Abstract
Urogenital schistosomiasis (UGS) is one of the important neglected tropical diseases, which requires global elimination programs. It is primarily diagnosed by urine microscopy (UM), but its sensitivity is not satisfactory. Ultrasonography (US) is an alternative screening method of UGS. The present study investigated the diagnostic feasibility of new criteria including echogenic snow sign, innumerable scattered small echogenic foci floating in bladder lumen, for UGS in White Nile State, Republic of Sudan, 2013–2014. A total of 1,462 participants were screened by US or UM, and 948 by both. The 948 subjects were 1–80 years of age, 485 (51.2%) of them were boys or men, and 648 (68.4%) were less than 15 years of age. Eggs were detected from 133 (14.0%) by UM. The US findings of bladder wall thickening, polypoid mass, and ureter dilatation were regarded as positive for UGS following the World Health Organization (WHO) guidelines. Of the 948 subjects, 155 (16.4%) were positive for US by the WHO criteria. The echogenic snow sign was detected in 75 participants, and was most frequently observed in age group of 10–14. It was more commonly observed in UM-positive participants (35/133; 26.3%) than in UM-negative participants (40/815; 4.9%), and the difference was statistically significant with an odds ratio of 6.92 (4.20–11.41). When the echogenic snow was added to the WHO criteria, 42 participants were additionally revealed to have UGS-related morbidity, reaching a total of 198 (20.9%) participants. The echogenic snow sign can be suggested as a new finding to the criteria of US for UGS.
Introduction
Urogenital schistosomiasis (UGS) is a parasitic helminthiasis caused by Schistosoma haematobium. Humans are usually infected when the skin comes in contact with contaminated freshwater. In 2012, 163 million individuals of sub-Saharan Africa were estimated to be infected with S. haematobium.1 Detecting S. haematobium eggs by urine microscopy (UM) has long been used as the gold standard diagnosis of UGS in both clinical case management and epidemiological purposes. Since the worms live in the blood vessel, the eggs pass through the urine when the infected vessels rupture through the mucosa. The irregularity of egg passing into the urine is a limitation of diagnosis of UGS by UM.
The ultrasonography (US) is widely accepted as a noninvasive and efficient technique for detecting urinary tract lesions related to UGS.2,3 The US images of the bladder are known to be correlated well with the urinary egg counts.4 Also, the US is used for monitoring of urinary tract lesions after treatment.5 The Niamey group suggested World Health Organization (WHO) guidelines to score UGS images by US. The WHO guidelines suggested US images of the urinary bladder by its shape, irregularity of the inner surface, wall thickening, and masses, or pseudopolyps for UGS diagnosis.6
In the present study, we screened schoolchildren and community people by both US and UM as one of implementation programs of schistosomiasis control in White Nile State, Sudan.7,8 We performed US to observe the morbidity of UGS in addition to UM for screening. The WHO guidelines of the urogenital US were modified, and we also evaluated diagnostic significance of one new US finding, the echogenic snow sign, for UGS morbidity.
Methods
Study area and population.
The study data were acquired from a schistosomiasis control program implemented from February 2013 to July 2014, in seven villages (Abu Sharif, Al Hideb, Al Jabalain, Al Zelit, Azhari, Campo, and Khor Ajwal) located in the White Nile State, Republic of Sudan. All villages were rural communities adjacent to the White Nile River or the irrigation canal from the river. There were no available clean water supply systems in those communities except one, Al Hideb, and their main source of water was the main or branches of the White Nile River.
The Sudanese Ministry of Health implemented integrated control projects for schistosomiasis in the study area, which was sponsored by the Korea International Cooperation Agency (KOICA).7,8 The projects included not only preventive chemotherapy with praziquantel subjecting schoolchildren and also village residents according to the egg positive rates, but also health education for behavioral change and construction of filtered water supply system. The US was implemented not for all target villages but for a part of them. Although US is not a common activity of the control program, it was included in the project to evaluate the disease morbidity. We screened not only schoolchildren but also village residents who voluntarily agreed to undergo both US and UM.
The schools included in this analysis were selected according to the annual schedule of the Ministry of Health (MOH) of White Nile State based on the geographical proximity. The schools were not systematically sampled representatives. However, we think the ecological condition important for the schistosomiasis transmission such as water source, weather condition, and snail distributions were similar between the schools included in our study. We collected as many urine samples as possible; however, US was performed to only those who voluntarily participated, and they could opt out at any time. Thus, there existed a possibility of selection bias, such as volunteer bias, thereby overestimating the disease prevalence by US.
The schoolchildren were the target of the mass drug administration and thus given the praziquantel irrespective of the survey results. The school-attached communities were given mass chemotherapy when the prevalence was more than 30%, and targeted chemotherapy, as treatment of the diagnosed person, when the prevalence was less than 30%. The praziquantel was given after the survey in most of the participants.
Ultrasonography.
