Abstract
Hepatitis B virus (HBV) infection accounts for about 1 million deaths worldwide annually. This study was to determine the prevalence, distribution of HBV, and factors associated with infection in an apparently healthy population in Nigeria. A cross-sectional study among the general population was conducted employing a multistage sampling technique. Data on demographic, social, and behavioral indicators were collected using questionnaires and blood samples tested for HBV seromarkers. Descriptive, bivariate, and multivariate analyses were done. Prevalence of hepatitis B infection was 12.2% (confidence interval [CI] = 10.3–14.5). Of the participants, more than half, 527 (54.6%), had evidence of previous exposure to HBV, while 306 (31.7%) showed no serologic evidence of infection or vaccination. Only 76 (7.9%) participants showed serologic evidence of immunity to HBV through vaccination. Factors associated with testing positive for HBV infection were dental procedure outside the health facility (odds ratios [OR] = 3.4, 95% CI = 1.52–7.70), local circumcision (OR = 1.73, 95% CI = 1.17–2.57), and uvulectomy (OR = 1.65, 95% = 1.06–2.57). With logistic regression, only dental procedure outside the health facility (adjusted OR = 3.32, 95% CI = 1.38–7.97) remained significant. This first national survey on seroprevalence of hepatitis B describes the epidemiology and high prevalence of HBV infection in Nigeria and highlights the need for improved vaccination against HBV.
Introduction
Viral hepatitis causes both acute and chronic infection with significant complications and sequelae. More than 2 billion people worldwide are estimated to have had hepatitis B virus (HBV) infection, with 350–400 million being chronic carriers of the virus.1,2 HBV accounts annually for an estimated 1 million deaths worldwide,3 and causes acute and chronic liver disease. Its prevalence varies throughout the world, but is highest in tropical regions.4–6 It is estimated that 5–15% of adults in sub-Saharan Africa are chronically infected with HBV.3 There is a 15–25% risk of dying prematurely in adulthood from HBV-related cirrhosis and hepatocellular carcinoma, while a small proportion of those with acute infections may also succumb to fulminant liver failure.1
In areas of high endemicity where at least 8% of the population are chronic HBV carriers,6 HBV is mainly contracted at birth and early childhood.6 Perinatal transmission from an infected mother to her baby is common. About 90% of those infected during the prenatal period, 30% of those infected in early childhood, and 6% of those infected after 5 years of age develop chronic infection.2 Transmission of HBV among adults occurs via contact with infected blood and body fluids such as semen, vaginal fluids, and saliva. Therefore transfusion of unscreened blood and its products, sexual activities, use of contaminated or inadequately sterilized instruments, sharing of sharp objects as could occur during some traditional or cultural practices, for example, local circumcision, are common means of spread. It could also occur by other means of iatrogenic or horizontal transmission such as long-term household contacts with no sexual involvements in regions of high endemicity. HBV infection is also recognized as an occupational health hazard for health-care practitioners.1,7–11
In many regions of the world, such as Europe, the Americas, and Australia, there has been a downward trend in the prevalence of chronic HBV infection mainly due to immunization against HBV and improved health-care practices, for example, screening of blood and blood products, injection safety, and infection control policies and practices.12,13 In Nigeria, 11.6% prevalence has been reported from Maiduguri among blood donors and pregnant women,14 4.3% from Port Harcourt among pregnant women,15 5.7% from Ilorin in mothers and their preschool children,16 8.3% from Zaria among pregnant women,17 17.1% from female sex workers in Nassarawa,18 14.9% from healthy blood donors in Yola,19 and 25.7% among surgeons in Lagos.8 Health-care workers have a 3- to 5-fold higher prevalence of HBV than the general population, with surgeons and dentists having higher reported cases.20
HBV infection is a vaccine-preventable disease. Vaccination with the monovalent HBV vaccine was introduced in Nigeria in 2004 as part of the National Program on Immunization (NPI), to be given at 6, 10, and 14 weeks of age.21 However in 2012, a pentavalent vaccine comprising diphtheria, tetanus, pertussis, HBV, and Haemophilus influenza type B was introduced.22 The prevalence of this disease in Nigeria as a whole is not known although the country has long been considered to be among the highly endemic countries of sub-Saharan Africa.3 Data for chronic viral hepatitis are not routinely collected by the Integrated Disease Surveillance and Response system, which collects only acute viral hepatitis cases; therefore, hepatitis infection remains largely underreported. In view of the advantage of decreasing the chronic carrier rate of HBV within the population, this study was carried out to determine the prevalence and distribution of HBV infection in an apparently healthy population who serve as an important source for new infections, identify subpopulations at risk who would benefit from vaccination, and explore factors associated with infection while providing baseline data for future assessment of the impact of HBV infection and vaccination in Nigeria.
Methods
This was a cross-sectional study that included the six geopolitical zones of Nigeria: namely north-west, north-central, north-east, south-west, south-east, and south-south regions. Nigeria, situated in west Africa, is the most populous black nation in the world with an estimated population of 170 million. The study was conducted among the general Nigerian population older than 2 years of age of unknown human immunodeficiency virus status and resident in the community at the time of the survey. A multistage sampling technique was used. Stages were the state level, the local government areas stratified into urban and rural, the ward level and the household level. At the household level, one respondent each was selected by simple random sampling through balloting. Using an expected prevalence of 0.103,23 our minimum sample size was 142 per state.
