Abstract
Histoplasmosis is common among persons living with human immunodeficiency virus/acquired immune deficiency syndrome (PLWHA) in Latin America, but its diagnosis is difficult and often nonspecific. We conducted prospective screening for histoplasmosis among PLWHA with signs or symptoms suggesting progressive disseminated histoplasmosis (PDH) and hospitalized in Hospital La María in Medellín, Colombia. The study's aim was to obtain a clinical and laboratory profile of PLWHA with PDH. During 3 years (May 2008 to August 2011), we identified 89 PLWHA hospitalized with symptoms suggestive of PDH, of whom 45 (51%) had histoplasmosis. We observed tuberculosis (TB) coinfection in a large proportion of patients with PDH (35%), so all analyses were performed adjusting for this coinfection and, alternatively, excluding histoplasmosis patients with TB. Results showed that the patients with PDH were more likely to have Karnofsky score ≤ 30 (prevalence ratio [PR] = 1.98, 95% confidence interval [CI] = 0.97–4.06), liver compromised with hepatomegaly and/or splenomegaly (PR = 1.77, CI = 1.03–3.06) and elevation in serum of alanine aminotransferase and aspartate aminotransferase to values > 40 mU/mL (PR = 2.06, CI = 1.09–3.88 and PR = 1.53, CI = 0.99–2.35, respectively). Using multiple correspondence analyses, we identified in patients with PDH a profile characterized by the presence of constitutional symptoms, namely weight loss and Karnofsky classification ≤ 30, gastrointestinal manifestations with alteration of liver enzymes and hepatosplenomegaly and/or splenomegaly, skin lesions, and hematological alterations. Study of the profiles is no substitute for laboratory diagnostics, but identifying clinical and laboratory indicators of PLWHA with PDH should allow development of strategies for reducing the time to diagnosis and thus mortality caused by Histoplasma capsulatum.
Introduction
Histoplasmosis, a disease caused by the dimorphic fungus Histoplasma capsulatum, is associated with high morbidity and mortality.1,2 Although histoplasmosis occurs globally, this mycosis is more frequently seen in the Americas and some regions of Asia and Africa.3 Despite advances in the treatment of persons living with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS)) (PLWHA), the incidence and mortality associated with H. capsulatum infection in this group remains high.1,4,5 In Colombia, the prevalence of HIV infection in the general population is less than 1%, but in populations at risk, this can rise to 5%. Official governmental data from Organización de las Naciones Unidas (ONU)/AIDS report that 32,864 of 37,325 (88%) patients diagnosed with HIV/AIDS are receiving highly active antiretroviral therapy.6 A study conducted by the Instituto Nacional de Salud of Colombia and the Corporación para Investigaciones Biológicas (CIB) identified 434 proven cases of the mycosis from 1992 to 2008, and 70% of these cases were among PLWHA.7 In a more recent study, the frequency of histoplasmosis in a cohort of PLWHA with clinical suspicion of histoplasmosis was estimated at 22%.8 In developing countries and in some regions of the United States that include resource-poor areas, high mortality rates are reported for PLWHA who have progressive disseminated histoplasmosis (PDH).9–20 For these reasons, health-care providers should be aware of the incidence of histoplasmosis in Colombia, and consider the possibility of this diagnosis in PLWHA patients with clinically compatible illness.
Diagnosis of PDH among PLWHA can be challenging.3,21 Symptoms and clinical manifestations of PDH are highly variable and similar to those produced by other infectious agents commonly affecting this population. Clinical suspicion of PDH is based on evaluating epidemiological risk factors and the presence of signs and symptoms in the patient, and on the use of laboratory tests with variable sensitivity/specificity such as direct examination, stains, culture, and immunological (antigen and antibody detection) and molecular tests, some of which are not widely available.5,8,21,22
Histoplasmosis is a common and important public health problem in these susceptible populations.9–20 The aim of this study was to identify a clinical and laboratory profile associated with histoplasmosis in PLWHA that could allow early suspicion of the disease, reducing the time to diagnosis and thus the disease's high mortality rate.
Materials and Methods
Study design.
From May 2008 to August 2011, we conducted a prospective study of PLWHA from one major hospital (Hospital La Maria) in Medellin, Colombia. To be included in the screening, PLWHA had to be hospitalized within the study period, had to be receiving no antifungal treatment when they enrolled in the study, and had to present with three of five of the following common indicators for suspicion of disseminated histoplasmosis: fever, pancytopenia, weight loss, radiological evidence of pulmonary involvement (interstitial infiltrate visible on chest X-ray), and skin or mucosal lesions. The diagnosis of histoplasmosis was made based on the recommendations of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG).23 A diagnosis was considered proven if the etiologic agent (H. capsulatum) could be isolated from any of the following samples: blood, tissue, sterile fluids, or respiratory specimens, or if the histopathological analyses showed presence of intracellular yeast compatible with H. capsulatum, identified by Wright staining. A diagnosis was considered probable if H. capsulatum could not be isolated, but there was serological evidence of infection, such as the presence of either H or M band or both bands by the immunodiffusion test and/or a titer of 1:32 or higher with histoplasmin antigen by the complement fixation test.23 Patients with a previous diagnosis of histoplasmosis were excluded from this report.
Diagnosis of tuberculosis (TB) was performed using conventional diagnostic tests such as auramine-rhodamine stain and Kinyoun stain and mycobacterial cultures BD Löwenstein–Jenssen, BD MGIT, and 7H11 thin layer agar.
This study was originally designed to evaluate the validity of an assay to detect histoplasmosis. We chose a cohort of PLWHA with high risk of having PDH to maximize our ability to diagnose histoplasmosis in this location.
Ethics statement.
This study was performed according to the terms agreed by and with the full approval of the ethical committees of the CIB and Hospital La María in Medellin, Colombia. All patients enrolled in this study signed an informed consent form designed in collaboration with the ethical committee of the CIB. All clinical information from the participants in the study was anonymized in a database using an alphanumerical code.
Data collection.