Participants were examined using a portable US device (Voluson-e®, General Electric Co., Milwaukee, WI) with a convex transducer of 5 MHz. The urinary bladder and the distal ureters were examined once under a full bladder state. The kidneys were not evaluated. All US examinations were performed by a single physician (Sung-Tae Hong), who was not a registered radiologist but was initially trained for 1 month on urogenital ultrasonography in Korea by a radiologist (Seung Hyup Kim) specializing in urogenital imaging, in 2011. After this, he began to perform US in Sudan and the images acquired were reviewed and discussed with the urogenital radiologist in 2011–2012. After 2 years of continuous training by review and discussion, the data produced in 2013–2014 were analyzed in the present study.
The abnormal US findings were grouped into four categories: bladder wall thickening with surface irregularity or elevation between 5 and 10 mm, polypoid mass protruding into the lumen or elevation more than 10 mm from the wall, distal ureter dilatation, and echogenic snow sign showing innumerable scattered small echogenic foci floating in the bladder lumen. The criteria of the Niamey group guidelines by WHO were adopted to define wall thickening, polypoid mass, and ureter dilatation.6 Although the last echogenic snow sign was not included in the WHO criteria, we watched this finding from many subjects and kept the records of the sign to evaluate its diagnostic significance. The results were described only qualitatively and thus, severity of the images was not scored.
Urine microscopy.
The urine samples were collected at the community after the US and transferred to the laboratory. They were grossly checked for macrohematuria, and were adjusted to the volume of 10 mL and centrifuged at 1,500 rpm for 5 minutes. All pellets were transferred onto the slide glasses and 2–4 slides were made for each sample. They were examined by light microscopy. The eggs of S. haematobium were identified and counted by trained technicians.
Statistical analysis.
The proportions of abnormal findings were compared using χ2 test. Statistical Package for the Social Sciences (SPSS, Armonk, NY) package version 18 was used for the statistical analysis.
Ethics statement.
Initially, the Sudanese Institutional Review Board (IRB) was missed because the US and UM screening was performed as part of the control program which was approved by the MOH of White Nile State, Sudan. Although US is not a common activity of the control program, it was included in this program as a supplementary diagnostic method to UM. With the help of the school teachers and chiefs of the villages, the program purpose and activity were explained to the schoolchildren and residents, and verbal consent was obtained from the parents of the children or school teachers. The program also contained the health education programs and the meaning of the test was also explained repeatedly. Only the community residents who wanted to undergo US were tested, and participants were free to opt out from the test.
The present study retrospectively analyzed the data produced by the control program, focusing on the diagnostic performance of the new echogenic snow sign. The study protocol was reviewed and approved by the IRB of the Korea Association of Health Promotion. Informed consent was waived by the board because it was a retrospective review of existing data produced from the schistosomiasis control program.
Results
Study population.
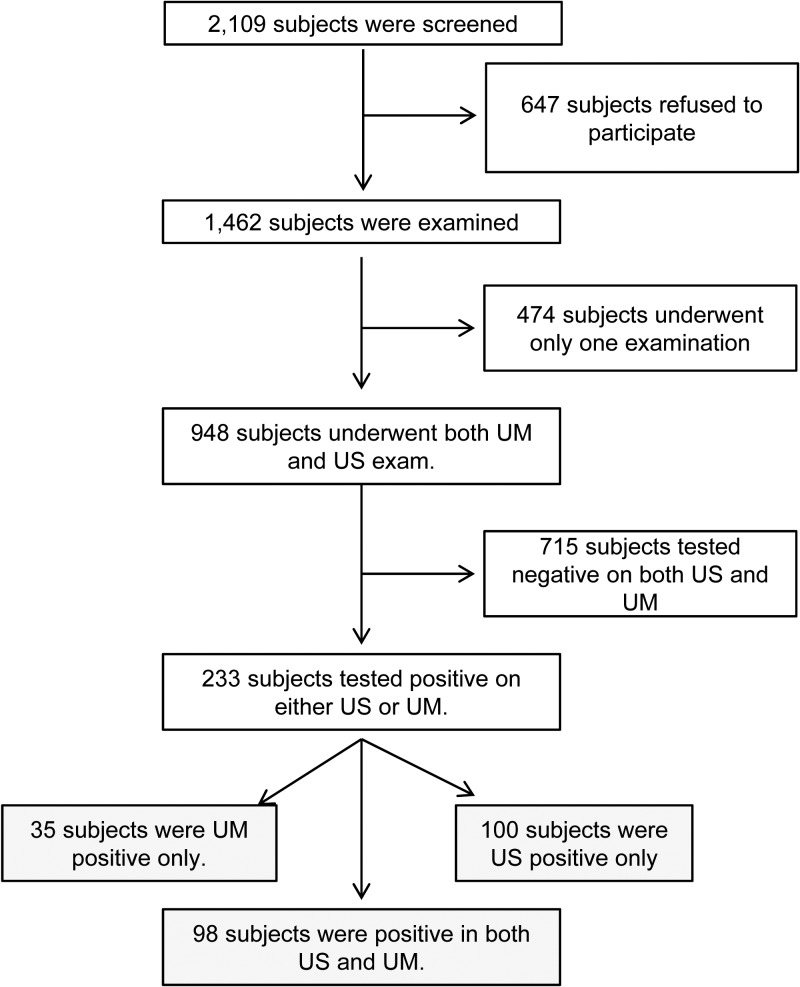
A total of 2,109 participants were screened and 1,462 were examined by either US scanning of their pelvis or UM for egg detection. Of them, 948 participants (men or boys 485, 51.2%) were examined by both US and UM, and only their findings were analyzed in the present study (Figure 1 ). More than half of them (631, 66.5%) were schoolchildren 6 to 15 years of age. When the study participants were grouped by age, men or boys comprised about 18.5–75% of each age group. The subset of UM results, especially those of the school-aged children, was already included in our previous report of UGS control in Sudan.7
Figure 1.