Training on the survey protocol, detailed methodology, infection control, safety issues, and consenting procedures was provided to all survey team members twice. The pretested interviewer-administered questionnaire comprised different sections to obtain sociodemographic data of interest, history of exposure to potential risk factors, hepatitis B vaccination, and previous testing history. When a young child was selected in any household, the parent or main caregiver was administered the questionnaire to answer on behalf of the child after due ethical processes.
Venous blood samples were obtained for testing of HBV seromarkers. The HBV surface and core antibodies (HBsAb and HBcAb, respectively) were assayed using an enzyme-linked immunosorbent assay (ELISA) kit by Diapro® (Diagnostic Bioprobes Srl, Milan, Italy), which has a sensitivity and specificity of 100% and 98.8%, respectively; while the HBV surface antigen (HBsAg) was assayed using the ELISA kit by Bio-Rad® (Bio-Rad Laboratories, Berkeley, CA), with sensitivity of 100% and specificity of 99.4%. The laboratory test results for individuals were anonymously linked to individual and household questionnaire information through their unique identifiers. Assay runs were strictly performed following standard operating procedures, including quality controls, developed for the survey, and were validated as per manufacturer's instructions.
Where quality control failed, the entire batch was rerun. Five (5%) of all negative and positive samples were reassayed independently for verification of the results using the same kits. A discordance of less than 5% between the quality control tests and the original test results was deemed acceptable. Results were interpreted as positive or negative after result validation based on predetermined cutoff values by kit manufacturers. A positive HBsAg test was considered evidence of HBV infection (chronic carrier state or infection) and used to calculate the prevalence; a positive HBcAb test was considered evidence of previous exposure to the HBV; and a positive HBsAb test was considered evidence of being immune to HBV, which when in combination with a positive HBcAb was considered due to natural infection and when alone due to vaccination. Being negative for all markers meant participant was susceptible to HBV infection.24
Ethical consideration.
Survey approval was granted by the Nigerian National Human Research Ethics Committee (NHREC/01/01/2007-28/07/2013). Written informed consent was obtained from each consenting participant 18 years and older, written assent from children aged 12 years to less than 18 years was obtained, in addition to consent provided by parent or caregiver, while for children less than 12 years, parental or caregiver written consent alone was obtained. All participants received their test result after the survey, and those who were found to be positive for HBsAg were contacted and referred to the nearest government hospital for further evaluation and management as deemed appropriate by the consulting physician. An information leaflet on hepatitis B and C infection was also produced and provided to all participants.
Completed questionnaires were checked for accuracy and completeness. Using Epi info 7 software (CDC, Atlanta, GA) for analysis, data were double entered to minimize data entry errors and later merged. Descriptive analysis and proportions were calculated for categorical data and to obtain prevalence. Bivariate analysis with calculation of odds ratios (ORs) and 95% confidence intervals (CIs) was done to examine the association between some exposure factors and HBV infection; logistic regression was done to determine predictors of infection. Statistical significance was determined with the χ2 test and the P value set at 0.05.
Results
The overall median age of the 965 participants interviewed across the nation was 35 years (range = 2–90 years); 525 (54.4%) were male, and 558 of 828 participants older than 18 years of age (67.4%) were married. Those who had completed at least a primary school education were 786 (83.7%), 663 of those older than 18 years (79.8%) had employment and about half (48.4%) earned less than 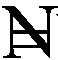 18, 000/month ($110/month), which is the national minimum wage (Table 1).
18, 000/month ($110/month), which is the national minimum wage (Table 1).
Table 1.
Demographic characteristics of participants
| Age mean age: 37.2 (±17) years; median: 35 years (interquartile range = 25–50); range: 2–90 years | ||
|---|---|---|
| Age group in years (N = 964) | Frequency | % (95% confidence intervals |
| < 10 | 41 | 4.3 (3.1–5.7) |
| 10–19 | 100 | 10.4 (8.6–12.4) |
| 20–29 | 202 | 21.0 (18.5–23.6) |
| 30–39 | 209 | 21.6 (19.2–23.4) |
| 40–49 | 154 | 16.0 (13.8–18.4) |
| 50–59 | 144 | 14.8 (12.8–17.3) |
| 60–69 | 70 | 7.3 (5.7–9.0) |
| ≥ 70 | 44 | 4.6 (3.4–6.0) |
| Marital status those aged 18 years and above (N = 828) | ||
| Married | 558 | 67.4 |
| Single | 194 | 23.4 |
| Widowed | 60 | 7.3 |
| Separated/divorced | 14 | 1.7 |
| Others | 2 | 0.2 |
| Sex (N = 965) | ||
| Males | 525 | 54.4 |
| Females | 440 | 45.6 |
| Education (N = 939) | ||
| No formal education | 153 | 16.2 |
| Primary | 230 | 24.5 |
| Secondary | 316 | 33.7 |
| Tertiary | 198 | 21.1 |
| Postgraduate | 42 | 4.5 |
| Occupation of those above 18 years (N = 831) | ||
| Self-used | 383 | 46.1 |
| Used for wages | 280 | 33.7 |
| Unemployed | 70 | 8.4 |
| Retired | 30 | 3.6 |
| Students | 68 | 8.2 |
Monthly income in naira (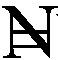 ) (N = 728) ) (N = 728) | ||
| < 18,000 | 352 | 48.4 |
| 18,000 to < 35,000 | 194 | 26.6 |
| 35,000 to < 70,000 | 101 | 13.9 |
| 70,000 to 120,000 | 42 | 5.8 |
| > 120,000 | 39 | 5.3 |
The prevalence of hepatitis B infection was 12.2% (CI = 10.3–14.5) depicting the level of hepatitis B endemicity in Nigeria. Of the participants, more than half, 527 (54.6%), had evidence of previous exposure to the HBV (HBcAb), 355 (36.8%) demonstrated the presence of protective antibodies (HBsAb) in their serum while 306 (31.7%) showed no serologic evidence of infection or vaccination (Table 2). Seventy-six (7.9%) participants showed serologic evidence of being immune to HBV through vaccination. Only seven (23.3%) of those who reported having received up to three doses of HBV vaccine showed serologic evidence of immunity due to vaccination; 279 (28.9%) of them showed serologic evidence of being immune to HBV through natural infection.