Basic demographic, laboratory (complete hemogram/complete blood count, hepatic profile, and indicators of HIV infection), and clinical information (constitutional, respiratory, gastrointestinal, and neuronal symptoms; presence of skin and oral lesions; and presence of lymphadenopathy) were collected for each patient enrolled. Hepatomegaly and splenomegaly were confirmed by physical examination using ultrasonography. Study personnel used a standardized data collection form to extract data from medical records. All data were entered into a Microsoft Access 2010 database (Microsoft Corp., Redmond, WA) for analysis.
Statistical analysis.
Absolute and relative frequencies were calculated and tested for normality. To identify differences in means or medians, we used the Student's t test or Mann–Whitney U test, as appropriate. Prevalence ratios (PR), crude and adjusted for the diagnosis of TB, and their respective 95% confidence intervals (CI) were calculated. We used multiple correspondence analysis (MCA)24 to evaluate the relationships among the selected variables and to obtain factors that best represent all variables, considering the level of significance (weight) of each, to explain total sample variability (inertia).25 All analyses were performed using the software EPIDAT 3.1 and STATA 11.0.
Results
Demographic characteristics.
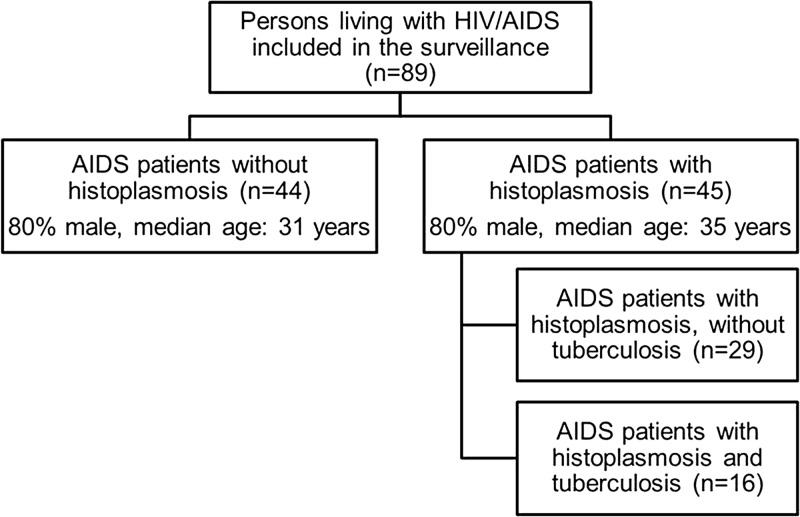
During 3 years of screening, we identified 89 PLWHA who met the inclusion criteria. Seventy-one were male (79%) and their median age was 33 years (interquartile range [IQR] = 51 years). A total of 45 patients met the EORTC/MSG criteria for PDH, of which 36 were male (80%) with a median age of 35 years (IQR = 34.7 years). Among patients without histoplasmosis (N = 44), 35 (80%) were male with a median age of 31 years (IQR = 51 years) (Figure 1 ). There were no significant differences in any demographics between these two groups (Table 1).
Figure 1.
Chart of study subjects grouped by histoplasmosis/tuberculosis status.
Table 1.
Characteristics of 89 AIDS patients with and without histoplasmosis
| Variable | With histoplasmosis (N = 45) | Without histoplasmosis (N = 44) | PR (95% CI) | P |
|---|---|---|---|---|
| Age*‡ | 35.3 (34.7) | 31.1 (51) | – | 0.980 |
| Sex (male)† | 36 (80) | 35 (79.5) | 1.00 (0.81–1.23) | 0.950 |
| Time of HIV diagnosis (years)*‡ | 0.7 (14.9) | 2.1 (18.2) | – | 0.370 |
| CD4 cell count*‡ | 30 (567) | 46 (564) | – | 0.200 |
| HIV viral load*‡ | 151,914 (6,678,500) | 127,000 (1,666,346) | – | 0.510 |
| Mortality† | 8 (17.7) | 6 (13.6) | 0.95 (0.79–1.13) | 0.590 |
| Diagnosis of TB† | 16 (35) | 24 (54) | 0.65 (0.40–1.05) | 0.070 |
AIDS = acquired immune deficiency syndrome; CI = confidence interval; HIV = human immunodeficiency virus; PR = prevalence ratio; TB = tuberculosis.
Data derived from Mann–Whitney U test.
Number (%).
Median (interquartile range).
Clinical and laboratory findings.
Most (67%) cases of histoplasmosis were proven cases, the median CD4 count was 30 cell/μL (range: 1–537), and the median HIV viral load was 151,914 copies/μL (range: 40–6,678,540) (Table 1). Most patients (87%) had weight loss at the time of enrollment, and 41% had a Karnofsky score ≤ 30. Gastrointestinal manifestations were the most common symptoms observed (84%), followed by pulmonary manifestations (76%), lymphadenopathy (58%), skin lesions (49%), and oral mucosal lesions (44%) (Table 2). Of the 45 patients with histoplasmosis, 16 (35%) also had a diagnosis of TB, and for that reason all analyses were adjusted for this common concomitant disease.
Table 2.