Inclusion and exclusion of subjects for the ultrasonography (US) and urine microscopy (UM) examinations.
Ultrasonography by WHO criteria.
Of the 948 participants who underwent both the US and UM, 155 participants (16.4%) showed abnormal US images by the WHO criteria(Table 1). Forty-six patients showed two or more findings. The most common finding was bladder wall thickening, followed by polypoid mass and ureter dilatation (Table 2, Figure 2 ). Some of the egg negatives by UM were US positive, and some of US negatives were US positives (Table 3). The US findings of wall thickening and polypoid masses were recognized more among subjects with higher egg counts (Table 3). The boys in the age group of 5–14 years observed the highest positive proportion of US abnormality and the proportion of US abnormal findings declined as the age increased (Table 4). Men or boys showed higher rates of positive US findings of UGS than women or girls, 21.9% versus 10.6% (P < 0.001). All the subjects with gross hematuria had positive US findings.
Table 1.
Number of subjects for examinations of the genitourinary schistosomiasis (N = 948)
| Examination method | No. of positive | Positive proportion (%) |
|---|---|---|
| US scanning by WHO criteria | 155 | 16.4 |
| US scanning including echogenic snow | 198 | 20.9 |
| Urine microscopy | 133 | 14.0 |
| Gross bloody urine | 24 | 2.5 |
US = ultrasonography; WHO = World Health Organization.
Table 2.
Number of subjects by positivity of US findings and UM
| US images | No. (%) of positive subjects by UM | |||
|---|---|---|---|---|
| Positive (N = 133) | Negative (N = 815) | Total (N = 948) | OR‡ (confidence interval) | |
| No abnormal findings | 35 (26.3) | 715 (87.7) | 750 (79.1) | N/A |
| Any of related findings by new criteria including echogenic snow | 98 (73.7) | 100 (12.3) | 198 (20.9) | 20.02 (12.91–31.05) |
| Echogenic snow | 35 (26.3)* | 40 (4.9)† | 75 (7.9) | 6.92 (4.20–11.41) |
| Any of related findings by WHO criteria | 86 (64.7) | 69 (8.5) | 155 (16.4) | 19.78 (12.83–30.49) |
| Bladder wall thickening | 81 (60.9) | 60 (7.4) | 141 (14.9) | 19.60 (12.67–30.32) |
| Polypoid mass | 9 (6.8) | 8 (1.0) | 17 (1.8) | 7.32 (2.77–19.33) |
| Ureter dilatation | 7 (5.3) | 4 (0.5) | 11 (1.2) | 11.26 (3.25–39.03) |
UM = urine microscopy; US = ultrasonography; WHO = World Health Organization.
One participant showed ureter dilatation, bladder wall thickening and echogenic snow sign. Another participant showed polypoid mass, bladder wall thickening and echogenic snow sign. Twenty-one participants showed bladder wall thickening and echogenic snow sign together. Twelve of the 35 demonstrated echogenic snow sign only.
One participant showed polypoid mass and echogenic snow sign together, eight participants showed bladder wall thickening and echogenic snow sign. Thirty-one of the 40 demonstrated echogenic snow sign only.
Odds ratio.
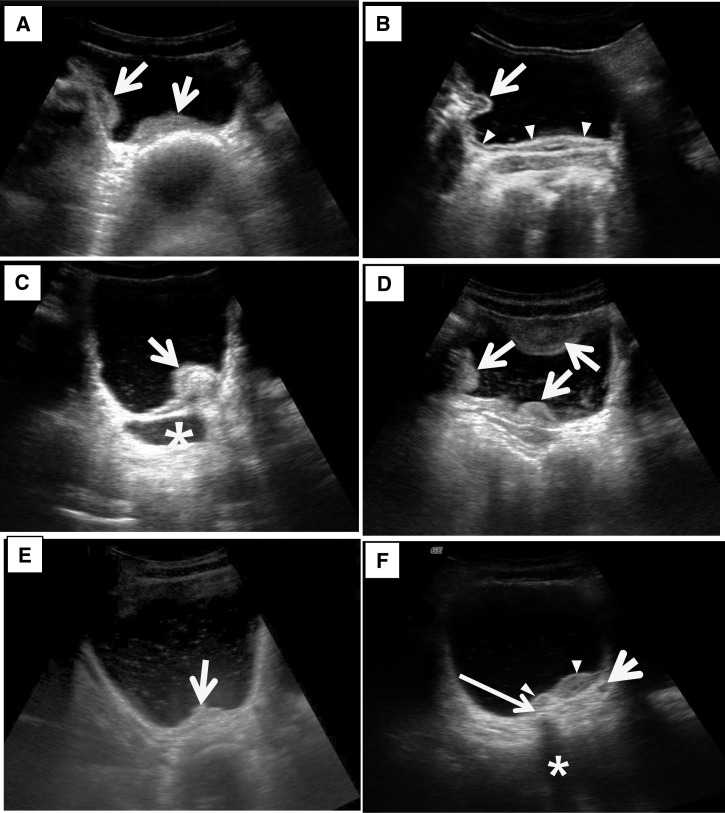
Figure 2.