Table 2.
Hepatitis B biomarkers among survey participants (N = 965)
| Biomarker | Frequency | % (95% CI) |
|---|---|---|
| HBsAg | 118 | 12.2 (10.3–14.5) |
| HBsAb | 355 | 36.8 (34.5–40.7) |
| HBcAb | 527 | 54.6 (52.6–58.9) |
| HBsAb +ve and HBsAg −ve and HBcAb −ve | 76 | 7.9 (16.1–24.1) |
| HBsAb +ve and HBsAg −ve and HBcAb +ve | 249 | 25.8 (23.6–29.2) |
| HBsAb −ve and HBsAg −ve and HBcAb −ve | 306 | 31.7 (29.5–35.4) |
+ve = positive test; −ve = negative test; CI = confidence interval; HBsAg = hepatitis B surface antigen; HBsAb = hepatitis B surface antibody; HBcAb = hepatitis B core antibody.
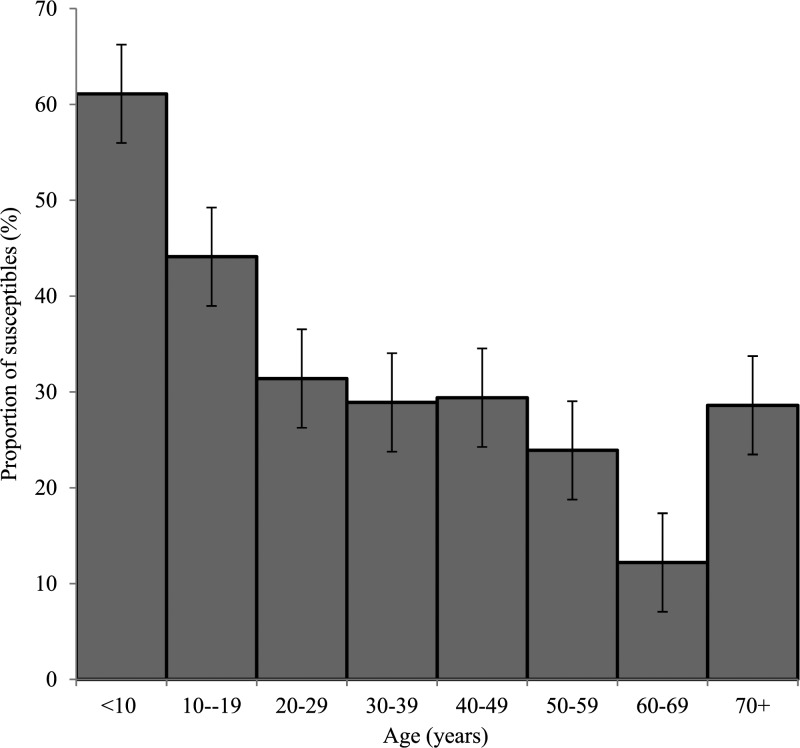
The proportion of persons susceptible to HBV infection decreased significantly with increasing age (χ2 for linear trend = 29.2, P < 0.0001; Figure 1 ) while there was a significantly higher proportion of susceptible persons among unmarried participants (divorced, single, widowed) than married ones (χ2 = 19, P < 0.0001). Most prevalent factors present in those positive for HBsAg were local circumcision, tribal marks, and uvulectomy (Table 3). There was no significant difference in the proportions of susceptible or immune persons across sex, level of educational attainment, and income brackets (Table 4). Among children aged 8 years and below, 21/35 (60%) did not have antibodies to HBsAg in their sera and were also HBcAb negative, therefore susceptible to hepatitis B infection.
Figure 1.
Proportion of hepatitis B virus susceptible persons by age group.
Table 3.