Clinical and laboratory findings in 89 AIDS patients with and without histoplasmosis, from La María Hospital in Medellín, Colombia
| Variable | Patients with histoplasmosis (N = 45) | Patients with histoplasmosis but without TB (N = 29) | Patients without histoplasmosis (N = 44) | PR (95% CI) adjusted for TB (N = 89) | P | PR (95% CI) excluding histoplamosis patients with TB (N = 73) | P |
|---|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||||
| Sex (male) | 36 (80) | 23 (79) | 35 (79) | 0.99 (0.60–1.62) | 0.864 | 1.01 (0.40–2.55) | 0.602 |
| Mortality | 8 (18) | 6 (21) | 6 (14) | 1.12 (0.69–1.83) | 0.818 | 1.15 (0.53–426) | 0.314 |
| Weight loss | 39 (87) | 25 (86) | 32 (73) | 1.18 (0.95–1.47) | 0.079 | 1.18 (1.51–0.92) | 0.141 |
| Karnofsky classification ≤ 30 | 18 (41) | 13 (46) | 8 (19) | 1.98 (0.97–4.06) | 0.047* | 2.43 (1.18–4.99) | 0.014*† |
| Gastrointestinal symptoms | 38 (84) | 23 (79) | 35 (79) | 1.07 (0.89–1.29) | 0.531 | 0.99 (0.78–1.26) | 0.602 |
| Abdominal pain | 14 (31) | 7 (24) | 14 (32) | 0.99 (0.54–1.83) | 0.942 | 0.75 (0.35–1.63) | 0.330 |
| Nausea | 16 (36) | 9 (31) | 6 (14) | 2.67 (1.15–6.19) | 0.015* | 2.27 (0.92–5.60) | 0.067 |
| Diarrhea | 22 (49) | 13 (45) | 26 (59) | 0.83 (0.56–1.21) | 0.348 | 0.75 (0.48–1.19) | 0.169 |
| Vomiting | 14 (31) | 11 (38) | 12 (27) | 1.07 (0.54–2.12) | 0.872 | 1.39 (0.70–2.74) | 0.240 |
| Hepatomegaly and/or splenomegaly | 24 (53) | 16 (55) | 13 (29) | 1.77 (1.03–3.06) | 0.030* | 1.86 (1.06–3.27) | 0.026*† |
| Skin lesions | 22 (49) | 16 (55) | 17 (39) | 1.10 (0.70–1.72) | 0.587 | 1.42 (0.86–2.36) | 0.125 |
| Ulcers | 10 (22) | 8 (27) | 8 (18) | 1.07 (0.46–2.47) | 0.868 | 1.51 (0.63–3.60) | 0.252 |
| Papules | 15 (33) | 13 (45) | 9 (20) | 1.46 (0.69–3.12) | 0.334 | 2.19 (1.09–4.40) | 0.025* |
| Crusted lesions | 15 (33) | 12 (41) | 8 (18) | 1.61 (0.76–3.41) | 0.203 | 2.27 (1.07–4.80) | 0.028* |
| Hyperpigmented lesions | 9 (20) | 6 (21) | 4 (9) | 1.85 (0.62–5.54) | 0.206 | 2.27 (0.72–7.19) | 0.144 |
| Hypopigmented lesions | 6 (13) | 4 (14) | 3 (7) | 1.25 (0.38–4.11) | 0.655 | 2.02 (0.49–8.23) | 0.275 |
| Oral lesions | 20 (44) | 12 (41) | 16 (36) | 1.18 (0.71–1.95) | 0.493 | 1.13 (0.62–2.05) | 0.425 |
| Tongue lesions | 4 (9) | 3 (10) | 3 (7) | 1.01 (0.21–4.72) | 0.980 | 1.51 (0.32–7.02) | 0.449 |
| Lips lesions | 2 (4) | 2 (7) | 4 (9) | 0.64 (0.15–2.70) | 0.545 | 0.75 (0.14–3.88) | 0.550 |
| Mucosal lesions | 16 (36) | 10 (34) | 9 (20) | 1.55 (0.77–3.10) | 0.161 | 1.68 (0.77–3.64) | 0.143 |
| Lymphadenopathy | 26 (58) | 17 (59) | 20 (45) | 1.26 (0.82–1.95) | 0.199 | 1.28 (0.81–2.03) | 0.194 |
| Neck lymph nodes | 22 (49) | 13 (45) | 16 (36) | 1.40 (0.85–2.30) | 0.171 | 1.23 (0.69–2.18) | 0.315 |
| Axillary lymph nodes | 7 (16) | 5 (17) | 7 (16) | 0.84 (0.32–2.17) | 0.758 | 1.08 (0.37–3.11) | 0.562 |
| Inguinal lymph nodes | 6 (13) | 4 (14) | 6 (14) | 0.95 (0.32–2.76) | 0.928 | 1.01 (0.30–3.30) | 0.621 |
| Epitrochlear | 1 (2) | 1 (3) | 2 (4) | 1.01 (0.15–6.90) | 0.986 | 0.75 (0.07–7.98) | 0.817 |
| Respiratory symptoms | 34 (76) | 23 (79) | 37 (84) | 0.90 (0.72–1.13) | 0.317 | 0.94 (0.75–1.17) | 0.412 |
| Cough | 31 (69) | 23 (79) | 34 (77) | 0.93 (0.70–1.24) | 0.311 | 1.02 (0.79–1.31) | 0.537 |
| Dyspnea | 16 (36) | 13 (45) | 22 (50) | 0.73 (0.43–1.24) | 0.117 | 0.89 (0.54–1.47) | 0.423 |
| Expectoration | 18 (40) | 15 (52) | 25 (57) | 0.76 (0.47–1.22) | 0.071 | 0.91 (0.59–1.40) | 0.425 |
| Rhonchi | 4 (9) | 4 (14) | 8 (18) | 0.53 (0.19–1.52) | 0.240 | 0.75 (0.25–2.28) | 0.437 |
| Crepitus | 9 (20) | 7 (24) | 13 (29) | 0.66 (0.30–1.45) | 0.255 | 0.81 (0.37–1.79) | 0.409 |
| Neurologic symptoms | 29 (64) | 16 (55) | 33 (75) | 0.91 (0.70–1.19) | 0.365 | 0.73 (0.52–1.03) | 0.066 |
| Headache | 19 (42) | 10 (34) | 21 (48) | 0.88 (0.56–1.40) | 0.600 | 0.72 (0.40–1.28) | 0.190 |
| Altered mental status | 6 (13) | 3 (10) | 7 (16) | 0.85 (0.31–2.33) | 0.742 | 0.65 (0.18–2.28) | 0.378 |
| Stiff neck | 3 (7) | 1 (3) | 2 (4) | 1.61 (0.27–9.39) | 0.566 | 0.76 (0.07–8.02) | 0.654 |
| Laboratory findings | |||||||
| Leukocyte counts < 4,000 cells/μL | 24 (53) | 14 (48) | 15 (35) | 1.31 (0.81–2.10) | 0.126 | 1.38 (0.78–2.43) | 0.186 |
| Platelet count < 100,000 cells/μL | 6 (13) | 2 (7) | 5 (11) | 1.36 (0.45–4.08) | 0.606 | 0.59 (0.12–2.79) | 0.407 |
| Hemoglobin concentration < 9 g/dL | 23 (51) | 13 (45) | 13 (30) | 1.01 (1.01–2.95) | 0.017* | 1.48 (0.80–2.74) | 0.155 |
| Bilirubin > 1 mg/100 mL | 4 (13) | 1 (6) | 7 (22) | 0.70 (0.23–2.12) | 0.540 | 0.27 (0.04–1.67) | 0.158 |
