control band (dog IDs 25 and 26) represent negative results, whereas strips with both a control band and a positive test band (dog IDs 27 and 28) reflect positive results.
ITS1-PCR and sequencing results.
Five (10%) of 50 dog samples were positive for Leishmania DNA by ITS1-PCR. Sequencing analysis of the amplified products revealed 100% similarity to L. donovani DNA sequences previously deposited in GenBank (accession nos. KT273408, KR858307).
Real-time PCR.
We obtained positive real-time PCR results for Leishmania kDNA amplification for 10 of 50 (20%) dog samples. The concentrations of parasite DNA were 0.005–4.344 pg, equivalent to 0.02–21.72 parasites.
Comparison of three diagnostic methods.
Table 2 provides a summary of rK39 ICT, ITS1-PCR, and real-time PCR results. Three dogs showed positive results by all the three diagnostic methods used in this study. Six dogs were serologically positive by rK39 ICT, in which Leishmania DNA could be detected by ITS1-PCR and/or real-time PCR in five dog samples. Of 10 samples that were positive by real-time PCR, only five were positive based on ITS1-PCR. A moderate agreement was obtained between rK39 ICT and ITS1-PCR results (κ = 0.50, 95% CI = 0.10–0.87) and between rK39 ICT and real-time PCR results (κ = 0.56, 95% CI = 0.25–0.87). A substantial agreement was found between ITS1-PCR and real-time PCR results (κ = 0.62, 95% CI = 0.32–0.91). We did not observe significant differences in infection rates between male and female dogs for any of the three diagnostic tests (Table 1).
Table 2.
Summary of the results of three different diagnostic tests
| rK39 ICT | ITS1-PCR | Real-time PCR | No. of dogs |
|---|---|---|---|
| + | + | + | 3 |
| + | + | – | 0 |
| + | – | + | 2 |
| + | – | – | 1 |
| – | + | + | 2 |
| – | – | + | 3 |
| – | – | – | 39 |
ICT = immunochromatographic test; ITS1 = internal transcribed spacer 1; PCR = polymerase chain reaction; + = positive; – = negative.
Discussion
We investigated the presence of Leishmania infection in stray dogs and found evidence that dogs play a role in the maintenance of Leishmania parasites in the VL-endemic areas of Bangladesh. Knowledge of reservoir hosts and their potential role in disease transmission is a prerequisite for understanding VL epidemiology and designing appropriate control strategies. Although VL in the Indian subcontinent is still thought to be anthroponotic, there is a good circumstantial evidence for a residual zoonotic reservoir. Disease emergence from stray dogs and other canids is of great concern, but the status of canine VL in Bangladesh is unclear.
In VL zoonotic foci, where dogs are the primary reservoir hosts, the disease is caused by L. infantum.20 However, there are also reports of canine infection with L. donovani,7,21 the causative agent of human VL in the Indian subcontinent and East Africa. It has been reported that the domestic dog may be an important reservoir host of L. donovani in eastern Sudan.22 Some recent studies also reported reservoir hosts for Leishmania parasites other than dogs, such as red foxes in central Greece,23 cats in the western provinces of Turkey,24 and Brazilian bats.25 In India, L. donovani DNA was recently detected in goats.26
Our observations of anti-Leishmania antibodies and Leishmania DNA in blood samples obtained from stray dogs corroborate the findings of previous studies in Sri Lanka,10 Sudan,2 and India.12 In Bangladesh, cattle that are seropositive for leishmaniasis have been found, but there is no evidence of Leishmania DNA,13 suggesting that cattle do not play a role as reservoir hosts. In a recent study, Leishmania DNA was detected in a single (1.2%) dog among 85 stray dogs using DNA extracted from whole blood spotted on filter paper.14 We found that 20% and 10% of stray dogs were positive based on real-time PCR and PCR using buffy coat DNA, respectively, despite sampling from the same VL-endemic foci. The higher positive rate in our study probably reflects a higher assay sensitivity using buffy coat DNA than whole-blood preparations, as demonstrated in previous studies.27,28