Prevalence of factors associated with risk of hepatitis B among participants (N = 965)
| Risk factor | Frequency | % (95% confidence interval) |
|---|---|---|
| Scarification/tattoo | 169 | 17.5 (15.2–20.0) |
| Tribal marks | 270 | 28.0 (25.2–30.8) |
| Received blood transfusion | 83 | 8.6 (6.9–10.5) |
| Received surgical procedure in health facility | 150 | 15.5 (13.4–17.9) |
| Local uvulectomy/tonsillectomy | 188 | 19.5 (17.1–22.1) |
| Local circumcision | ||
| Male | 341 | 65.0 (60.8–69.1) |
| Female | 125 | 28.4 (24.3–32.9) |
| Delivery of child at home | 125 | 13.0 (10.9–15.2) |
| Dental procedure at health facility | 100 | 10.4 (8.5–12.4) |
| Dental procedure outside health facility | 29 | 3.0 (2.1–4.2) |
| Blood oaths | 6 | 0.6 (0.3–1.3) |
| Body piercing | 178 | 18.5 (16.1–21.0) |
| Intravenous drug use | 48 | 5.0 (3.7–6.5) |
| Sharing of sharp objects | 189 | 19.6 (17.2–22.2) |
| Cupping/blood letting | 69 | 7.1 (5.6–8.9) |
| Liver transplantation | 2 | 0.2 (0.0–0.7) |
| Parents with hepatitis infection | 23 | 2.4 (1.6–3.5) |
| Living with and sharing facilities and material with IDU | 16 | 1.7 (1.0–2.7) |
| Needle-stick injury | 167 | 17.3 (15.0–19.8) |
| Exposure to blood, body fluids, or tissues | 100 | 10.4 (8.6–12.4) |
| On dialysis treatment | 2 | 0.2 (0.0–0.7) |
| Sharing of toothbrush/chewing stick | 25 | 2.6 (1.7–3.7) |
| Local hair shaving | 83 | 8.6 (6.9–13.0) |
| Local manicure/pedicure | 105 | 10.9 (9.0–13.0) |
IDU = injection drug users.
Table 4.
Prevalence of HBV biomarkers by sociodemographic characteristics of survey respondents (N = 965)
| HBsAg | HBsAb | HBcAb | ||||
|---|---|---|---|---|---|---|
| Frequency | % | Frequency | % | Frequency | % | |
| Sex | ||||||
| Male | 76 | 14.5 | 192 | 36.6 | 298 | 56.8 |
| Female | 37 | 8.4 | 159 | 36.1 | 219 | 49.8 |
| Age (years) | ||||||
| < 10 | 4 | 9.8 | 11 | 26.8 | 5 | 12.2 |
| 10–19 | 13 | 13.0 | 32 | 32.0 | 30 | 30.0 |
| 20–29 | 27 | 13.4 | 73 | 36.1 | 111 | 55.0 |
| 30–39 | 26 | 12.4 | 83 | 39.7 | 125 | 59.8 |
| 40–49 | 20 | 13.0 | 61 | 39.6 | 87 | 56.5 |
| 50–59 | 13 | 9.0 | 46 | 31.9 | 97 | 67.4 |
| 60–69 | 11 | 15.7 | 30 | 42.9 | 44 | 62.9 |
| ≥ 70 | 4 | 9.1 | 14 | 31.8 | 20 | 45.5 |
| Marital status | ||||||
| Single | 38 | 19.6 | 109 | 56.2 | 130 | 67.0 |
| Married | 71 | 12.7 | 212 | 38.0 | 349 | 62.6 |
| Divorced/separate | 1 | 7.1 | 9 | 64.3 | 10 | 71.4 |
| Widowed | 5 | 8.3 | 22 | 36.7 | 35 | 58.3 |
| Others | 1 | 50.0 | 1 | 50.0 | 1 | 50.0 |
| Education | ||||||
| No formal education | 20 | 13.1 | 71 | 46.4 | 100 | 65.4 |
| Primary | 25 | 10.9 | 76 | 33.0 | 119 | 51.7 |
| Secondary | 39 | 12.3 | 113 | 35.8 | 165 | 52.2 |
| Tertiary | 24 | 12.1 | 77 | 38.9 | 117 | 59.1 |
| Post graduate | 6 | 14.3 | 9 | 21.4 | 17 | 40.5 |
| Income (Naira) | ||||||
| < 18,000 | 40 | 11.4 | 150 | 42.6 | 211 | 59.9 |
| 18,000 to < 35,000 | 27 | 13.9 | 68 | 35.1 | 129 | 66.5 |
| 35,000 to < 70,000 | 11 | 10.9 | 34 | 33.7 | 56 | 55.5 |
| 70,000 to < 120,000 | 8 | 19.0 | 10 | 23.8 | 20 | 47.6 |
| ≥ 120, 000 | 5 | 12.8 | 13 | 33.3 | 21 | 53.9 |
| Occupational status | ||||||
| Used for wages | 34 | 12.1 | 95 | 33.9 | 151 | 53.9 |
| Self-used | 47 | 12.3 | 154 | 40.2 | 251 | 65.5 |
| Retired | 3 | 10.0 | 14 | 46.7 | 20 | 66.7 |
| Unemployed | 12 | 17.1 | 30 | 42.9 | 43 | 61.4 |
HBsAg = hepatitis B surface antigen; HBsAb = hepatitis B surface antibody; HBcAb = hepatitis B core antibody; HBV = hepatitis B virus.