| LDH > 500 mU/mL | 20 (64) | 15 (75) | 15 (48) | 1.28 (0.75–2.17) | 0.292 | 1.55 (0.97–2.45) | 0.054 |
| AST > 40 mU/mL | 29 (70) | 18 (72) | 16 (45) | 1.53 (0.99–2.35) | 0.028* | 1.57 (1.01–2.45) | 0.038*† |
| ALT > 40 mU/mL | 23 (56) | 15 (60) | 9 (26) | 2.06 (1.09–3.88) | 0.015* | 2.33 (1,24–4.36) | 0.008*† |
| Alkaline phosphatase > 190 mU/mL | 19 (51) | 10 (45) | 10 (41) | 1.20 (0.69–2.10) | 0.294 | 1.09 (0.56–2.12) | 0.515 |
| CD4 cell counts below 50 cells/μL | 22 (63) | 12 (57) | 18 (53) | 1.16 (0.77–1.73) | 0.426 | 1.07 (0.65–1.77) | 0.490 |
AIDS = acquired immune deficiency syndrome; ALT = alanine aminotransferase; AST = aspartate aminotransferase concentration; CI = confidence interval; LDH = lactic dehydrogenase; PR = prevalence ratio; TB = tuberculosis .
Statistically significant difference (P < 0.05).
Both analyses show statistically significant differences (P < 0.05). Analysis without excluding or adjusting for TB gave similarly significant results: Karnofsky classification ≤ 30 (P = 0.027), hepatomegaly and/or splenomegaly (P = 0.022), AST > 40 mU/mL (P = 0.027), ALT > 40 mU/mL (P = 0.007).
For the clinical variables, the following adjusted PR values were higher in patients with histoplasmosis: lymphadenopathy (PR = 1.26, CI = 0.82–1.95), skin lesions (PR = 1.10, CI = 0.70–1.72), oral lesions (PR = 1.18, CI = 0.71–1.95), and gastrointestinal symptoms (PR = 1.07, CI = 0.89–1.29), and patients with histoplasmosis were less likely to have respiratory manifestations (PR = 0.90, CI = 0.72–1.13), but none of these differences were statistically significant (Table 2). However, when we analyzed the results in more detail, we observed several significant differences. In patients with histoplasmosis, nausea (PR = 2.67, CI = 1.15–6.19; P = 0.015), hepatomegaly and/or splenomegaly (PR = 1.77, CI = 1.03–3.06; P = 0.030), and the presence of a Karnofsky score ≤ 30 (PR = 1.98, CI = 0.97–4.06; P = 0.047) were more frequently observed (Table 2).
The most notable differences in laboratory variables distinguishing PLWHA with histoplasmosis were increases in both transaminases and a decrease in hemoglobin concentration. Aspartate aminotransferase (AST) concentration was significantly > 40 mU/mL in 70% of the histoplasmosis cases, versus 45% in patients without histoplasmosis; alanine aminotransferase (ALT) was significantly > 40 mU/mL in 56% of the cases, versus 26%; and hemoglobin was significantly less than 9 g/dL in 51% versus 30%. Differences for laboratory variables that did not reach significance included lactic dehydrogenase > 500 mU/mL in 64% of histoplasmosis patients versus 48%, and leukocyte counts < 4,000/μL in 53% versus 35%. Clinical and laboratory findings are summarized with their P values in Table 2.
To check robustness of the results, we also recalculated the PR after excluding patients with histoplasmosis and concomitant diagnosis of TB (N = 16). In this analysis, we compared the 29 patients who had histoplasmosis without TB with the 44 patients who did not have histoplasmosis. The results obtained in this analysis were similar to those observed above when we adjusted for TB, with some differences: significant differences were found for the presence of papules and crusted lesions (PR = 2.19, CI = 1.09–4.40 and PR = 2.27, CI = 1.07–4.80, respectively), but were no longer found for nausea (PR = 2.27, CI = 0.92–5.50) and hemoglobin concentration < 9 g/dL (PR = 1.48, CI = 0.80–2.74) (Table 2).
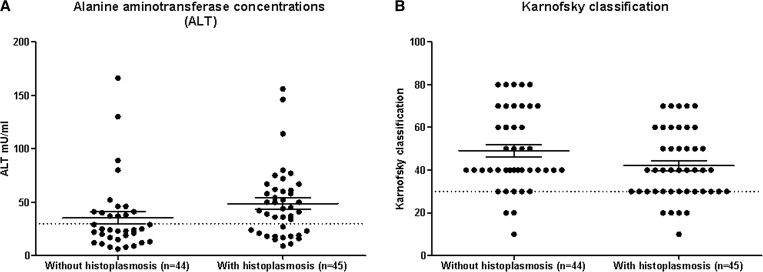
For two of the four robust indicators with differences in histoplasmosis patients, namely Karnofsky score ≤ 30 and ALT > 40 mU/mL, we also observed global distributional differences when we plotted the corresponding continuous variables. Figure 2 shows the plots for the full cohort including all patients with TB. Patients with histoplasmosis (N = 45) had higher ALT values than patients without histoplasmosis (N = 44; 44 versus 24 mU/mL mean difference; P = 0.012), suggesting more hepatic compromise. This result was not partly due to TB coinfection, since the patients with histoplasmosis actually had a lower proportion of TB diagnosis than patients without histoplasmosis (16/45 = 36% versus 24/44 = 55%; P = 0.112). Karnofsky score distributions showed that results were not sensitive to the choice of threshold at 30, and mean values were also not significantly different (P = 0.065; Figure 2).
