Abstract
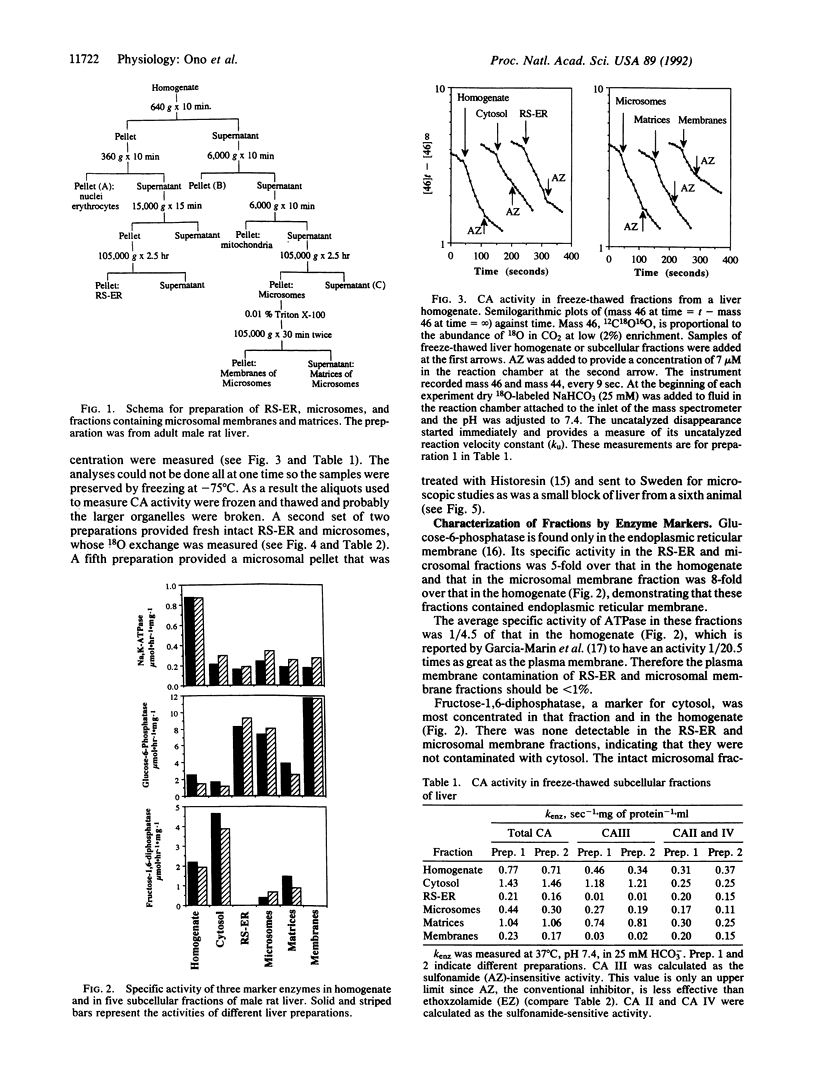
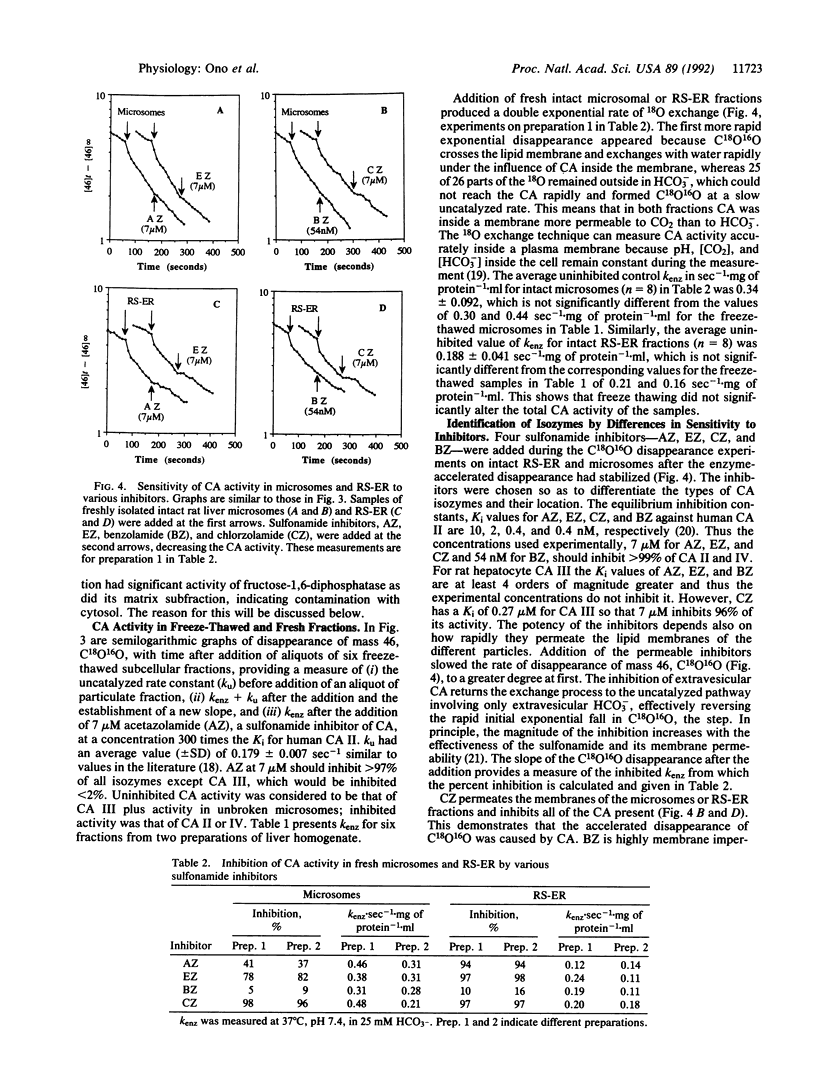
We have prepared subcellular fractions of male rat liver homogenate by the method of Lewis and Tata [Lewis, J. A. & Tata, J. R. (1973) J. Cell Sci. 23, 447-459], further purifying the membranes of the microsomal fraction by exposure to 0.01% Triton X-100 and centrifugation. We determined the purity of the fractions with marker enzymes and measured carbonic anhydrase (CA; EC 4.2.1.1) activity in intact and solubilized particulates with 18O exchange between CO2/HCO3- and water. We measured the concentration of CA by titration with a sulfonamide inhibitor, ethoxzolamide, obtaining an average value of 3.8 mumol/mg of microsomal membrane protein. The equilibrium constant for binding ethoxzolamide was 0.49 x 10(-9) M. The Km for CO2 was 1.7 mM and the turnover number was 560,000 sec-1, characterizing this as a membrane-bound, high-activity isozyme of type IV. By electron microscopy of tissue sections after staining with a cobalt precipitation technique, CA was seen in small cytoplasmic vesicles in hepatocytes and in microsomal particles and membranes. There was a sulfonamide-resistant (isozyme type III) and a sulfonamide-sensitive (isozyme type II) CA in the cytosol but none in the rapidly sedimenting endoplasmic reticulum. We conclude that there is no CA normally within the matrix of the cell endoplasmic reticulum but that the CA type III found in the microsome may have been captured from the cytosol during resealing. Thus the adult male rat hepatocyte contains CA type IV in the membrane of the endoplasmic reticulum and CA type II and CA type III in the cytoplasm.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dodgson S. J., Contino L. C. Rat kidney mitochondrial carbonic anhydrase. Arch Biochem Biophys. 