Abstract
Placental malaria causes low birth weight and neonatal mortality in malaria-endemic areas. The diagnosis of placental malaria is important for program evaluation and clinical care, but is compromised by the suboptimal performance of current diagnostics. Using placental and peripheral blood specimens collected from delivering women in Malawi, we compared estimation of the operating characteristics of microscopy, rapid diagnostic test (RDT), polymerase chain reaction, and histopathology using both a traditional contingency table and a latent class analysis (LCA) approach. The prevalence of placental malaria by histopathology was 13.8%; concordance between tests was generally poor. Relative to histopathology, RDT sensitivity was 79.5% in peripheral and 66.2% in placental blood; using LCA, RDT sensitivities increased to 93.7% and 80.2%, respectively. Our results, if replicated in other cohorts, indicate that RDT testing of peripheral or placental blood may be suitable approaches to detect placental malaria for surveillance programs, including areas where intermittent preventive therapy in pregnancy is not used.
Antenatal Plasmodium falciparum infection can cause placental malaria, which causes low birth weight (LBW), stillbirths, premature births, and postnatal morbidity and mortality.1,2 Therefore, antenatal malaria prevention is an important component of malaria control programs in sub-Saharan Africa, and most countries recommend intermittent preventive therapy with antifolate antimalarials during pregnancy (IPTp) to prevent placental malaria and LBW. However, routine monitoring of the effectiveness of IPTp against LBW is challenging, owing to the multifactorial determinants of LBW and the barriers to routine detection of placental malaria.
The optimal approach to diagnose placental malaria remains unclear. Histopathologic analysis by trained personnel of fixed placental tissue is often considered as the reference standard,1 but its reliability can be undermined by multiple factors.3 Other approaches, using placental or peripheral blood, include parasite detection by traditional microscopy, rapid diagnostic tests (RDTs), or polymerase chain reaction (PCR) assays. Compared with histopathology, peripheral blood testing by microscopy4 or by RDT has been insensitive for placental malaria.5 Similarly, in placental specimens, the summary sensitivity of placental blood microscopy was 54% compared with placental histopathology in a large meta-analysis which identified only one study which compared RDT or PCR to histology4; in a subsequent report, results of a commercial RDT targeting the parasite histidine-rich protein 2 (HRP2) on peripheral blood at delivery had a sensitivity of only 81% compared with histopathology.6 Rapidly and reliably testing peripheral or placental blood for parasites would enable efficient surveillance for the efficacy of antenatal prevention strategies.
We used peripheral and placental specimens collected from delivering women in Malawi to compute the operating characteristics of microscopy, RDT, and PCR to detect placental malaria using both the classical contingency table and latent class analysis (LCA). Unlike the contingency table, LCA does not assume a gold standard, but constructs one based on the outcomes of the individual, imperfect tests.7 With this approach, we hypothesized that RDT would be comparable to PCR for the diagnosis of placental P. falciparum infection.
Specimens were collected from consecutive delivering women recruited between November 2009 and January 2011 at three sites near Blantyre, Malawi, where P. falciparum transmission is perennial and intense.8 At delivery, parasites were detected by microscopy, RDT, and PCR in maternal peripheral and placental blood; there was no formal quality assurance for parasite detection after initial training in each method. Thick blood smears were read on-site and the RDT used was First Response Malaria Ag. (Plasmodium lactate dehydrogenase [pLDH]/HRP2) Combo (Premier Medical Corp, Daman, India). PCR was performed on genomic DNA extracted by Chelex-100 (Bio-Rad, Hercules, CA) from dried blood spots in real-time PCR assays targeting the Plasmodium spp. 18s ribosomal RNA.9 Placental histology was analyzed by formalin fixing a 2 × 2 × 1 cm specimen from the maternal side of the placenta and staining with Gurr's modified Giemsa or hematoxylin and eosin; these were examined by a trained technician and scored on a standard 5-point scale for placental malaria.10
We performed statistical analyses using SAS version 9.4 (SAS Institute, Cary, NC). We computed pairwise kappa coefficients between each test method using standard approaches.11 We computed the operating characteristics of individual tests using two approaches: 1) a traditional dichotomy format contingency table, in which histopathological evidence of acute or chronic infection was considered the reference standard,12 and 2) LCA, in which the operating characteristics of each method (including histopathology) were computed against a statistically inferred latent class that was estimated based upon individual test results.7 The LCA approach assumes that each test method is an imperfect representation of the specimen's membership in the “latent class” (which in this case is an infected placenta). The LCA-estimated operating characteristics were computed with the Proc LCA package for SAS.13 Both sets of operating characteristics were computed separately for peripheral and placental specimens.