Figure 2.
Distributions of values of (A) alanine aminotransferase concentration (ALT) and (B) Karnofsky score, in patients without and with histoplasmosis. Distributions are shown for all patients (with or without tuberculosis). Dotted lines indicate thresholds used in the analyses of this study.
Multivariate analysis.
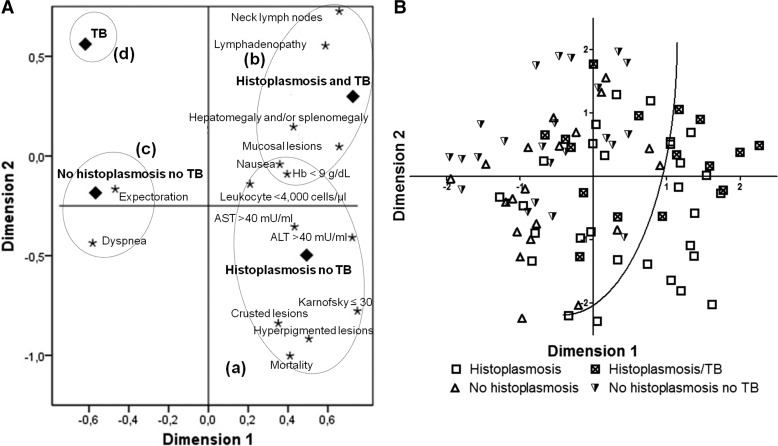
Using a two-dimensional MCA, we identified four patterns, based on clinical and laboratory characteristics (Figure 3A ), corresponding to the four main groups of patients: (a) with histoplasmosis and no TB (N = 29), (b) with both histoplasmosis and TB (N = 16), (c) patients without diagnosis of histoplasmosis without TB (N = 20), and (d) patients without diagnosis of histoplasmosis and with TB (N = 24). In the first pattern (a), we observed elevated values of liver enzymes (AST and ALT), the presence of skin lesions (hyperpigmented and crusted lesions), hematological disorders (anemia and leukopenia) and constitutional symptoms such as weight loss, and a Karnofsky score ≤ 30. The second pattern we observed (b), above the first pattern in the figure, was associated with the presence of lymph nodes, hepatomegaly and/or splenomegaly, and mucosal lesions. The third pattern observed (c), distant to the other two and corresponding to the group of patients without histoplasmosis, was characterized by the presence of respiratory symptoms (expectoration and dyspnea). Finally, the fourth pattern (d) was not associated with clinical and laboratory findings (Figure 3A).
Figure 3.
(A) Multiple correspondence analysis (MCA) showing patterns, based on clinical and laboratory characteristics, defining four main groups of patients: (a) patients with histoplasmosis and not tuberculosis (TB) (N = 29), (b) patients with histoplasmosis and TB (N = 16), (c) patients without diagnosis of histoplasmosis and without TB (N = 20), and (d) patients with TB (N = 24). The horizontal and vertical axes represent the first and the second principal components, respectively. (B) Scatter plot of patients' distribution according to the first two components identified by the MCA, after excluding mortality and diagnoses to retain only clinical indicators. The curve demarcates a region in the lower right part of the plot in which only patients with histoplasmosis are observed.
We used the same procedure, applied to all variables except mortality and the diagnosis information, to see how well the first two components of the MCA could distinguish the main groups (patients with histoplasmosis but no TB, histoplasmosis and TB, without histoplasmosis and TB, and patients without histoplasmosis with TB). The results are shown in Figure 3B. We also observed that the two robust indicators shown in Table 2 and illustrated in Figure 2, that is, ALT > 40 mU/mL and a Karnofsky score ≤ 30, were among the four largest contributors to the total inertia in the first component, and AST > 40 mU/mL was among the three largest contributors to the second component, showing concordance among the different analyses presented here. In both components, the two main contributing indicators were lymphadenopathy and palpable neck lymph nodes.
Discussion
The diagnosis of patients with histoplasmosis remains a challenge, especially among immunocompromised persons resident in resource-limited regions where HIV/AIDS is an important public health problem and histoplasmosis is endemic.26,27 To the best of our knowledge, this is the first prospective study in Latin America to describe and compare the clinical and laboratory findings among PLWHA with and without histoplasmosis. We described the clinical and laboratory profile we observed in patients with PDH. These patients were characterized by 1) presence of constitutional symptoms, in particular Karnofsky classification ≤ 30, 2) gastrointestinal alteration of liver enzymes (AST and ALT) and hepatosplenomegaly and/or splenomegaly, and 3) the presence of skin lesions. These findings were identified using bivariate and multivariate statistical methods.
Constitutional symptoms were more frequent in PLWHA with PDH, as shown by Karnofsky scores ≤ 30 at the time of enrollment, indicating that PDH patients were very ill at the time of hospital admission. This finding has not been reported in similar studies.
In our study, we also observed that a large proportion (84%) of PDH patients had gastrointestinal symptoms; other reports indicate much lower frequencies (ranging from 2% to 46%).9,11,28,29 Patients in our cohort had lower socioeconomic status than prior reports, and this finding may be related to economic inability to seek clinical care, and thereby preventing patients from presenting for care in a timely manner.