We detected some discrepancies among the results of the three diagnostic methods used in this study. We obtained the highest positive rate (20%) using kDNA-based real-time PCR, consistent with several previous studies showing that kDNA-based PCR is more sensitive than serological and ITS1-based PCR.29,30 kDNA is considered the most sensitive target for leishmaniasis diagnosis, because it contains ∼10,000 minicircles per parasite.31 Samples that were positive based on PCR and/or real-time PCR, but negative based on rK39 ICT, might have a low infection burden and, therefore, lower levels of anti-Leishmania antibodies, consistent with previous studies32,33 in which some seronegative dogs were PCR positive. In our study, we observed one serologically positive dog that was negative for Leishmania DNA. This might be attributable to a past infection that was controlled via an immune response, as discussed elsewhere.34 However, the possibility of false-positive results of each diagnostic test should also be considered, which might have led to the discrepancies among the diagnosing tests. For example, Mohammadiha and others reported that 3.6% (1/28) and 10.7% (3/28) of dogs from Leishmania nonendemic areas were positive by real-time PCR and ITS-based PCR, respectively.30 The specificity of rK39 ICT with sera of dogs from nonendemic regions ranged from 94% to 100% according to some previous studies and a few false-positive reactions were also reported in dogs infected with Ehrlichia canis, Trypanosoma cruzi, or Neospora caninum.35–38
It is important to isolate viable Leishmania from naturally exposed animals to clarify their role in the maintenance and transmission of VL. After a Leishmania-infected sand fly bites a mammalian host, promastigotes (flagellated forms) are phagocytized by dermal macrophages and transformed into round-shaped amastigotes, which replicate in macrophages, leading to cell destruction and the progressive infection of more phagocytes.39 Once an infection is established, Leishmania tends to localize in all tissues in which monocytic–macrophagic cells exist in high numbers, such as the liver, spleen, lymph nodes, bone marrow, gastrointestinal tract, and skin.40 Several strains of L. donovani, L. infantum, and Leishmania archibaldi were isolated from lymph node cultures of dog samples in eastern Sudan.9 In the United States and Canada, L. infantum zymodeme MON1 was isolated from tissue specimen cultures of dogs.41 As part of a preliminary study, we attempted to detect Leishmania amastigotes in the spleen, liver, and lymph nodes of serologically positive dogs; however, we did not observe the parasites in the hematoxylin/eosin-stained tissue sections (data not shown), probably owing to the low number of parasites in the reservoir host. Further studies with an increased sample size are required to demonstrate the existence of parasites in tissue specimens with more sensitive tools and to isolate viable Leishmania from naturally exposed dogs.
We observed a higher infection rate in male dogs than in females, in agreement with the results of previous studies.42,43 Traditionally, canine leishmaniasis is transmitted directly from sand flies to dogs, but dog to dog transmission of L. infantum via direct contamination with blood and secretions was recently detected in the United States and Canada.41 The possible interaction between dogs and sand flies is an important issue with respect to the transmission of VL to humans. New and Old World sand fly species have varying degrees of host preferences, and hence are opportunistic feeders.44,45 In eastern Sudan, Phlebotomus orientalis and other sand flies are more attracted to dogs than to the mongoose, genet, and Nile rat.22 Although there is a lack of information about the host preference of P. argentipes, the only known vector of L. donovani in Bangladesh, the feeding behavior of P. argentipes is mainly zoophilic46 and animals act as the preferred blood meal source.47 Hence, we recommend that further studies should examine the host preferences of P. argentipes to dogs and other animals in the study area.
In conclusion, we confirmed the presence of anti-rK39 antibodies and Leishmania DNA in several stray dogs in the VL-endemic focus of Bangladesh. Although the number of animals examined was not adequate to incriminate dogs as a reservoir, our findings imply that dogs are probable animal reservoirs for VL transmission in this endemic focus. However, detailed analyses of Leishmania infection in dogs and the ability of dogs to transmit the parasite to the vector sand fly in nature are needed to reveal the potential role of dogs in VL epidemiology in Bangladesh.