1988 Jan;260(1):334–341. doi: 10.1016/0003-9861(88)90457-2. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Gros G., Krawiec J. A., Lin L., Bitterman N., Forster R. E. Comparison of 18O exchange and pH stop-flow assays for carbonic anhydrase. J Appl Physiol (1985) 1990 Jun;68(6):2443–2450. doi: 10.1152/jappl.1990.68.6.2443. [DOI] [PubMed] [Google Scholar]
- Garcia-Marin J. J., Perez-Barriocanal F., Garcia A., Serrano M. A., Regueiro P., Esteller A. Evidence for the presence of carbonic anhydrase in the plasma membrane of rat hepatocytes. Biochim Biophys Acta. 1988 Nov 3;945(1):17–22. doi: 10.1016/0005-2736(88)90357-4. [DOI] [PubMed] [Google Scholar]
- Gierow P., Jergil B. Heterogeneity of smooth endoplasmic reticulum from rat liver studied by two-phase partitioning. Biochem J. 1989 Aug 15;262(1):55–61. doi: 10.1042/bj2620055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson H. P. Histochemical demonstration of carbonic anhydrase activity. Histochemie. 1967;11(2):112–128. doi: 10.1007/BF00571716. [DOI] [PubMed] [Google Scholar]
- Itada N., Forster R. E. Carbonic anhydrase activity in intact red blood cells measured with 18O exchange. J Biol Chem. 1977 Jun 10;252(11):3881–3890. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurila A. L., Parvinen E. K., Slot J. W., Vänänen H. K. Consecutive expression of carbonic anhydrase isoenzymes during development of rat liver and skeletal muscle differentiation. J Histochem Cytochem. 1989 Sep;37(9):1375–1382. doi: 10.1177/37.9.2504813. [DOI] [PubMed] [Google Scholar]
- Lewis J. A., Tata J. R. A rapidly sedimenting fraction of rat liver endoplasmic reticulum. J Cell Sci. 1973 Sep;13(2):447–459. doi: 10.1242/jcs.13.2.447. [DOI] [PubMed] [Google Scholar]