At bivariate analysis, factors found to be significantly associated with testing positive for HBsAg were history of dental procedure outside the health facility (OR = 3.4, 95% CI = 1.52–7.70), local circumcision (OR = 1.73, 95% CI = 1.17–2.57), and uvulectomy (OR = 1.65, 95% CI = 1.06–2.57) (Table 5).
Table 5.
Factors associated with risk of positivity for HBsAg
| Variable | HbsAg | |||||||
|---|---|---|---|---|---|---|---|---|
| Response | Positive | % | Negative | % | OR | 95% CI | P value | |
| Local circumcision | Yes | 71 | 15 | 395 | 85 | 1.73 | 1.17–2.57 | 0.0075 |
| No | 47 | 9 | 453 | 91 | ||||
| Dental procedure outside health facility | Yes | 9 | 31 | 20 | 69 | 3.4 | 1.52–7.70 | 0.0043 |
| No | 109 | 12 | 828 | 88 | ||||
| Uvulectomy | Yes | 32 | 17 | 156 | 83 | 1.65 | 1.06–2.57 | 0.0342 |
| No | 86 | 11 | 692 | 89 | ||||
| Local manicure/pedicure | Yes | 41 | 15.5 | 223 | 84.5 | 1.51 | 1.00–2.28 | 0.06 |
| No | 73 | 10.8 | 600 | 89.2 | ||||
| Blood transfusion | Yes | 15 | 18.1 | 68 | 81.9 | 1.67 | 0.92–3.03 | 0.1263 |
| No | 103 | 11.7 | 780 | 88.3 | ||||
| Work involves blood contact | Yes | 8 | 9.5 | 76 | 90.5 | 0.71 | 0.33–1.52 | 0.4757 |
| No | 109 | 12.9 | 737 | 87.1 | ||||
| Delivery at home | Yes | 11 | 8.8 | 114 | 91.2 | 0.66 | 0.35–1.27 | 0.2699 |
| No | 107 | 12.7 | 734 | 87.3 | ||||
| Condom use in extra-marital sex | Never or sometimes | 50 | 11.7 | 376 | 88.3 | 0.64 | 0.32–1.28 | 0.2835 |
| Always | 12 | 17.1 | 58 | 82.9 | ||||
| Local hair shaving | Yes | 36 | 14.0 | 222 | 88.0 | 1.26 | 0.82–1.92 | 0.338 |
| No | 78 | 11.4 | 606 | 88.6 | ||||
| Needle-stick injury | Yes | 20 | 12.0 | 147 | 88.0 | 0.97 | 0.58–1.63 | 0.9792 |
| No | 98 | 12.3 | 701 | 87.7 | ||||
| Scar/tattoo | Yes | 19 | 11.2 | 150 | 88.8 | 0.89 | 0.53–1.50 | 0.7674 |
| No | 99 | 12.4 | 698 | 87.6 | ||||
| Tribal marks | Yes | 30 | 11.1 | 240 | 88.9 | 0.86 | 0.56–1.34 | 0.5869 |
| No | 88 | 12.6 | 608 | 87.4 | ||||
CI = confidence interval; HBsAg = hepatitis B surface antigen; OR = odds ratio.
With multivariable logistic regression, only dental procedure outside the health facility (adjusted OR = 3.32, 95% CI = 1.38–7.97) remained statistically significant (Table 6).
Table 6.
Unconditional logistic regression for risk factors for HBsAg that achieved statistical significance at bivariate analysis
| Variable | aOR | 95% CI | P value |
|---|---|---|---|
| Dental procedure outside health facility | 3.32 | 1.38–7.97 | 0.007 |
| Local circumcision | 1.40 | 0.89–2.19 | 0.143 |
| Local manicure/pedicure | 1.24 | 0.80–1.92 | 0.336 |
| Uvulectomy | 1.48 | 0.92–2.36 | 0.105 |
| Sex (male/female) | 1.38 | 0.87–2.17 | 0.168 |
aOR = adjusted odds ratio; CI = confidence interval; HBsAg = hepatitis B surface antigen.