Ultrasonography images of the urinary bladder with urogenital schistosomiasis. (A) Thickening of bladder wall. US image of the bladder in transverse plane shows marked thickening of the right lateral and posterior wall of the bladder (arrows). (B) Thickening of bladder wall. US image of the bladder in transverse plane shows diffuse thickening of the bladder wall (arrowheads) and a mass-like lesion (arrow) in the right lateral wall. Note that echogenic line of bladder mucosa is well preserved. (C) Bladder mass. US image of the bladder in oblique longitudinal plane shows a mass (arrow) in the posterior wall of the bladder. Note that echogenic line of the bladder mucosa is preserved indicating that the mass is in submucosal location. Also note that distal ureter is markedly dilated (asterisk) and there are fine scattered echoes (echogenic snow) in the dependent portion of the bladder. (D) Multifocal thickening of the bladder wall and echogenic snow in the lumen. US image of the bladder in transverse plane shows multifocal thickening of the bladder wall (arrows) and scattered echoes in the bladder lumen. (E) Focal thickening of the bladder wall and echogenic snow in the lumen. US image of the bladder in transverse plane shows focal thickening of the posterior wall of the bladder (arrow) and scattered echoes in the bladder lumen. (F) Focal thickening and probable calcification of the bladder wall and dilated ureter. US image of the bladder in transverse plane shows focal thickening of the posterior wall of the bladder (arrowheads). Note focal echogenic lesion (thin long arrow) with posterior sonic shadowing (asterisk) suggesting calcification of the bladder wall. Also note slightly dilated left distal ureter (short arrow).
Table 3.
Distribution of US image findings by egg counts per 10 mL (N = 948)
| US images of urinary bladder | No. (%) of subjects by egg counts | ||||
|---|---|---|---|---|---|
| Negative | 1–10 | 11–20 | > 21 | Total | |
| Negative | 715 | 26 | 5 | 4 | 750 |
| Wall thickening | 60 (7.4) | 57 (60.6) | 11 (52.8) | 13 (72.2) | 141 (14.9) |
| Echogenic snow | 40 (4.9) | 22 (23.4) | 8 (38.1) | 5 (27.8) | 75 (7.9) |
| Polypoid mass | 8 (1.0) | 4 (4.3) | 0 (0) | 5 (27.8) | 16 (1.8) |
| Ureter dilatation | 4 (0.5) | 5 (5.3) | 2 (9.5) | 0 (0) | 11 (1.2) |
| Total | 815 | 94 | 21 | 18 | 948 |
US = ultrasonography.
Table 4.
Positive proportions of examinations by age and sex
| Age and sex | Ultrasonography findings* | Urine microscopy findings | ||||
|---|---|---|---|---|---|---|
| No. of examinations | No. of positive | Proportion (%) | No. of examinations | No. of positive | Proportion (%) | |
| 0–9 | ||||||
| Male | 120 | 37 | 30.8 | 120 | 31 | 25.8 |
| Female | 98 | 21 | 21.4 | 98 | 14 | 14.3 |
| Total | 218 | 58 | 26.6 | 218 | 45 | 20.6 |
| 10–14 | ||||||
| Male | 228 | 70 | 30.7 | 228 | 37 | 16.2 |
| Female | 176 | 41 | 23.3 | 176 | 29 | 16.5 |
| Total | 404 | 111 | 27.5 | 404 | 66 | 16.3 |
| 15–19 | ||||||
| Male | 64 | 14 | 21.9 | 64 | 7 | 10.9 |
| Female | 21 | 4 | 19.0 | 21 | 5 | 23.8 |
| Total | 85 | 18 | 21.2 | 85 | 12 | 14.1 |
| 20– | ||||||
| Male | 73 | 10 | 13.7 | 73 | 5 | 6.8 |
| Female | 168 | 1 | 0.6 | 168 | 5 | 3.0 |
| Total | 241 | 11 | 4.6 | 241 | 10 | 4.1 |
| Total | ||||||
| Male | 485 | 131 | 27.0 | 485 | 80 | 16.5 |
| Female | 463 | 67 | 14.5 | 463 | 53 | 11.4 |
| Total | 948 | 198 | 20.9 | 948 | 133 | 14.0 |
The images include echogenic snow.