A total of 1,141 women were enrolled in the study, and 713 of these were tested in peripheral blood by RDT; these women were the analytic population. For 21 women (2.9%), histopathology scores were missing; the numbers of other missing data were peripheral microscopy = 4 (0.6%) or PCR = 36 (5%), and placental microscopy = 4 (0.6%), RDT = 1 (0.1%), and PCR = 13 (1.8%). Overall, by histopathologic examination, 77 (11.13%) had evidence of active (acute or chronic) placental malaria (Table 1). In maternal peripheral blood, the prevalence of parasites was 3.5% by microscopy, 18.7% by RDT, and 5.3% by PCR. In placental blood specimens, only 0.9% were positive by microscopy, 12.6% by RDT, and 10.6% by PCR.
Table 1.
Operating characteristics of diagnostics for placental malaria
| Diagnostic test | Plasmodium falciparum prevalence, % (n/N) | Contingency analysis* | Latent class analysis | ||
|---|---|---|---|---|---|
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | Sensitivity, % (95% CI) | Specificity, % (95% CI) | ||
| Peripheral | |||||
| Microscopy | 3.53 (25/709) | 20.51 (11.55–29.47) | 98.85 (98.01–99.70) | 26.03 (16.19–35.87) | 99.68 (99.17–100) |
| RDT | 18.65 (133/713) | 79.49 (70.53–88.45) | 89.25 (86.80–91.70) | 92.74 (84.41–100) | 91.83 (89.24–94.42) |
| PCR | 5.32 (36/677) | 34.21 (23.54–44.88) | 98.28 (97.23–99.34) | 38.56 (27.02–50.10) | 99.51 (98.86–100) |
| Histopathology† | 11.27 (78/692) | NA | NA | 77.75 (65.13–90.37) | 98.03 (96.64–99.42) |
| Placental | |||||
| Microscopy | 0.85 (6/709) | 7.79 (1.81–13.78) | 100 (99.40–100) | 6.35 (1.35–11.35) | 100 (99.98–100) |
| RDT | 12.64 (90/712) | 66.23 (55.67–76.80) | 93.98 (92.10–95.86) | 79.31 (68.37–90.25) | 97.55 (95.98–99.12) |
| PCR | 10.57 (74/700) | 64.38 (53.40–75.37) | 95.89 (94.32–97.47) | 78.46 (66.97–89.95) | 99.45 (98.33–100) |
| Histopathology† | 11.13 (77/692) | NA | NA | 69.36 (57.70–81.02) | 97.76 (96.37–99.15) |
CI = confidence interval; NA = not applicable; PCR = polymerase chain reaction; RDT = rapid diagnostic test.
Estimated with active placental histopathology as the reference diagnostic standard.
A positive histopathologic analysis defined as showing evidence of acute/chronic infection.
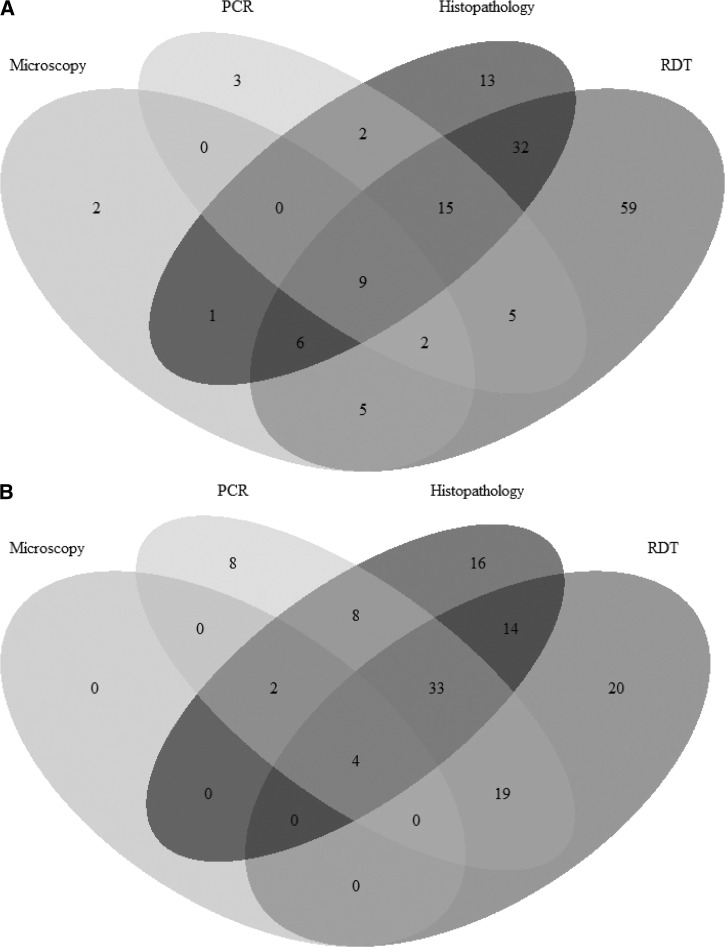
The concordance between positive tests are illustrated in Figure 1 . To quantify this, we computed pairwise kappa coefficients on placental tests to quantify agreement between diagnostics for active placental infection. Kappa values with microscopy were poor for RDT, PCR, and histopathology (each < 0.14), but agreement was substantial between RDT and PCR (0.66) or histopathology (0.57), and between PCR and histopathology (0.63).
