Abstract
Inflammation has been associated with cardiovascular disease and other health outcomes in children and adults, yet few longitudinal data are available on prevalence and predictors of inflammation in infants. We aimed to identify the prevalence of inflammation in a cohort of Bolivian infants and estimate its association with acute (recent illnesses) and chronic (overweight, stunting) morbidities and potential pathogen exposure (represented by water, sanitation, and hygiene [WASH] resources). We measured plasma concentrations of two acute phase proteins (C-reactive protein [CRP], marking acute inflammation, and alpha(1)-acid-glycoprotein [AGP], marking chronic inflammation) at three time points (target 2, 6–8, and 12–18 months). Of 451 singleton infants enrolled in the parent study, 272 had the first blood draw and complete data. Anthropometry and sociodemographic and recent illness data (2-week recall of cough, diarrhea, and fever) were collected at each visit. Inflammation was defined as CRP > 5 mg/L or AGP > 1 g/L. The prevalence of inflammation increased from early infancy (3% at first blood draw) to later infancy (15–22% at later blood draws). Recent cough, recent fever, and age in months were significantly associated with relative increases in CRP (7–44%) and AGP (5–23%), whereas recent diarrhea was only significantly associated with an increase in CRP (48%). Neither anthropometry nor WASH was significantly associated with inflammation. Results confirm the role of recent acute illness in inflammation in infants, and indicate that adiposity and WASH are not as important to inflammation in this age category.
Introduction
Inflammation has been associated with numerous adverse health outcomes ranging from cardiovascular disease1–3 to psychiatric and mood disorders,4,5 to linear growth failure,6 and potentially even some cancers.7,8 Inflammation is often described by the acute phase response (APR), a process activated by the body in response to stress, trauma, or infection. The APR is triggered by cytokines such as interleukin-1 and interleukin-6, which in turn induce the liver to produce C-reactive protein (CRP), alpha(1)-acid glycoprotein (AGP), and other proteins that can be measured in serum. CRP rises rapidly (within 1–2 days) and remains elevated for about 1week after symptom resolution, whereas AGP rises more slowly (after 4–5 days) and remains elevated for several weeks.9 Although this response resolves within 1–2 weeks in a healthy system, it can remain activated in situations of chronic stress or repeated immunological insult.10,11 Further, in minor illnesses or in cases where the body's immune response is particularly robust, this inflammatory process may be subclinical and not manifest overt symptoms of illness such as fever; however, it can still affect nutritional biomarkers and other health outcomes.12 For example, inflammation can contribute to invalid nutrition measurements given that multiple biomarkers of micronutrient status (e.g., ferritin, retinol) are affected by inflammation.9,12 Since various stimuli may cause clinical or subclinical inflammation, inflammatory status cannot be predicted simply by the presence or absence of recent infection or trauma.
Various studies have sought to assess different correlates of inflammation, to better understand the role of acute as well as chronic exposures in this process, and identify points of intervention so as to prevent harmful consequences of inflammation. Adiposity is one known correlate of inflammation, and it is thought that adipose tissue is in fact proinflammatory; this association has been demonstrated in both children and adults.13–15 Socioeconomic status has also been frequently studied as a potential correlate of inflammation, with measures of increasing household wealth typically associated with reduced likelihood of elevated CRP.16–19 Indeveloping-country settings, models of inflammation also often incorporate potential sources of pathogen exposure (and thus, infection), often represented by access to water, sanitation, and hygiene (WASH) resources such as where the family obtains water (and whether it is treated), the type of sanitation facilities, and the general cleanliness level of the house and surrounding area, with mixed findings.16,18–20 Indoor and outdoor pollutants have also been associated with inflammation in children and adults.20–22
Much research on correlates of inflammation has taken place in developed-country settings, which may not only have a different distribution of inflammation and risk factors, but also potentially a different relationship of risk factors to inflammation.19 Furthermore, some studies have suggested that prevalence and correlates of inflammation may differ by sex as well as by age.16,18,19,23,24 Although a number of studies have examined inflammation in schoolchildren, and others have described extreme states such as sepsis in neonates, very few studies have been published on prevalence or correlates of inflammation in healthy infants in community settings, particularly in developing countries where inflammation may be prevalent.16,25 Nonetheless, this is an important population to study given the potential adverse consequences of inflammation and immune dysregulation even in infants.26,27
The present study leverages data from a longitudinal birth cohort of Bolivian infants to identify the prevalence and correlates of inflammation in young infants and examine how these may change with increasing infant age. Bolivia, as a lower-middle-income country in the midst of the epidemiologic transition and suffering the “double burden” of malnutrition and overweight, is a location well suited to studying varied contributors to inflammation.28 This work will focus on the role of recent illness, anthropometry, and pathogenic exposures (represented by access to WASH resources); these factors have been shown to be correlated with inflammation in adults and schoolchildren, but there are very few data in infants. The present work will help to elucidate different inflammatory factors in a community population of healthy infants in a developing-country urban setting. Furthermore, it will add to knowledge regarding correlates of inflammation in infants, whose innate immune function differs from that of adults.29 Also, nearly all studies of correlates of inflammation have only taken into account CRP, a marker of the earlier stages of inflammation. In the present study, we also add AGP, a marker of later stages of inflammation, as a way to better understand how correlates may differ by acute versus chronic inflammatory processes.