Echogenic snow sign.
Echogenic snows were observed in 75 subjects. It was the second frequent US abnormal finding after bladder wall thickening. The echogenic snow sign was identified dominantly in the school-aged children (71/75). Of the 75 participants with echogenic snow sign, 46 were boys or men. The echogenic snow sign was the only abnormal finding in 43 participants, whereas others showed multiple abnormal findings. Of these 43 participants, 31 were UM negative and 12 were UM positive. Including this echogenic snow sign, a total of 198 (20.9%) subjects showed US abnormalities (Table 2).
Urine microscopy.
One urine sample was collected from each participant. Of the 948 urine samples examined, 133 (14.0%) were positive for S. haematobium eggs by UM, and 24 samples (2.5%) were grossly bloody (Table 1). Urinary egg counts were in the range of 0–100 (median = 6, interquartile range = 3–15). The positive proportion was the highest in the age group 0–9 years (20.8%) and decreased in the older age groups, similar to the trend of the US findings (Table 4). The positive proportion was 16.5% in men or boys, and 11.4% in women or girls (P = 0.031). One of the 24 participants with gross hematuria was UM negative. Any participant with either positive urine for S. haematobium eggs or abnormal ultrasonography findings were medicated with one dose of 40 mg/kg praziquantel.
Comparison of US and UM findings.
Among UM egg-positive participants, 73.7% (98/133) revealed US abnormalities. In comparison, among UM egg-negative participants, only 12.3% (100/815) showed abnormal US findings. The difference was statistically significant, and the odds ratio (OR) was 20.02 (confidence interval = 12.91–31.05). Each of the US abnormal findings was also more commonly observed in the UM-positive groups. Each OR was in the range of 6.92–19.78.
When the US results were analyzed according to the urinary egg counts, all the four abnormal findings were more prevalent in the population with higher egg excretion (Table 3). Wall thickening and the polypoid mass were most prevalent in the population with the highest urinary egg excretion. Echogenic snow sign and the ureter dilatation were common in population with urinary egg excretion of 11–20.
When the relation between gross hematuria and the echogenic snow sign was analyzed, the echogenic snow sign was more common in persons with hematuria (25.0%; 6/24) than in those without hematuria (7.5%; 69/924). The difference was statistically significant with P = 0.009 and OR of 4.130 (1.588–10.744).
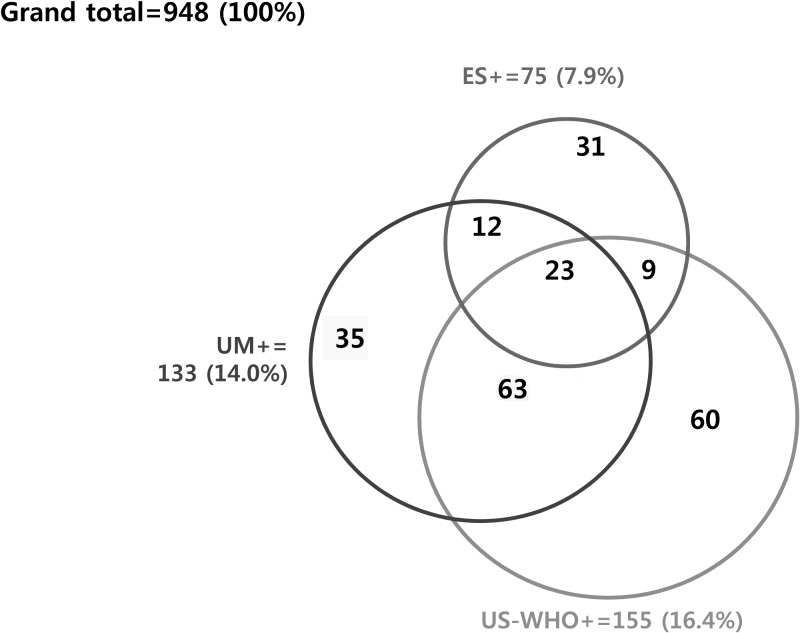
When the 233 participants who were positive by either US (including echogenic snow) or UM are regarded as the gold standard among the 948 subjects, the sensitivity of US is 85.0% (198/233) and that of UM is 57.1% (133/233). When the echogenic snow is excluded for diagnostic images of UGS, a total of 202 subjects become positive as gold standard. The sensitivity of US decreases to 76.7% (155/202) and that of UM increases to 65.8% (133/202). The detailed composition of the study population is shown in Figure 3 .
Figure 3.
Composition of the participants tested positive by urine microscopy (UM+), ultrasonography by WHO criteria (US−WHO+) and echogenic snow sign (ES+).