We found significantly higher levels of AST and ALT > 40 mU/mL, and higher presence of hepatosplenomegaly and/or splenomegaly among PDH cases than non-PDH cases. It is important to note that individuals presenting comorbidities with hepatitis B and C viruses and drug interactions were not observed in the study. Similar findings have also been reported in other studies,9,14,18,28,30 and high AST and ALT levels frequently correlate with hepatomegaly. Splenomegaly and hepatomegaly have been reported frequently among PDH patients (10–93%) in similar cohorts from Europe, United States, Panama, French Guyana, Brazil, and Argentina.9,10,12,13,18,28,30,31 The signs of hepatomegaly and/or splenomegaly are often nonspecific and are frequently present in PLWHA having other opportunistic coinfections, especially TB.32–36
When we excluded patients with histoplasmosis and TB, we observed a significant statistical association between the presence of skin lesions and the diagnosis of PDH. These lesions include ulcers, papules, crusted lesions, and hyperpigmented and hypopigmented lesions. A high proportion of skin manifestations had been previously reported in studies conducted in Latin American countries such as French Guyana (13%), Panama (17%), and Brazil (43–66%),9–11,14,18,30,31 in contrast to much lower rates reported among patients in the United States (1–7%).28,29,37 Karimi and others11 have proposed an association between these skin and mucosal lesions and genetic variations occurring in the etiological agent infecting the patient and suggest that more studies are needed to further evaluate this observation.
Hemoglobin abnormality (concentration < 9 g/dL) was more pronounced in PDH patients, before (P = 0.046) and after (P = 0.017) adjusting for TB. These alterations were present in 51% of patients with histoplasmosis. Our results are in agreement with a similar study in French Guyana where anemia was observed in 41% of PLWHA and PDH.14
The presence of lymphadenopathy, respiratory manifestations, and neurological symptoms were similar in patients with and without histoplasmosis. Similar findings were described in previous studies.9–11,14,18,30,31,34,35 The similarity between the two groups of patients in this respect could well be due to other diseases in the non-PDH patients affecting the respective organs (e.g., caused by Mycobacterium species, Pneumocystis jirovecii, Cryptococcus neoformans, and Toxoplasma gondii).
This report is subject to a limitation characteristic of hospital descriptive studies. This study was performed at a single hospital in Colombia (Hospital La María), and thus results are not generalizable. Nevertheless, this study highlights the importance of histoplasmosis as a neglected, opportunistic infection in PLWHA in Colombia, and is the first report to date describing PLWHA with and without PDH in Latin America. In addition, we pointed that diagnosis of histoplasmosis was performed by histopathology, fungal cultures, and serology; the Histoplasma antigen test is more sensitive for diagnosing PDH, but at the time of the study, it was not available in our laboratory.
In the cohort studied here, 51% had PDH, and one-third of the patients had concomitant TB, which can complicate diagnosis in any setting, and especially in resource-limited settings. We provide characteristic differences between PLWHA with and without PDH. Taking the identified clinical and laboratory profile of PLWHA into account may prompt clinicians to give particular attention to the possibility of concomitant histoplasmosis if they would otherwise not have done so. It is, however, important to emphasize that study of the profiles is certainly no substitute for laboratory diagnostics. Proper diagnostic procedures should preferably be done whenever there is any suspicion of histoplasmosis and/or TB, regardless of profile studies and their outcomes. The use of specific and rapid diagnosis procedures such as fungal cultures, histopathologic testing, serological tests (for antibody and antigen detection), and molecular tests should ensure prompt diagnosis, reduce the time to treatment, and thus impact mortality.
ACKNOWLEDGMENTS
We thank the medical staff of La Maria Hospital, Carlos A. Agudelo, Carlos A. Restrepo, Diego A. Molina, and Carolina Muñoz. We also express our thanks to Lucia Correa, Fernando Bedoya, Alejandra Medina, Federico Rodriguez, Luisa Orozco, and Juliana Marin from the same institution. Our thanks also to the laboratory diagnosis staff of the Medical and Experimental Mycology group (CIB), Catalina de Bedout, Alejandra Zuluaga, Karen Arango, and Angela Tabares. We also thank Blanca Samayoa (Clinica Familiar Luis ÁngelGarcía, Guatemala City, Guatemala) and Oliver K. Clay (Universidad del Rosario) for critical reading of the manuscript.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Financial support: This work was supported in part by the Global Disease Detection Program, Centers for Disease Control and Prevention, Atlanta, GA; Colciencias, Bogotá, Colombia, via the program for young investigators and innovators Virginia Gutierrez de Pineda; the Corporacioìn para InvestigacionesBioloìgicas (CIB), Medellín, Colombia; and the Fondo de Investigaciones de la Universidad del Rosario (FIUR), Bogota, Colombia.
Authors' addresses: Diego H. Caceres, Medical and Experimental Mycology Group, Corporación para Investigaciones Biológicas (CIB), Medellin, Colombia, and School of Medicine, Universidad CES, Medellín, Colombia, E-mail: diegocaceres84@gmail.com. Angela M. Tobón, Medical and Experimental Mycology Group, Corporación para Investigaciones Biológicas, Medellín, Colombia, and Hospital La María, Medellín, Colombia, E-mail: atobon@cib.org.co. Angela Ahlquist Cleveland, Christina M. Scheel, Mary E. Brandt, and Tom Chiller, Mycotic Diseases Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: ara0@cdc.gov, zsr3@cdc.gov, mbb4@cdc.gov, and tnc3@cdc.gov. Dedsy Y. Berbesi, School of Medicine, Universidad CES, Medellín, Colombia, E-mail: dberbesi@ces.edu.co. Jesús Ochoa, Facultad Nacional de Salud Pública, Universidad de Antioquia, Medellín, Colombia, E-mail: jochoa@saludpublica.udea.edu.co. Angela Restrepo, Medical and Experimental Mycology, Corporación Investigaciones Biológicas (CIB), Medellín, Colombia, E-mail: angelares@une.net.co. Beatriz L. Gómez, School of Medicine and Health Sciences, Universidad del Rosario, Bogota, Colombia, and Medical and Experimental Mycology Group, Corporación para Investigaciones Biológicas (CIB), Medellin, Colombia, E-mail: beatrizlgomez@hotmail.com.