ACKNOWLEDGMENTS
We would like to thank Kaori Igarashi for technical help and the staffs from Bangladesh Agricultural University and Mymensingh Municipality for their help during sample collection in the field.
Footnotes
Financial support: This work was supported in part by the Global Center of Excellence Program for International Collaboration Centers for Zoonosis Control, JSPS KAKENHI grant nos. 22405037 and 24380163 from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) and a special grant by the Program for Leading Graduate Schools “Fostering Global Leaders in Veterinary Science for Contributing to One Health” (F01), MEXT, Japan.
Authors' addresses: Shirin Akter, Laboratory of Parasitology, Department of Disease Control, Graduate School of Veterinary Medicine, Hokkaido University, Hokkaido, Japan, and Department of Parasitology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, Bangladesh, E-mail: shirin@vetmed.hokudai.ac.jp. Mohammad Zahangir Alam and Md. Golam Yasin, Department of Parasitology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, Bangladesh, E-mails: mzalam@bau.edu.bd and golam.yasin@yahoo.com. Ryo Nakao, Hirotomo Kato, and Ken Katakura, Laboratory of Parasitology, Department of Disease Control, Graduate School of Veterinary Medicine, Hokkaido University, Hokkaido, Japan, E-mails: ryo.nakao@vetmed.hokudai.ac.jp, hkato@vetmed.hokudai.ac.jp, and kenkata@vetmed.hokudai.ac.jp.
References
- 1.Joshi A, Narain JP, Prasittisuk C, Bhatia R, Hashim G, Jorge A, Banjara M, Kroeger A. Can visceral leishmaniasis be eliminated from Asia? J Vector Borne Dis. 2008;45:105–111. [PubMed] [Google Scholar]
- 2.Sundar S, Mondal D, Rijal S, Bhattacharya S, Ghalib H, Kroeger A, Boelaert M, Desjeux P, Richter-Airijoki H, Harms G. Implementation research to support the initiative on the elimination of kala azar from Bangladesh, India and Nepal—the challenges for diagnosis and treatment. Trop Med Int Health. 2008;13:2–5. doi: 10.1111/j.1365-3156.2007.01974.x. [DOI] [PubMed] [Google Scholar]
- 3.Bern C, Chowdhury R. The epidemiology of visceral leishmaniasis in Bangladesh: prospects for improved control. Indian J Med Res. 2006;123:275–288. [PubMed] [Google Scholar]
- 4.Salam MA, Khan MG, Bhaskar KR, Afrad MH, Huda MM, Mondal D. Peripheral blood buffy coat smear: a promising tool for diagnosis of visceral leishmaniasis. J Clin Microbiol. 2012;50:837–840. doi: 10.1128/JCM.05067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dantas-Torres F, de Brito ME, Brandão-Filho SP. Seroepidemiological survey on canine leishmaniasis among dogs from an urban area of Brazil. Vet Parasitol. 2006;140:54–60. doi: 10.1016/j.vetpar.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Shamboul KM, El Bagir AM, El Sayed MO, Saeed SAK, Abdalla H, Omran OF. Identification of Leishmania donovani from infected dogs at a dormant focus of VL in Blue Nile State, Sudan. J Genet Eng Biotechnol. 2009;7:27–31. [Google Scholar]
- 8.Kalayou S, Tadelle H, Bsrat A, Abebe N, Haileselassie M, Schallig HD. Serological evidence of Leishmania donovani infection in apparently healthy dogs using direct agglutination test (DAT) and rk39 dipstick tests in Kafta Humera, north-west Ethiopia. Transbound Emerg Dis. 2011;58:255–262. doi: 10.1111/j.1865-1682.2011.01209.x. [DOI] [PubMed] [Google Scholar]