Discussion
We analyzed cross-sectional screening data of US and UM subjecting schoolchildren and community residents to estimate the morbidity of the urinary bladder caused by S. haematobium by US and to identify the diagnostic significance of the echogenic snow sign in the White Nile State of Sudan. The cross-sectional screening was included in the public health program of mass drug administration and health education implemented by the Korea Association of Health Promotion (KAHP) and the MOH, Sudan, sponsored by KOICA.8
A total of 948 participants underwent both examinations, and 198 (20.9%) were US positive and 133 (14.0%) were UM positive. The Niamey working group criteria suggested by WHO are widely accepted and used for identification of UGS-related pathologic findings.6 Our categorization of the US findings generally agreed with the Niamey criteria although they were simplified. Adopting the Niamey criteria, the US image–positive subjects were 155 (16.4%) of 948. Bladder wall calcification was observed only in one.
The echogenic snow sign was scattered small particle-like echogenicity in the bladder lumen. We frequently noticed the echogenic particles during US examination of the bladder. We suspected that this finding may be one of the pathologic changes possibly related to the S. haematobium infection, which reflects tissue debris or blood clots mixed in urine. Along with other US abnormalities, the echogenic snow sign was significantly frequently observed in the UM-positive groups than the UM-negative group, with a statistical significance of P < 0.001 and an OR of 6.92. Of the 75 echogenic snow sign–positive participants, 35 (46.7%) were UM positive and 32 (42.7%) were combined with other related findings. A total of 31 of the 75 subjects were positive by this sign only, and a part of the 31 may be involved by UGS. Since it is very hot in Sudan and the urine must be highly concentrated, some other unknown etiology can produce the echogenic snow sign. However, the diagnostic sensitivity of US can be increased when this sign is included in the criteria of US findings. This sign must be a topic of further studies on US findings of UGS. A recent study also mentioned the sign as an abnormality related to UGS.9
Usually, the urinary bladder is examined by US under full state and congestive change of the ureter and the kidney is evaluated after voiding. We confined the US examination only to the bladder and distal ureter under the full bladder state. Because we have limited information on subjected people, our US data may include false-positive findings due to other diseases with similar pathogenesis. However, the positive US images of the bladder are not so commonly encountered in children. In this context, it is reasonable to accept that the bladder wall images by US in children suggest morbidity of UGS in its endemic areas.
We investigated the diagnostic performance of the echogenic snow sign in an area where S. haematobium prevalence is approximately 16–20%. In lower prevalence areas, where the control programs were successful, the diagnostic tests are required to be sensitive enough to find most of the lightly infected persons. We anticipate that the echogenic snow sign would help to increase the sensitivity in the lightly infected person. Another issue regarding US is that it is impractical as a screening method due to high cost, need of electricity, and expertise. With the progression of technology, mobile US with good resolutions are under development, and some are already in the market currently. Thus, we believe it would become easier to use US in a remote setting in endemic countries. We need to actively test new US findings which do not require complex measurements and also can be reproduced by basically trained persons. We think the echogenic snow sign could be one of such findings after more studies on its diagnostic significance.
It is promising that the US recognizes UGS with higher sensitivity than the UM by any criteria of US images. The higher diagnostic sensitivity of US suggests that the detection of pathological lesions in the bladder wall is easier than observing the eggs. Since the worms and eggs of S. haematobium are in the vein of the bladder wall, the eggs are released into urine when the vessel wall is ruptured. However, rupture of the blood vessel wall is irregular and the egg release is more irregular. Detection of eggs by UM may depend on the number of released eggs within a few hours before the urine collection. That makes the UM less sensitive. The US-positive findings could also reflect the remnant chronic changes after the treatment. However, because more than 90% of subjects with abnormal US findings are under 15 years of age, the false-positive US finding by other pathological conditions is supposed to be low.
Of course, the method of egg detection may influence the sensitivity of microscopic examination. When compared with the urine filtration method (the standard method by WHO guidelines), urine sedimentation is known to be less sensitive.10 Furthermore, in the previous studies that used the UM examination as gold standard, urine samples were collected for three–seven consecutive days to increase the sensitivity.4,11 These two aspects could have contributed to low sensitivity in UM in our results. Conversely, it also highlights the usefulness of US for epidemiological survey. Another study performed in Gezira State in Sudan also detected more schistosomiasis-related morbidity by US than UM after filtration.9
The prevalence of S. haematobium in the White Nile State was reported to be 21.4% by UM in 1996,12 28.5% in 2009, and 13.5% in 2011,13 but it was 14.0% in our study. The subjects were screened and medicated with praziquantel by a control program supported by the KOICA.8 Other studies performed in other states of Sudan reported the prevalence as 1.7% in River Nile, 23.7% in southern Kordofan, 73% in Upper Nile, and 54.2% and 71% in Gezira among the schoolchildren.9,13–16 The studies performed surveys on the UGS prevalence by investigating schoolchildren in the age group of 10–19 years. Our study confirmed that the prevalence and the age group with the highest disease burden were 20.8% in 0–9 years, 16.3% in 10–14 years, and 14.1% in 15–19 years. Boys were more infected than girls, which was same as that in previous studies. Our survey included 241 community people of ≥ 20 years of age in addition to schoolchildren, which was different from other studies. Of the 241 adults, 11 (4.6%) were US positive and 10 (4.1%) were UM positive. Adults were not free from UGS, although their positive proportion was lower than that of children. Moreover, we observed a wide variation of the positive proportions by locality in the White Nile State (data not shown). Although there have been intermittent chemotherapeutic control programs in White Nile State, UGS has been prevalent continuously especially among schoolchildren.