Discussion and Conclusion
This national survey on seroprevalence of hepatitis B infection confirms that HBV infection is highly endemic in Nigeria providing a prevalence within the estimated prevalence in sub-Saharan Africa.4 Furthermore, our study revealed that common risk factors for hepatitis B infection among our participants were uvulectomy, presence of tribal marks, sharing of sharp objects, and circumcision, which is performed traditionally on all Nigerian males and is one of the oldest surgical procedures carried out in home settings by traditional “circumcisionists,” with some regions still practicing group circumcision.25,26 This factor may therefore suggest why the prevalence of HBV was higher in males although not statistically significant but similar to Uganda and other sub-Saharan African countries, where chronic HBV infections have been found to be more common in males.23,27–29 However in Vilibic's study and a similar study done in Cambodia, sex was not associated with HBV seropositivity.30,31
Invasive dental procedure done outside the health facility was shown to be a predictor of HBV infection. Main risk factors for both hepatitis C virus and HBV were exposure to dental procedures and surgery in one study.32 Although in other studies,33,34 reuse of razor blades and needle-stick injuries have been documented as risk factors for HBV infection, we did not find this so in our study. In yet another study, risk factors such as unprotected sex, mouth-to-mouth kissing, blood transfusion, public barbing salon clipper cuts, manicure and pedicure cuts, and scarification were not significantly associated with the presence of HBsAg. However, they reported that students who had been cut with reused razor blades and who had needle-stick injuries were more likely to be infected.33 We found out that the majority of those married demonstrated evidence of past infection by the presence of anti-HBc while there was a higher proportion of susceptible persons among unmarried respondents. Unprotected sex is known to be a means of transmission of HBV, and marriage provides a means of unprotected sex, which could increase the chances of exposure and transmission of HBV. Similarly in a study among students, married students had higher prevalence of HBsAg compared with single students also possibly substantiating the role of sexual transmission of HBV.11
More than half of our participants showed evidence of previous or current HBV infection, and there was a corresponding increase in seropositivity to anti-HBc as age increased. In addition, we found out that susceptibility to infection decreased with age. In some studies, it was also evident that HBV seropositivity increased progressively with age; and this was adduced to increasing exposure to the virus by a significant proportion of the children as they grew older.30,31,35
More than one-third of our respondents demonstrated the presence of circulating protective antibodies in their sera; however, few of these were as a result of vaccination. A very small proportion of people had been vaccinated in the first place, and some were unsure of their vaccination status. Other studies reported higher proportions of persons with circulating antibodies from vaccination, and this was felt to still be suboptimal in terms of coverage in view of the fact that hepatitis B vaccines have been available since 1991.11,35 Even though we had a small number of children aged 8 years or younger among our participants, less than one-third of them were protected as detected by the presence of HBsAb and majority of them were susceptible. The low rate of vaccination against HBV is poor although HBV vaccines have been available since the 1990s36 and had been incorporated in the NPI in Nigeria since 2004—8 years previous to this study.21 This finding is similar to that in a study among preschool children where less than one-fifth of those who had received the three doses recommended were serologically protected, and the response to vaccination was considered low.35
In many countries, a comparison of the proportion of those vaccinated 13 years before and after demonstrated an increase from less than 30% to greater than 90% after 13 years of a successful national HBV immunization program. We however consider the findings in this study as the national baseline value for we were unable to access any published record of a previous estimation or assessment. A study in Nigeria showed a poor vaccination status among operating theater personnel,37 a subset of health-care workers obviously at high risk of occupational exposure to HBV. This needs to be addressed as previous studies in the country have consistently shown low vaccination status among health-care workers.10,38 There is currently no national HBV vaccination program for health-care workers in the country.
Limitations to this first national survey included the small sample size and the inability to differentiate between those that had a current acute infection, and those that had chronic infection or an occult HBV infection as IgM serology and polymerase chain reaction were not carried out. However, the validity of our findings is supported by the national representativeness of our participants as samples were obtained from the six geopolitical zones of the country, and the testing method which involved the use of quality controlled ELISA kits with high sensitivity and specificity. With the cross-sectional design used, it was difficult to definitively ascertain if the identified putative risk factors were specific for HBV infection. The study was however community based and able to identify those with evidence of lifetime exposure and those that had presence of circulating antibodies.
This study describes the epidemiology and high prevalence of HBV infection in Nigeria on a national level and highlights the need for improved vaccination against HBV. There is a strong age-related aspect in relation to time in seropositivity of HBV. As such, there is a need to strengthen the national immunization program for infants but the government should also consider the vaccination of older children and people at risk such as health-care workers. Indigenous or local practitioners of dental procedures, circumcision, scarification, pedicure, and other body-based procedures need to be educated on health risks and infection control regarding their techniques. Information to increase awareness about HBV prevention and transmission especially by sexual means needs to be disseminated to the public. Finally, it would be useful to identify the circulating genotypes across the country as such genetic characterization may help to explain differences encountered at community levels.
ACKNOWLEDGMENTS
We thank Gabrielle Poggensee and the residents of the Nigeria Field Epidemiology and Laboratory Training Programme Abdullahi Musa, Adaora Offor, Amina Kazaure, Celestine Ameh, Charles Akataobi, Chidinma Agbai, Emmanuel Eze, Funke Fagbemiro, Habila Ismaila, Janada Dimas, Lydia Taiwo, Samuel Sha'aibu, and Saude Abdullahi who helped with data entry and cleaning. We also appreciate Kabir Sabitu and O. Fawole for their support during the survey period.
Footnotes
Financial support: Funding was received from Roche Nigeria Ltd. for the nonlaboratory component of the survey.
Authors' addresses: Adebola T. Olayinka, Nigeria Field Epidemiology and Laboratory Training Programme, Abuja, Nigeria, and Department of Medical Microbiology, Ahmadu Bello University, Zaria, Nigeria, E-mail: debolaola@yahoo.com. Akin Oyemakinde and Anthonia Ajudua, Epidemiology Division, Federal Ministry of Health, Abuja, Nigeria, E-mails: gbekeloluwa2003@yahoo.com and maamahnwendu@yahoo.com. Muhammad S. Balogun, Patrick Nguku, Moses Aderinola, Abiodun Egwuenu-Oladejo, Simeon W. Ajisegiri, Samuel Sha'aibu, and Saheed Gidado, Nigeria Field Epidemiology and Laboratory Training Programme, Abuja, Nigeria, E-mails: shakirmuhammad@yahoo.co.uk, drnguku@gmail.com, olaoluaderinolam@gmail.com, a.jumoke19@yahoo.com, doctorajisegiri@yahoo.com, samuelshybu@gmail.com, and gsosuccess@yahoo.com. Bolanle O. P. Musa, Immunology Unit, Department of Medicine, Ahmadu Bello University Teaching Hospital, Zaria, Nigeria, E-mail: bolamusa2002@yahoo.com. Abdulsalami Nasidi, Nigeria Center for Disease Control, Abuja, Nigeria, E-mail: nasidi@gmail.com.