- 9.Dereure J, El-Safi SH, Bucheton B, Boni M, Kheir MM, Davoust B, Pratlong F, Feugier E, Lambert M, Dessein A, Dedet JP. Visceral leishmaniasis in eastern Sudan: parasite identification in humans and dogs; host-parasite relationships. Microbes Infect. 2003;5:1103–1108. doi: 10.1016/j.micinf.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Nawaratna SS, Weilgama DJ, Rajapaksha K. Cutaneous leishmaniasis in Sri Lanka: a study of possible animal reservoirs. Int J Infect Dis. 2009;13:513–517. doi: 10.1016/j.ijid.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 11.Bhattarai NR, Auwera GV, Rijal S, Picado A, Speybroeck N, Khanal B, De Doncker S, Das ML, Ostyn B, Davies C, Coosemans M, Berkvens D, Boelaert M, Dujardin JC. Domestic animals and epidemiology of visceral leishmaniasis, Nepal. Emerg Infect Dis. 2010;16:231–237. doi: 10.3201/eid1602.090623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma NL, Mahajan VK, Negi AK, Verma GK. The rK39 immunochromatic dipstick testing: a study for K39 seroprevalence in dogs and human leishmaniasis patients for possible animal reservoir of cutaneous and visceral leishmaniasis in endemic focus of Satluj river valley of Himachal Pradesh (India) Indian J Dermatol Venereol Leprol. 2009;75:52–55. doi: 10.4103/0378-6323.45221. [DOI] [PubMed] [Google Scholar]
- 13.Alam MS, Ghosh D, Khan MG, Islam MF, Mondal D, Itoh M, Haque R. Survey of domestic cattle for anti-Leishmania antibodies and Leishmania DNA in a visceral leishmaniasis endemic area of Bangladesh. BMC Vet Res. 2011;7:27. doi: 10.1186/1746-6148-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam MZ, Yasin MG, Kato H, Sakurai T, Katakura K. PCR-based detection of Leishmania donovani DNA in a stray dog from a visceral leishmaniasis endemic focus in Bangladesh. J Vet Med Sci. 2013;75:75–78. doi: 10.1292/jvms.12-0134. [DOI] [PubMed] [Google Scholar]
- 15.Burns JM, Jr, Shreffler WG, Benson DR, Ghalib HW, Badaro R, Reed SG. Molecular antibody in African and American visceral leishmaniasis. Proc Natl Acad Sci USA. 1993;90:775–779. doi: 10.1073/pnas.90.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schönian G, Nasereddin A, Dinse N, Schweynoch C, Schallig HD, Presber W, Jaffe CL. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349–358. doi: 10.1016/s0732-8893(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 17.Mary C, Faraut F, Lascombe L, Dumon H. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J Clin Microbiol. 2004;42:5249–5255. doi: 10.1128/JCM.42.11.5249-5255.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall T. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- 19.Altman DG. Practical Statistics for Medical Research. London, United Kingdom: Chapman and Hall; 2001. [Google Scholar]
- 20.Gavgani AS, Mohite H, Edrissian GH, Mohebali M, Davies CR. Domestic dog ownership in Iran is a risk factor for human infection with Leishmania infantum. Am J Trop Med Hyg. 2002;67:511–515. doi: 10.4269/ajtmh.2002.67.511. [DOI] [PubMed] [Google Scholar]
- 21.Dereure J, Boni M, Pratlong F, Osman M, Boucheton B, El-Safi S, Feugier E, Musa MK, Daroust B, Dessein A, Dededt JP. Visceral leishmaniasis in Sudan: first identification of Leishmania from dogs. Trans R Soc Trop Med Hyg. 2000;94:154–155. doi: 10.1016/s0035-9203(00)90253-0. [DOI] [PubMed] [Google Scholar]
- 22.Hassan MM, Osman OF, El-Raba'a FM, Schallig HD, Elnaiem DE. Role of the domestic dog as a reservoir host of Leishmania donovani in eastern Sudan. Parasit Vectors. 2009;2:26. doi: 10.1186/1756-3305-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karayiannis S, Ntais P, Messaritakis I, Tsirigotakis N, Dokianakis E, Antoniou M. Detection of Leishmania infantum in red foxes (Vulpes vulpes) in Central Greece. Parasitology. 2015;142:1574–1578. doi: 10.1017/S0031182015001158. [DOI] [PubMed] [Google Scholar]