References
- 1.Colombo AL, Tobon A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of endemic systemic fungal infections in Latin America. Med Mycol. 2011;49:785–798. doi: 10.3109/13693786.2011.577821. [DOI] [PubMed] [Google Scholar]
- 2.Knox KS, Hage CA. Histoplasmosis. Proc Am Thorac Soc. 2010;7:169–172. doi: 10.1513/pats.200907-069AL. [DOI] [PubMed] [Google Scholar]
- 3.Deepe GS. In: Principles and Practice of Infectious Diseases. Bennett JE, Dolin R, Blaser MJ, editors. Philadelphia, PA: Elsevier; 2015. pp. 2949–2962. (Histoplasma capsulatum). [Google Scholar]
- 4.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53. doi: 10.3109/10408410903241444. [DOI] [PubMed] [Google Scholar]
- 5.Scheel CM, Gómez BL. Diagnostic methods for histoplasmosis: focus on endemic countries with variable infrastructure levels. Curr Trop Med Rep. 2014;1:129–137. doi: 10.1007/s40475-014-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaviria A, Muñoz NJ, Ruiz F, Burgos G, Osorio EJ, Ospina ML. Informe GARPR–2014: Seguimiento de la Declaración de compromiso sobre el VIH/sida. 2014. http://www.unaids.org/sites/default/files/country/documents//file%2C94471%2Ces.pdf Available at. Accessed April 11, 2014.
- 7.Arango M, Castañeda E, Agudelo CI, De Bedout C, Agudelo CA, Tobón A, Linares M, Valencia Y, Restrepo A. Colombian Histoplasmosis Study Group Histoplasmosis: results of the Colombian National Survey, 1992–2008. Biomédica. 2011;31:344–356. doi: 10.1590/S0120-41572011000300007. [DOI] [PubMed] [Google Scholar]
- 8.Caceres DH, Zuluaga A, Arango-Bustamante K, de Bedout C, Tobón ÁM, Restrepo Á, Gómez BL, Cano LE, González Á. Implementation of a training course increased the diagnosis of histoplasmosis in Colombia. Am J Trop Med Hyg. 2015;93:662–667. doi: 10.4269/ajtmh.15-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huber F, Nacher M, Aznar C, Pierre-Demar M, El Guedj M, Vaz T, Vantilcke V, Mahamat A, Magnien C, Chauvet E, Carme B, Couppié P. AIDS-related Histoplasma capsulatum var. capsulatum infection: 25 years experience of French Guiana. AIDS. 2008;22:1047–1053. doi: 10.1097/QAD.0b013e3282ffde67. [DOI] [PubMed] [Google Scholar]
- 10.Mora DJ, dos Santos CT, Silva-Vergara ML. Disseminated histoplasmosis in acquired immunodeficiency syndrome patients in Uberaba, MG, Brazil. Mycoses. 2008;51:136–140. doi: 10.1111/j.1439-0507.2007.01459.x. [DOI] [PubMed] [Google Scholar]
- 11.Karimi K, Wheat LJ, Connolly P, Cloud G, Hajjeh R, Wheat E, Alves K, Lacaz Cd Cda S, Keath E. Differences in histoplasmosis in patients with acquired immunodeficiency syndrome in the United States and Brazil. J Infect Dis. 2002;186:1655–1660. doi: 10.1086/345724. [DOI] [PubMed] [Google Scholar]
- 12.Pietrobon D, Negro-Marquinez L, Kilstein J, Galindez J, Greca A, Battagliotti C. Disseminated histoplasmosis and AIDS in an Argentine hospital: clinical manifestations, diagnosis and treatment. Enferm Infecc Microbiol Clin. 2004;22:156–159. doi: 10.1016/s0213-005x(04)73056-6. [DOI] [PubMed] [Google Scholar]
- 13.Corti ME, Negroni R, Esquivel P, Villafañe MF. Histoplasmosis diseminada en pacientes con SIDA: análisis epidemiológico, clínico, microbiológico e inmunológico de 26 pacientes. Enf Emerg. 2004;6:8–15. [PubMed] [Google Scholar]
- 14.Couppié P, Sobesky M, Aznar C, Bichat S, Clyti E, Bissuel F, El Guedj M, Alvarez F, Demar M, Louvel D, Pradinaud R, Carme B. Histoplasmosis and acquired immunodeficiency syndrome: a study of prognostic factors. Clin Infect Dis. 2004;38:134–138. doi: 10.1086/379770. [DOI] [PubMed] [Google Scholar]
- 15.Scheel CM, Samayoa B, Herrera A, Lindsley MD, Benjamin L, Reed Y, Hart J, Lima S, Rivera BE, Raxcaco G, Chiller T, Arathoon E, Gómez BL. Development and evaluation of an enzyme-linked immunosorbent assay to detect Histoplasma capsulatum antigenuria in immunocompromised patients. Clin Vaccine Immunol. 2009;16:852–858. doi: 10.1128/CVI.00066-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang MR, Taira CL, Paniago AM, Taira DL, Cunha RV, Wanke B. Study of 30 cases of histoplasmosis observed in the Mato Grosso do Sul State, Brazil. Rev Inst Med Trop Sao Paulo. 2007;49:37–39. doi: 10.1590/s0036-46652007000100007. [DOI] [PubMed] [Google Scholar]
- 17.de Francesco Daher E, de Sousa Barros FA, da Silva Júnior GB, Takeda CF, Mota RM, Ferreira MT, Martins JC, Oliveira SA, Gutiérrez-Adrianzén OA. Risk factors for death in acquired immunodeficiency syndrome-associated disseminated histoplasmosis. Am J Trop Med Hyg. 2006;74:600–603. [PubMed] [Google Scholar]
- 18.Gutierrez ME, Canton A, Sosa N, Puga E, Talavera L. Disseminated histoplasmosis in patients with AIDS in Panama: a review of 104 cases. Clin Infect Dis. 2005;40:1199–1202. doi: 10.1086/428842. [DOI] [PubMed] [Google Scholar]
- 19.Velásquez Uribe G, Rueda ZV, Vélez LA, Aguirre DC, Gómez-Arias RD. Histoplasmosis in AIDS patients: a cohort study in Medellín, Colombia. Infectio. 2010;14:s99–s106. [Google Scholar]
- 20.Cáceres DH, Gómez BL, Restrepo Á, Tobón ÁM. Histoplasmosis y sida: factores de riesgo clínico y de laboratorio asociados al pronóstico de la enfermedad. Infectio Supl. 2012;3:44–50. [Google Scholar]