References
- 1.Ganem D, Prince AM. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 2.Lok ASF, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 3.WHO Hepatitis B. 2014. http://www who.inf/inf-s/en/fact204.html Available at. Accessed July 28, 2013.
- 4.Kramvis A, Kew M. Epidemiology of hepatitis B virus in Africa, its genotypes and clinical associations of genotypes. Hepatol Res. 2007;37((Suppl 1)):9–19. doi: 10.1111/j.1872-034X.2007.00098.x. [DOI] [PubMed] [Google Scholar]
- 5.Apurva AM, Jordan JF. Viral hepatitis and HIV in Africa. AIDS Rev. 2007;9:25–39. [PubMed] [Google Scholar]
- 6.Hou J, Liu Z, Gu F. Epidemiology and prevention of hepatitis B virus infection. Int J Med Sci. 2005;2:50–57. doi: 10.7150/ijms.2.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray Davis L, Weber DJ, Lemon SM. Horizontal transmission of hepatitis B virus. Lancet. 1989;1:889–893. doi: 10.1016/s0140-6736(89)92876-6. [DOI] [PubMed] [Google Scholar]
- 8.Belo AC. Prevalence of hepatitis B virus markers in surgeons in Lagos, Nigeria. East Afr Med J. 2000;77:283–285. doi: 10.4314/eamj.v77i5.46634. [DOI] [PubMed] [Google Scholar]
- 9.Chang MH. Natural history of hepatitis B virus infection in children. J Gastroenterol Hepatol. 2000;15:16–19. doi: 10.1046/j.1440-1746.2000.02096.x. [DOI] [PubMed] [Google Scholar]
- 10.Sofola OO, Folayan MO, Denloye OO, Okeigbemen SA. Occupational exposure to bloodborne pathogens and management of exposure incidents in Nigerian dental schools. J Dent Educ. 2007;71:832–837. [PubMed] [Google Scholar]
- 11.Bhattarai S, KC S, Pradhan PM, Lama S, Rijal S. Hepatitis B vaccination status and needle-stick and sharps-related injuries among medical school students in Nepal: a cross-sectional study. BMC Res Notes. 2014;7:774. doi: 10.1186/1756-0500-7-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahoney FJ, Kane M. Hepatitis B vaccine. In: Plotkin SA, Orenstein WA, editors. Vaccines. 3rd edition. Philadelphia, PA: W.B. Saunders Company; 1999. pp. 158–182. [Google Scholar]
- 13.Ott JJ, Stevens G A, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 14.Harry TO, Bajani MD, Moses AE. Hepatitis B virus infection among blood donors and pregnant women in Maiduguri, Nigeria. East Afr Med J. 1994;70:596–597. [PubMed] [Google Scholar]
- 15.Akani CI, Ojule AC, Opurum HC, Ejilemele AA. Seroprevalence of HBsAg in pregnant women in Port Harcourt. Niger Postgrad Med J. 2005;12:266–270. [PubMed] [Google Scholar]
- 16.Agbede OO, Iseniyi JO, Kolewale MO, Ojuawo A. Risk factors and seroprevalence of hepatitis B antigenemia in mothers and their preschool children in Ilorin, Nigeria. Therapy. 2007;4:67–72. [Google Scholar]
- 17.Luka SA, Ibrahim MB, Iliya SN. Seroprevalence of hepatitis B surface antigen among pregnant women attending Ahmadu Bello University Teaching Hospital Zaria. Niger J Parasitol. 2008;29:38–41. [Google Scholar]
- 18.Forbi JC, Onyemauwa N, Gyar SD, Oyeleye AO, Entonu P, Agwale SM. High prevalence of hepatitis B virus among female sex workers in Nigeria. Rev Inst Med Trop Sao Paulo. 2008;50:219–221. doi: 10.1590/s0036-46652008000400006. [DOI] [PubMed] [Google Scholar]
- 19.Olokoba AB, Salawu FK, Danburam A, Desalu OO, Olokoba LB, Wahab KW. Viral hepatitis in voluntary blood donors in Yola, Nigeria. Euro J Scientific Res. 2009;31:329–334. [Google Scholar]
- 20.Minuk GY, Cohen AJ, Assy N, Moser M. Viral hepatitis and the surgeon. HPB (Oxford) 2005;7:56–64. doi: 10.1080/13651820410016633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Programme on Immunization and Partners . Five Years NationaStrategic Plan 2003–2007. Abuja, Nigeria: National Programme on Immunization; 2002. pp. 19–20. [Google Scholar]
- 22.GAVI Nigeria Launches Pentavalent Vaccine. 2015. http://www.gavi.org/Library/News/GAVI-features/2012/Nigeria-launches-pentavalent-vaccine/ Available at. Accessed June 24, 2015.