- 24.Pasa S, Vardarl AT, Erol N, Karakus M, Toz S, Atasoy A, Balc𝚤oğlu IC, Tuna GE, Ermis OV, Ertabaklar H, Ozbel Y. Detection of Leishmania major and Leishmania tropica in domestic cats in the Ege Region of Turkey. Vet Parasitol. 2015;212:389–392. doi: 10.1016/j.vetpar.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Oliveira FM, Costa LH, Barros TL, Ito PKRK, Colombo FA, Carvalhoa C, Pedroa WA, Queiroza LH, Nunesa CM. First detection of Leishmania spp. DNA in Brazilian bats captured strictly in urban areas. Acta Trop. 2015;150:176–181. doi: 10.1016/j.actatropica.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Singh N, Mishra J, Singh R, Singh S. Animal reservoirs of visceral leishmaniasis in Bihar. Indian J Parasitol. 2013;99:64–67. doi: 10.1645/GE-3085.1. [DOI] [PubMed] [Google Scholar]
- 27.Lachaud L, Chabbert E, Dubessay P, Reynes J, Lamothe J, Bastein P. Comparison of various sample preparation methods of PCR diagnosis of visceral leishmaniasis using peripheral blood. J Clin Microbiol. 2001;38:613–617. doi: 10.1128/JCM.39.2.613-617.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9:951–958. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fallah E, Khanmohammadi M, Rahbari S, Farshchian M, Farajnia S, Hamzavi F, Mohammadpour A. Serological survey and comparison of two polymerase chain reaction (PCR) assays with enzyme-linked immunosorbent assay (ELISA) for the diagnosis of canine visceral leishmaniasis in dogs. Afr J Biotechnol. 2011;10:648–656. [Google Scholar]
- 30.Mohammadiha A, Mohebali M, Haghighi A, Mandian R, Abadi AR, Zarei Z, Yeganeh F, Kazemi B, Taghipour N, Akhoundi B. Comparison of real-time PCR and conventional PCR with two DNA targets for detection of Leishmania (Leishmania) infantum infection in human and dog blood samples. Exp Parasitol. 2013;133:89–94. doi: 10.1016/j.exppara.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Bensoussan E, Nasereddin A, Jonas F, Schnur LF, Jaffe CL. Comparison of PCR assays for diagnosis of cutaneous leishmaniasis. J Clin Microbiol. 2006;44:1435–1439. doi: 10.1128/JCM.44.4.1435-1439.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamarsheh O, Nasereddin A, Damaj S, Sawalha S, Al-Jawabreh H, Azmi K, Amro A, Ereqat S, Abdeen Z, Al-Jawabreh A. Serological and molecular survey of Leishmania parasites in apparently healthy dogs in the West Bank, Palestine. Parasit Vectors. 2012;5:183. doi: 10.1186/1756-3305-5-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JY, Ha Y, Gao CH, Wang Y, Yang YT, Chen HT. The prevalence of canine Leishmania infantum infection in western China detected by PCR and serological tests. Parasit Vectors. 2011;4:69. doi: 10.1186/1756-3305-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massunari GK, Voltarelli EM, dos Santos DR, dos Santos AR, Poiani LP, de Oliveira O, Violato RJ, Matsuo R, Teodoro U, Lonardoni MV, Silveira TG. A serological and molecular investigation of American cutaneous leishmaniasis in dogs, three years after an outbreak in the northwest of Paran'a State, Brazil. Cad Saude Publica. 2009;25:97–104. doi: 10.1590/s0102-311x2009000100010. [DOI] [PubMed] [Google Scholar]