Gross or microhematuria is also a well-known finding of UGS. The present screening recognized gross hematuria in 24 (2.5%) of the 948 examined. All but one were positive by both UM and US in the present subjects. A man 26 years of age showed gross hematuria with US findings of a mass and echogenic snow in the bladder, but UM was negative. He was later confirmed to suffer not from UGS, but hemorrhagic cystitis by a urologist after cystoscopy in a university hospital. His bladder US findings were produced by aggregated blood cells or clots. Blood cells or clots in urine can produce the echogenic snow sign.
Our study had several limitations. First, we could not certainly suggest the clinical implication of the single positive echogenic snow sign observed in 31 subjects. To clarify its significance, comparison with other diagnostic tests such as serologic antibody test, urine dip stick test, or urinary antigen detection could be helpful. Follow-up US after treatment can also make supportive data. Second, although we suggested the sensitivity and specificity of US findings, their significance is limited due to lack of the gold standard test. More sophisticated statistical models supplemented with the results of the above test will more accurately define how sensitivity and specificity would change with the addition of the echogenic snow sign.17 Third, the severity of the US abnormality was not quantitatively scored according to the WHO guidelines. Generally, the bladder disease is known to be correlated with the urinary egg counts.4 The quantitative outcomes would have helped to identify the participants with severe disease and also to elucidate the clinical and pathologic significance of the echogenic snow sign. Fourth, we did not collect the history of praziquantel intake at individual level. Some of the schoolchildren were subjected to mass drug administration before this survey and were repeatedly examined, but correct identification of their history was impossible. Finally, since the study population was not systematically sampled, our data contain a possibility of selection bias such as volunteer bias as limitation. However, our study only included those who underwent both US and UM, and focuses more on the diagnostic performance of each test rather than estimating the disease prevalence.
Despite these limitations, our study adds valuable information about UGS in Sudan. It updates the disease prevalence and confirms the highest risk group in White Nile State. It also highlights the usefulness of US in the rapid assessment of schistosomiasis-related morbidity. When the echogenic snow sign is added to the known criteria, diagnostic sensitivity of US can be increased. To investigate the clinical significance of the echogenic snow sign, further studies with a more large population are required. Follow-up US after the treatment and results of other diagnostic tests of the participants with the echogenic snow sign would also help to elucidate the clinical meaning.
ACKNOWLEDGMENTS
We thank all the staff involved in this project. This study was part of “The Project for Combating Schistosomiasis in Sudan,” carried by Korea Association of Health Promotion (KAHP) and the Ministry of Health (MoH) of the White Nile State.
Footnotes
Financial support: The project was supported by the Korea International Cooperation Agency (KOICA).
Authors' addresses: Min Jae Kim, Kyungshick Ryu, Yan Jin, and Sung-Tae Hong, Department of Parasitology and Tropical Medicine, Seoul National University College of Medicine, Institute of Endemic Diseases Medical Research Center, Seoul National University, Seoul, Korea, E-mails: nahani99@gmail.com, ryu.ks91@gmail.com, jinyan1024@gmail.com, and hst@snu.ac.kr. Young Ha Lee, Department of Infection Biology, Chungnam National University School of Medicine, Daejeon, Korea, E-mail: yhalee@cnu.ac.kr. Hoo Gn Jeoung, Korea Association of Health Promotion, Seoul, Korea, E-mail: hoo0667@hanmail.net. Adl Al Wahab Saeed, Center for Neglected Tropical Disease Control, White Nile State, Republic of Sudan, E-mail: abdosaed8374@gmail.com. Seung Hyup Kim, Department of Radiology, Seoul National University College of Medicine, Seoul, Korea, E-mail: kimshrad@snu.ac.kr.