- Maren T. H., Couto E. O. The nature of anion inhibition of human red cell carbonic anhydrases. Arch Biochem Biophys. 1979 Sep;196(2):501–510. doi: 10.1016/0003-9861(79)90302-3. [DOI] [PubMed] [Google Scholar]
- Nioka S., Henry R. P., Forster R. E. Total CA activity in isolated perfused guinea pig lung by 18O-exchange method. J Appl Physiol (1985) 1988 Nov;65(5):2236–2244. doi: 10.1152/jappl.1988.65.5.2236. [DOI] [PubMed] [Google Scholar]
- PALADE G. E. THE ORGANIZATION OF LIVING MATTER. Proc Natl Acad Sci U S A. 1964 Aug;52:613–634. doi: 10.1073/pnas.52.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderstråle Y. Intracellular localization of carbonic anhydrase in some vertebrate nephrons. Acta Physiol Scand Suppl. 1980;488:1–22. [PubMed] [Google Scholar]
- Ridderstråle Y. Intracellular localization of carbonic anhydrase in the frog nephron. Acta Physiol Scand. 1976 Dec;98(4):465–469. doi: 10.1111/j.1748-1716.1976.tb10337.x. [DOI] [PubMed] [Google Scholar]
- Sanyal G., Pessah N. I., Maren T. H. Kinetics and inhibition of membrane-bound carbonic anhydrase from canine renal cortex. Biochim Biophys Acta. 1981 Jan 15;657(1):128–137. doi: 10.1016/0005-2744(81)90136-4. [DOI] [PubMed] [Google Scholar]
- Sanyal G., Swenson E. R., Pessah N. I., Maren T. H. The carbon dioxide hydration activity of skeletal muscle carbonic anhydrase. Inhibition by sulfonamides and anions. Mol Pharmacol. 1982 Jul;22(1):211–220. [PubMed] [Google Scholar]
- Scharschmidt B. F., Keeffe E. B., Blankenship N. M., Ockner R. K. Validation of a recording spectrophotometric method for measurement of membrane-associated Mg- and NaK-ATPase activity. J Lab Clin Med. 1979 May;93(5):790–799. [PubMed] [Google Scholar]
- Zhu X. L., Sly W. S. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. J Biol Chem. 1990 May 25;265(15):8795–8801. [PubMed] [Google Scholar]