- 23.Bwogi J, Braka F, Makumbi I, Mishra V, Bakamutumaho B, Nanyunja M, Opio A, Downing R, Biryahwaho B, Lewis RF. Hepatitis B infection is highly endemic in Uganda: findings from a national serosurvey. Afr Health Sci. 2009;9:98–108. [PMC free article] [PubMed] [Google Scholar]
- 24.Center for Disease Control and Prevention . Chapter 4: Hepatitis B. In: Finelli L, Bell BP, editors. Manual for the Surveillance of Vaccine Preventable Diseases. 2012. www.cdc.gov/vaccines/pubs/surv-manual/chpt04-hepb.html Available at. Accessed July 7, 2012. [Google Scholar]
- 25.Osifo OD, Ovueni ME. Current views, level of acceptance, and practice of male circumcision in Africa subregion. Ann Pediatr Surg. 2009;5:254–260. [Google Scholar]
- 26.Abdur-Rahman LO, Musa OI, Oshagbemi GK. Community-based study of circumcision practices in Nigeria. Ann Trop Med Public Health. 2012;5:231–235. [Google Scholar]
- 27.Kiire CF. African Regional Study Group Hepatitis B infection in sub-Saharan Africa. Vaccine. 1990;8((Suppl 1)):107–112. doi: 10.1016/0264-410x(90)90229-f. [DOI] [PubMed] [Google Scholar]
- 28.Martinson FEA, Weigle KA, Royce RA. Risk factors for horizontal transmission of hepatitis B in a rural district in Ghana. Am J Epidemiol. 1998;147:478–487. doi: 10.1093/oxfordjournals.aje.a009474. [DOI] [PubMed] [Google Scholar]
- 29.Burnett RJ, Francois G, Kew MC. Hepatitis B virus and human immunodeficiency virus co-infection in sub Saharan Africa: a call for further investigation. Liver Int. 2005;25:201–213. doi: 10.1111/j.1478-3231.2005.01054.x. [DOI] [PubMed] [Google Scholar]
- 30.Vilibić-Cavlek T, Kucinar J, Ljubin-Sternak S, Kaić B, Lazarić-Stefanović L, Kolarić B. Prevalence of viral hepatitis in Croatian adult population undergoing routine check-up, 2010–2011. Cent Eur J Public Health. 2014;22:29–33. doi: 10.21101/cejph.a3844. [DOI] [PubMed] [Google Scholar]
- 31.Yamada H, Fujimoto M, Svay S, Lim O, Hok S, Goto N, Ohisa M, Akita T, Matsuo J, Do SH, Katayama K, Miyakawa Y, Tanaka J. Seroprevalence, genotypic distribution and potential risk factors of hepatitis B and C virus infections among adults in Siem Reap, Cambodia. Hepatol Res. 2014;45:480–487. doi: 10.1111/hepr.12367. [DOI] [PubMed] [Google Scholar]
- 32.Edris A, Nour MO, Zedan OO, Mansour AE, Ghandour AA, Omran T. Seroprevalence and risk factors for hepatitis B and C virus infection in Damietta Governorate, Egypt. East Mediterr Health J. 2014;20:605–613. [PubMed] [Google Scholar]
- 33.Aminu M, Okachi EE, Abubakar SM, Yahaya A. Prevalence of hepatitis B virus surface antigen among healthy asymptomatic students in a Nigerian University. Ann Afr Med. 2013;12:55–56. doi: 10.4103/1596-3519.108257. [DOI] [PubMed] [Google Scholar]
- 34.Eroglu C, Zivalioglu M, Esen S, Sunbul M, Leblebicioglu H. Detection of hepatitis B virus in used razor blades by PCR. Hepat Mon. 2010;10:22–25. [PMC free article] [PubMed] [Google Scholar]
- 35.Black AP, Nouanthong P, Nanthavong N, Souvannaso C, Vilivong K, Jutavijittum P, Samountry B, Lütteke N, Hübschen JM, Goossens S, Quet F, Buisson Y, Muller CP. Hepatitis B virus in the Lao People's Democratic Republic: a cross sectional serosurvey in different cohorts. BMC Infect Dis. 2014;14:457–460. doi: 10.1186/1471-2334-14-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen TT, McPhee SJ, Stewart S, Gildengorin G, Zhang L, Wong C. Factors associated with hepatitis B testing among Vietnamese Americans. J Gen Intern Med. 2010;25:694–700. doi: 10.1007/s11606-010-1285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogoina D, Pondei K, Adetunji B. Prevalence of hepatitis B vaccination among health care workers in Nigeria in 2011–12. Int J Occup Environ Med. 2014;5:51–56. [PMC free article] [PubMed] [Google Scholar]
- 38.Adebamowo CA, Odukogbe AA, Ajuwon AJ. Knowledge, attitude, and practices related to hepatitis B virus infection among Nigerian obstetricians and midwives. J Obstet Gynaecol. 1998;18:528–532. doi: 10.1080/01443619866255. [DOI] [PubMed] [Google Scholar]