- 21.Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007;20:115–132. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson GR, Gómez BL. Histoplasma, blastomyces, coccidioides, and other dimorphic fungi causing systemic mycoses. In: Jorgensen JH, Pfaller MA, Carroll KC, Landry ML, Funke G, Richter SS, Warnock DW, editors. Manual of Clinical Microbiology. 11th. Washington, DC: ASM Press; 2015. pp. 2109–2127. Chapter 122. [Google Scholar]
- 23.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Muñoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE. European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group, National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briand S, Beresniak A, Nguyen T, Yonli T, Duru G, Kambire C, Perea W. Yellow Fever Risk Assessment Group (YF-RAG) Assessment of yellow fever epidemic risk: an original multi-criteria modeling approach. PLoS Negl Trop Dis. 2009;14:e483. doi: 10.1371/journal.pntd.0000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno ES, Barata Rde C. Methodology for definition of yellow fever priority areas, based on environmental variables and multiple correspondence analyses. PLoS Negl Trop Dis. 2012;6:e1658. doi: 10.1371/journal.pntd.0001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nacher M, Adenis A, Mc Donald S, Do Socorro Mendonca Gomes M, Singh S, Lopes Lima I, Malcher Leite R, Hermelijn S, Wongsokarijo M, Van Eer M, Marques Da Silva S, Mesquita Da Costa M, Silva M, Calvacante M, do Menino Jesus Silva Leitao T, Gómez BL, Restrepo A, Tobon A, Canteros CE, Aznar C, Blanchet D, Vantilcke V, Vautrin C, Boukhari R, Chiller T, Scheel C, Ahlquist A, Roy M, Lortholary O, Carme B, Couppié P, Vreden S. Disseminated histoplasmosis in HIV-infected patients in South America: a neglected killer continues on its rampage. PLoS Negl Trop Dis. 2013;7:e2319. doi: 10.1371/journal.pntd.0002319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adenis AA, Aznar C, Couppié P. Histoplasmosis in HIV-infected patients: a review of new developments and remaining gaps. Curr Trop Med Rep. 2014;28:119–128. doi: 10.1007/s40475-014-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baddley JW, Sankara IR, Rodriquez JM, Pappas PG, Many WJ., Jr Histoplasmosis in HIV-infected patients in a southern regional medical center: poor prognosis in the era of highly active antiretroviral therapy. Diagn Microbiol Infect Dis. 2008;62:151–156. doi: 10.1016/j.diagmicrobio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 29.Wheat LJ, Chetchotisakd P, Williams B, Connolly P, Shutt K, Hajjeh R. Factors associated with severe manifestations of histoplasmosis in AIDS. Clin Infect Dis. 2000;30:877–881. doi: 10.1086/313824. [DOI] [PubMed] [Google Scholar]
- 30.Daher EF, Silva GB, Jr, Barros FA, Takeda CF, Mota RM, Ferreira MT, Oliveira SA, Martins JC, Araújo SM, Gutiérrez-Adrianzén OA. Clinical and laboratory features of disseminated histoplasmosis in HIV patients from Brazil. Trop Med Int Health. 2007;12:1108–1115. doi: 10.1111/j.1365-3156.2007.01894.x. [DOI] [PubMed] [Google Scholar]
- 31.Casotti JA, Motta TQ, Ferreira CU, Jr, Cerutti C., Jr Disseminated histoplasmosis in HIV positive patients in Espirito Santo state, Brazil: a clinical-laboratory study of 12 cases (1999–2001) Braz J Infect Dis. 2006;10:327–330. doi: 10.1590/s1413-86702006000500005. [DOI] [PubMed] [Google Scholar]
- 32.Antinori S, Magni C, Nebuloni M, Parravicini C, Corbellino M, Sollima S, Galimberti L, Ridolfo AL, Wheat LJ. Histoplasmosis among human immunodeficiency virus-infected people in Europe: report of 4 cases and review of the literature. Medicine (Baltimore) 2006;85:22–36. doi: 10.1097/01.md.0000199934.38120.d4. [DOI] [PubMed] [Google Scholar]
- 33.Agudelo CA, Restrepo CA, Molina DA, Tobón AM, Kauffman CA, Murillo C, Restrepo A. Tuberculosis and histoplasmosis co-infection in AIDS patients. Am J Trop Med Hyg. 2012;87:1094–1098. doi: 10.4269/ajtmh.2012.12-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adenis A, Nacher M, Hanf M, Basurko C, Dufour J, Huber F, Aznar C, Carme B, Couppie P. Tuberculosis and histoplasmosis among human immunodeficiency virus-infected patients: a comparative study. Am J Trop Med Hyg. 2014;90:216–223. doi: 10.4269/ajtmh.13-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nacher M, Adenis A, Sambourg E, Huber F, Abboud P, Epelboin L, Mosnier E, Vantilcke V, Dufour J, Djossou F, Demar M, Couppié P. Histoplasmosis or tuberculosis in HIV-infected patients in the amazon: what should be treated first? PLoS Negl Trop Dis. 2014;8:e3290. doi: 10.1371/journal.pntd.0003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sterling TR, Chaisson RE. General clinical manifestations of human immunodeficiency virus infection (including the acute retroviral syndrome and oral cutaneous, renal, ocular, metabolic and cardiac diseases) In: Mandel GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 2010. pp. 1705–1725. [Google Scholar]
- 37.Hajjeh RA, Pappas PG, Henderson H, Lancaster D, Bamberger DM, Skahan KJ, Phelan MA, Cloud G, Holloway M, Kauffman CA, Wheat LJ. National Institute of Allergy and Infectious Diseases Mycoses Study Group Multicenter case-control study of risk factors for histoplasmosis in human immunodeficiency virus-infected persons. Clin Infect Dis. 2001;32:1215–1220. doi: 10.1086/319756. [DOI] [PubMed] [Google Scholar]