Figure 1.
Overlap in test positivity for (A) maternal peripheral and (B) placental blood specimens.
We calculated the operating characteristics of peripheral or placental tests using traditional contingency tables by setting histopathologic evidence of active infection as the reference standard for each analysis (Table 1). In peripheral blood specimens, specificities of each test exceeded 89%, but sensitivities were below 80%. In placental specimens, specificities of microscopy, RDT, and PCR each exceeded 93%, but sensitivities were low, even for RDT (66.2%) and PCR (64.4%), which are typically considered to have a low limit of detection. The sensitivity of microscopy, compared with histopathology, was very poor (7.3%), underscoring the unreliability of this approach, even in research settings. Furthermore, based upon our contingency tables, neither RDT nor PCR performed on peripheral or placental blood appear tohave adequate sensitivity to diagnose histopathologically proven placental malaria.
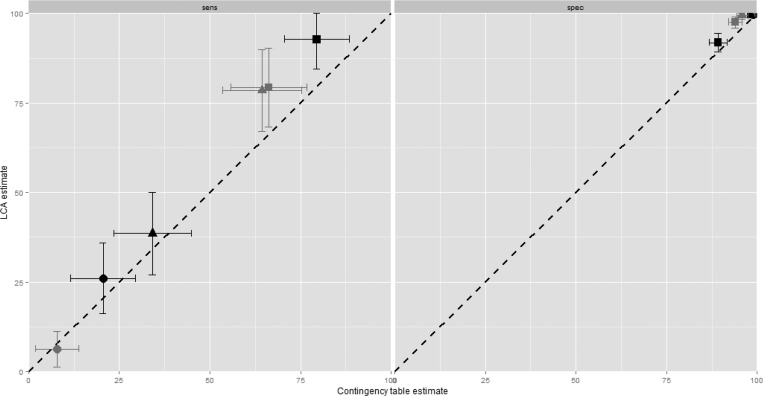
We next assumed that each test (including histopathology) was imperfect, and performed LCA to construct an “alloyed” reference standard against which to reestimate operating characteristics (Table 1). Notably, despite its consideration as reference standard, the LCA-computed sensitivities of histopathology were low in models of both peripheral (77.8%) and placental (69.4%) specimens. Using LCA, sensitivities remained poor for microscopy of either peripheral (26%) or placental blood (6.4%), and PCR sensitivity was poor in peripheral blood (38.6%) and moderate in placental blood (78.5%). However, RDT sensitivity was increased by LCA estimation in both peripheral (79.5–92.7%) and placental (66.2–79.3%) specimens, with increased estimates of specificity (compared with contingency estimation) in both peripheral (89.3–91.8%) and placental (94–97.6%) specimens. Overall, compared with traditional contingency analysis, LCA estimated higher sensitivities and specificities of each of the component tests (Figure 2 ).
Figure 2.
Pairwise comparisons of the sensitivities (left) and specificities (right) of each test method estimated by traditional contingency table (x axes) or latent class analysis (LCA; y axes). Black = peripheral maternal blood; gray = placental blood; circle = microscopy, square = rapid diagnostic test (RDT), triangle = polymerase chain reaction (PCR). Lines are 95% confidence intervals of each point estimate. Contingency table estimates were computed using active placental histopathology as the reference standard.
Our LCA approach to placental malaria diagnostics suggests that active, histologically evident placental malaria can be routinely diagnosed by RDT or PCR testing of placental blood specimens or RDT testing of peripheral blood. In our analysis, the LCA-estimated sensitivities for each exceeded that for histopathology, and each is less operator dependent than histopathology. If our findings are replicated in other studies, the minimal resources and training needed for RDT use and rapid turnaround (compared with PCR), would make RDT of maternal peripheral or placental blood an efficient and reliable diagnostic test for active placental malaria.