References
- 1.Lai YS, Biedermann P, Ekpo UF, Garba A, Mathieu E, Midzi N, Mwinzi P, N'Goran EK, Raso G, Assare RK, Sacko M, Schur N, Talla I, Tchuente LA, Toure S, Winkler MS, Utzinger J, Vounatsou P. Spatial distribution of schistosomiasis and treatment needs in sub-Saharan Africa: a systematic review and geostatistical analysis. Lancet Infect Dis. 2015;15:927–940. doi: 10.1016/S1473-3099(15)00066-3. [DOI] [PubMed] [Google Scholar]
- 2.Degremont A, Burki A, Burnier E, Schweizer W, Meudt R, Tanner M. Value of ultrasonography in investigating morbidity due to Schistosoma haematobium infection. Lancet. 1985;1:662–665. doi: 10.1016/s0140-6736(85)91327-3. [DOI] [PubMed] [Google Scholar]
- 3.Hatz C, Jenkins JM, Meudt R, Abdel-Wahab MF, Tanner M. A review of the literature on the use of ultrasonography in schistosomiasis with special reference to its use in field studies. 1. Schistosoma haematobium. 1992;51:1–14. doi: 10.1016/0001-706x(92)90016-q. [DOI] [PubMed] [Google Scholar]
- 4.Kouriba B, Traore HA, Dabo A, Sangare L, Guindo H, Keita AS, Reimert CM, van Dam GJ, Deelder AM, Doumbo O, Dessein AJ. Urinary disease in 2 Dogon populations with different exposure to Schistosoma haematobium infection: progression of bladder and kidney diseases in children and adults. J Infect Dis. 2005;192:2152–2159. doi: 10.1086/498214. [DOI] [PubMed] [Google Scholar]
- 5.Richter J. Evolution of schistosomiasis-induced pathology after therapy and interruption of exposure to schistosomes: a review of ultrasonographic studies. Acta Trop. 2000;77:111–131. doi: 10.1016/s0001-706x(00)00125-x. [DOI] [PubMed] [Google Scholar]
- 6.Richter J, Hatz C, Campagne G, Bergquist NR, Jenkins J. Ultrasound in Schistosomiasis: A Practical Guide to the Standardized Use of Ultrasonography for the Assessment of Schistosomiasis-Related Morbidity. Geneva, Switzerland: 2000. –World Health Organization.pp. 1–51. World Health Organization/TDR/STR/SCH/WHO-document. [Google Scholar]
- 7.Ismail HA, Hong ST, Babiker AT, Hassan RM, Sulaiman MA, Jeong HG, Kong WH, Lee SH, Cho HI, Nam HS, Oh CH, Lee YH. Prevalence, risk factors, and clinical manifestations of schistosomiasis among school children in the White Nile River basin, Sudan. Parasit Vectors. 2014;7:478. doi: 10.1186/s13071-014-0478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YH, Jeong HG, Kong WH, Lee SH, Cho HI, Nam HS, Ismail HA, Alla GN, Oh CH, Hong ST. Reduction of urogenital schistosomiasis with an integrated control project in Sudan. PLoS Negl Trop Dis. 2015;9:e3423. doi: 10.1371/journal.pntd.0003423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasmelseed N, Karamino NE, Abdelwahed MO, Hamdoun AO, Elmadani AE. Genetic diversity of Schistosoma haematobium parasite is not associated with severity of disease in an endemic area in Sudan. BMC Infect Dis. 2014;14:469. doi: 10.1186/1471-2334-14-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dazo BC, Biles JE. Two new field techniques for detection and counting of Schistosoma haematobium eggs in urine samples, with an evaluation of both methods. Bull World Health Organ. 1974;51:399–408. [PMC free article] [PubMed] [Google Scholar]
- 11.Hatz C, Mayombana C, de Savigny D, MacPherson CN, Koella JC, Degremont A, Tanner M. Ultrasound scanning for detecting morbidity due to Schistosoma haematobium and its resolution following treatment with different doses of praziquantel. Trans R Soc Trop Med Hyg. 1990;84:84–88. doi: 10.1016/0035-9203(90)90392-r. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed ES, Daffalla A, Christensen NO, Madsen H. Patterns of infection and transmission of human schistosomiasis mansoni and schistosomiasis haematobium in White Nile Province, Sudan. Ann Trop Med Parasitol. 1996;90:173–180. doi: 10.1080/00034983.1996.11813041. [DOI] [PubMed] [Google Scholar]
- 13.Abou-Zeid AH, Abkar TA, Mohamed RO. Schistosomiasis infection among primary school students in a war zone, southern Kordofan State, Sudan: a cross-sectional study. BMC Public Health. 2013;13:643. doi: 10.1186/1471-2458-13-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deganello R, Cruciani M, Beltramello C, Duncan O, Oyugi V, Montresor A. Schistosoma haematobium and S. mansoni among children, southern Sudan. Emerg Infect Dis. 2007;13:1504–1506. doi: 10.3201/eid1310.070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elmadani AE, Hamdoun AO, Monis A, Karamino NE, Gasmelseed N. Ultrasound findings in urinary shistosomaisis infection in school children in the Gezira State, Central Sudan. Saudi J Kidney Dis Transpl. 2013;24:162–167. doi: 10.4103/1319-2442.106362. [DOI] [PubMed] [Google Scholar]
- 16.Elmadhoun WM, Msmar AH, Elnoby OA, Noor SK, Suliman AA, Bushara SO. Situation analysis of schistosomiasis and soil-transmitted helminthes in River Nile State, Sudan. Trans R Soc Trop Med Hyg. 2013;107:195–199. doi: 10.1093/trstmh/trs088. [DOI] [PubMed] [Google Scholar]
- 17.Koukounari A, Webster JP, Donnelly CA, Bray BC, Naples J, Bosompem K, Shiff C. Sensitivities and specificities of diagnostic tests and infection prevalence of Schistosoma haematobium estimated from data on adults in villages northwest of Accra, Ghana. Am J Trop Med Hyg. 2009;80:435–441. [PMC free article] [PubMed] [Google Scholar]