- 35.Lemos EM, Laurenti MD, Moreira MAB, Reis AB, Giunchetti RC. Canine visceral leishmaniasis: performance of a rapid diagnostic test (Kalazar Detect™) in dogs with and without signs of the disease. Acta Trop. 2008;107:205–207. doi: 10.1016/j.actatropica.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 36.Lima VMF, Fattori KR, Michelin AF, Neto LS, Vasconcelos RO. Comparison between ELISA using total antigen and immunochromatography with antigen rK39 in the diagnosis of canine visceral leishmaniasis. Vet Parasitol. 2010;173:330–333. doi: 10.1016/j.vetpar.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Quinnell RJ, Carson C, Reithinger R, Garcez LM, Courtenay O. Evaluation of rK39 rapid diagnostic tests for canine visceral leishmaniasis: longitudinal study and meta-analysis. PLoS Negl Trop Dis. 2013;7:e1992. doi: 10.1371/journal.pntd.0001992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mettler M, Grimm F, Capelli G, Camp H, Deplazes P. Evaluation of enzyme-linked immunosorbent assays, an immunofluorescent-antibody test, and two rapid tests (immunochromatographic-dipstick and gel tests) for serological diagnosis of symptomatic and asymptomatic Leishmania infections in dogs. J Clin Microbiol. 2005;43:5515–5519. doi: 10.1128/JCM.43.11.5515-5519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chance ML, Evans DA. The leishmaniases—the agent. In: Gilles HM, editor. Protozoal Diseases. London, United Kingdom: Arnold; 1999. pp. 419–425. [Google Scholar]
- 40.Paltrinieri S, Solano-Gallego L, Fondati A, Lubas G, Gradoni L, Castagnaro M, Crotti A, Maroli M, Oliva G, Roura X, Zatelli A, Zini E. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J Am Vet Med Assoc. 2010;236:1184–1191. doi: 10.2460/javma.236.11.1184. [DOI] [PubMed] [Google Scholar]
- 41.Duprey ZH, Steurer FJ, Rooney JA, Kirchhoff LV, Jackson JE, Rowton ED, Schantz PM. Canine visceral leishmaniasis, United States and Canada, 2000–2003. Emerg Infect Dis. 2006;12:440–446. doi: 10.3201/eid1203.050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haddadzade HR, Fattahi R, Mohebali M, Akhoundi B, Ebrahimzade E. Seroepidemiological investigation of visceral leishmaniasis in stray and owned dogs in Albroz Province, central Iran using direct agglutination test. Iran J Parasitol. 2013;8:152–157. [PMC free article] [PubMed] [Google Scholar]
- 43.Mohebali M, Hajjaran H, Hamzavi Y, Mobedi I, Arshi S, Zarei Z, Akhoundi B, Naeini KM, Avizeh R, Fakhar M. Epidemiological aspects of canine visceral leishmaniosis in the Islamic Republic of Iran. Vet Parasitol. 2005;129:243–251. doi: 10.1016/j.vetpar.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Quinnell RJ, Dye C, Shaw JJ. Host preferences of the phlebotomine sandfly Lutzomyia longipalpis (Diptera: Psychodidae) in Amazonian Brazil. Med Vet Entomol. 1992;6:195–200. doi: 10.1111/j.1365-2915.1992.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 45.Lane RP, Pile MM, Amerasinghe FP. Anthropophagy and aggregation behaviour of the sandfly Phlebotomus argentipes in Sri Lanka. Med Vet Entomol. 1990;4:79–88. doi: 10.1111/j.1365-2915.1990.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 46.Palit A, Bhattacharya SK, Kundu SN. Host preference of Phlebotomus argentipes and Phlebotomus papatasi in different biotopes of West Bengal, India. Int J Environ Health Res. 2005;15:449–454. doi: 10.1080/09603120500392525. [DOI] [PubMed] [Google Scholar]
- 47.Mukhopadhyay AK, Chakravarty AK. Bloodmeal preference of Phlebotomus argentipes and Ph. papatasi of north Bihar, India. Indian J Med Res. 1987;86:475–480. [PubMed] [Google Scholar]