In a prior study in Mozambique,5 the sensitivity of an HRP2-based RDT used on peripheral blood to detect histologically confirmed active placental malaria was 76%, very similar to our estimate of 79.5%, suggesting that RDT testing of peripheral blood will underestimate placental malaria infections. However, when we estimated the sensitivity of this approach using LCA, which does not assume that histopathology is a perfect test, the sensitivity increased to 93.7%, suggesting that peripheral blood RDT testing could be an adequate approach to placental diagnosis. Indeed, the sensitivity of peripheral blood RDT exceeded any other approach estimated by LCA, including histopathology. This may reflect, in part, the persistence of HRP2 antigenemia after effective treatment, which results in HRP2 detection being a proxy for cumulative parasite exposure in the prior several weeks; indeed, we noted that, among RDT-positive samples, HRP2 was the only positive antigen in 58.6% (78/133) of maternal peripheral and 30% (27/90) of placental specimens.
Our results also underscore the suboptimal performance of current individual approaches to diagnose placental malaria. Although often considered the gold standard strategy, the sensitivity of histopathologic analysis was only 76% when estimated by LCA. Similarly, in a prior study in Malawi which used Bayesian LCA models to assess the operating characteristics of microscopy, PCR, and histopathology, the LCA-estimated sensitivity of histopathology was only 50%.14 Because of this, future studies of placental malaria prevention should incorporate multiple approaches to diagnosis and test alternate approaches, including enhanced immunohistochemistry,15,16 large-volume specimen preparation for PCR,17 or novel nucleic acid detection assays.18
Our study suggests that peripheral or placental blood RDT testing at delivery can be useful tools for the routine diagnosis of placental malaria for surveillance. Our results also illustrate the potential value of LCA estimation of the operating characteristics of diagnostic tests when, as in placental malaria, individual diagnostic approaches are each imperfect. RDTs are increasingly available throughout malaria-endemic Africa19; if our findings are replicated in other settings, this could enable the surveillance of placental malaria by the routine use of RDTs on delivering women, and thereby provide a tool to evaluate measures to prevent the morbidity of malaria in pregnancy.
ACKNOWLEDGMENTS
We thank three colleagues in the Malaria in Pregnancy Consortium for their comments on the manuscript. We are indebted to the women who participated in the clinical study.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This work was supported by the Malaria in Pregnancy Consortium, which is funded through a grant from the Bill & Melinda Gates Foundation to the Liverpool School of Tropical Medicine (to Feiko O. ter Kuile), and by the National Institute of Allergy and Infectious Diseases under award no. K08AI100924 (to Steve M. Taylor).
Authors' addresses: Yunhao Liu, Kyaw L. Thwai, and Steven R. Meshnick, Department of Epidemiology, University of North CarolinaGillings School of Public Health, Chapel Hill, NC, E-mails: yl6b@live.unc.edu, klthwai@gmail.com, and meshnick@unc.edu. Victor Mwapasa, Malawi-Liverpool-Wellcome Trust Clinical Research Programme, Department of Clinical Sciences, Blantyre, Malawi, and Department of Community Health, College of Medicine, Blantyre, Malawi, E-mail: vmwapasa69@gmail.com. Carole Khairallah and Feiko O. ter Kuile, Department of Clinical Sciences, Liverpool School of Tropical Medicine, Liverpool, United Kingdom, E-mails: carole.khairallah@liverpool.ac.uk and feiko.terkuile@lstmed.ac.uk. Linda Kalilani-Phiri, Department of Community Health, College of Medicine, Blantyre, Malawi, E-mail: lkalilani@medcol.mw. Steve M. Taylor, Department of Medicine, Duke University School of Medicine, Duke University Medical Center (DUMC), Durham, NC, E-mail: steve.taylor@duke.edu.
References
- 1.Fried M, Muehlenbachs A, Duffy PE. Diagnosing malaria in pregnancy: an update. Expert Rev Anti Infect Ther. 2012;10:1177–1187. doi: 10.1586/eri.12.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mouatcho JC, Goldring JP. Malaria rapid diagnostic tests: challenges and prospects. J Med Microbiol. 2013;62:1491–1505. doi: 10.1099/jmm.0.052506-0. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Griffin JB, Muehlenbachs A, Rogerson SJ, Bailis AJ, Sharma R, Sullivan DJ, Tshefu AK, Landis SH, Kabongo JM, Taylor SM, Meshnick SR. Diagnosis of placental malaria in poorly fixed and processed placental tissue. Malar J. 2016;15:272. doi: 10.1186/s12936-016-1314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kattenberg JH, Ochodo EA, Boer KR, Schallig HD, Mens PF, Leeflang MM. Systematic review and meta-analysis: rapid diagnostic tests versus placental histology, microscopy and PCR for malaria in pregnant women. Malar J. 2011;10:321. doi: 10.1186/1475-2875-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayor A, Moro L, Aguilar R, Bardají A, Cisteró P, Serra-Casas E, Sigaúque B, Alonso PL, Ordi J, Menéndez C. How hidden can malaria be in pregnant women? Diagnosis by microscopy, placental histology, polymerase chain reaction and detection of histidine-rich protein 2 in plasma. Clin Infect Dis. 2012;54:1561–1568. doi: 10.1093/cid/cis236. [DOI] [PubMed] [Google Scholar]
- 6.Kyabayinze DJ, Tibenderana JK, Nassali M, Tumwine LK, Riches C, Montague M, Counihan H, Hamade P, Van Geertruyden JP, Meek S. Placental Plasmodium falciparum malaria infection: operational accuracy of HRP2 rapid diagnostic tests in a malaria endemic setting. Malar J. 2011;10:306. doi: 10.1186/1475-2875-10-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hui SL, Walter SD. Estimating the error rates of diagnostic tests. Biometrics. 1980;36:167–171. [PubMed] [Google Scholar]
- 8.Patel JC, Mwapasa V, Kalilani L, Ter Kuile FO, Khairallah C, Thwai KL, Meshnick SR, Taylor SM. Absence of association between sickle trait hemoglobin and placental malaria outcomes. Am J Trop Med Hyg. 2016;94:1002–1007. doi: 10.4269/ajtmh.15-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor SM, Messina JP, Hand CC, Juliano JJ, Muwonga J, Tshefu AK, Atua B, Emch M, Meshnick SR. Molecular malaria epidemiology: mapping and burden estimates for the Democratic Republic of the Congo, 2007. PLoS One. 2011;6:e16420. doi: 10.1371/journal.pone.0016420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–119. [PubMed] [Google Scholar]
- 11.Fleiss JL, Cohen J, Everitt BS. Large sample standard errors of kappa and weighted kappa. Psychol Bull. 1969;72:323. [Google Scholar]
- 12.Fletcher RH, Fletcher SW. Clinical Epidemiology: The Essentials. 4th edition. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. [Google Scholar]
- 13.Lanza ST, Collins LM, Lemmon DR, Schafer JL. PROC LCA: a SAS procedure for latent class analysis. Struct Equ Modeling. 2007;14:671–694. doi: 10.1080/10705510701575602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Z, Mitchell RM, Gutman J, Wiegand RE, Mwandama DA, Mathanga DP, Skarbinski J, Shi YP. Pooled PCR testing strategy and prevalence estimation of submicroscopic infections using Bayesian latent class models in pregnant women receiving intermittent preventive treatment at Machinga District Hospital, Malawi, 2010. Malar J. 2014;13:509. doi: 10.1186/1475-2875-13-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brahmbhatt H, Sullivan D, Kigozi G, Askin F, Wabwire-Mangenm F, Serwadda D, Sewankambo N, Wawer M, Gray R. Association of HIV and malaria with mother-to-child transmission, birth outcomes, and child mortality. J Acquir Immune Defic Syndr. 2008;47:472–476. doi: 10.1097/QAI.0b013e318162afe0. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Griffin JB, Muehlenbachs A, Rogerson SJ, Bailis AJ, Sharma R, Sullivan DJ, Tshefu AK, Landis SH, Kabongo JM, Taylor SM, Meshnick SR. Diagnosis of placental malaria in poorly fixed and processed placental tissue. Malar J. 2016;15:272. doi: 10.1186/s12936-016-1314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imwong M, Hanchana S, Malleret B, Rénia L, Day NP, Dondorp A, Nosten F, Snounou G, White NJ. High-throughput ultrasensitive molecular techniques for quantifying low-density malaria parasitemias. J Clin Microbiol. 2014;52:3303–3309. doi: 10.1128/JCM.01057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med. 2015;12:e1001788. doi: 10.1371/journal.pmed.1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization World Malaria Report 2014. 2014 http://www.who.int/malaria/publications/world_malaria_report_2014/en/ Available at. Accessed July 26, 2016. [Google Scholar]