Methods
Study population and design.
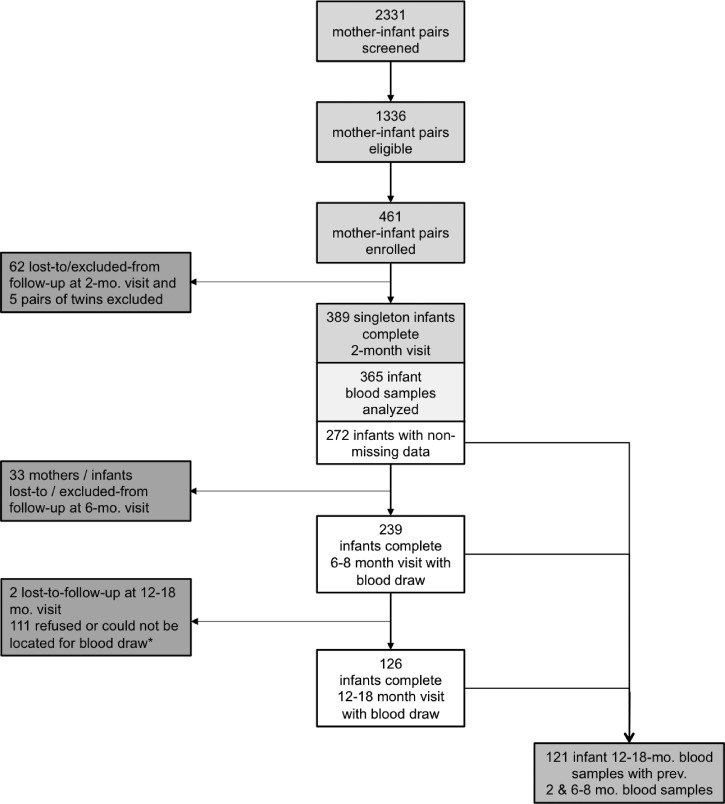
Data for the present study were drawn from the Nutrición, Inmunología, y Diarrea Infantil study, the primary aim of which was to assess the effect of nutritional status on response to the rotavirus vaccine. For this reason, all enrolled infants were required to receive both doses of the rotavirus vaccine (scheduled at 2 and 4 months of age). In brief, 461 healthy infants (2–4 weeks of age) and their mothers were recruited from two hospitals in El Alto, Bolivia (altitude 4,000 m), during well-child or vaccination visits. This is a primarily urban and largely indigenous population; although most have access to sanitation and improved water, socioeconomic resources are typically low. Exclusion criteria included infant illness at recruitment, infants suspected to have immunodeficiency (e.g., human immunodeficiency virus), congenital malformations, and maternal inability to speak and understand Spanish or Aymara. Recruitment took place from May 2013 to March 2014, and infant–mother dyads were followed for 12–18 months, with final data collected in March 2015. The study comprised seven hospital visits and two in-home visits, with blood drawn from mothers at a target schedule of 1 and 6–8 months postpartum, and from infants at a target schedule of 2, 6–8, and (optionally) 12–18 months of age. However, despite interviewer efforts, some blood draws fell outside the target ranges due to participant family travel or faulty contact information. The third blood draw, added to support a newly funded secondary aim, was approved only after 50% of infants had already completed the study; these mothers were contacted to participate optionally in an extra visit and blood draw. Although infants were required to be healthy at the first visit (0–2 months of age), no infants were excluded from blood draws based on concurrent or recent illness. For some infants, the first blood draw coincided with Rotarix® vaccination, but no association with inflammatory status was detected. For the present study, only singleton infants who had completed at least the first blood draw (N = 365) were eligible, and of these, 272 had nonmissing data for exposures and outcomes (Figure 1 ).
Figure 1.
Of 2,331 screened mother–infant pairs, 1,336 were eligible and 461 enrolled. A total of 365 singleton infants provided samples at 2months, but only 272 were included in the present analysis due to missing data. Of these, 239 had blood drawn at 6–8 months, and 126 at 12–18months of age. There were 121 infants with samples at all three time points. *Third blood draw was added after a secondary aim was funded, see Methods section.
Ethical approval.
The protocol and instruments for this study were approved by the Emory University Institutional Review Board (IRB00056127) and the Bolivian “Comité de Etica de la Investigación” (Research Ethics Committee). Mothers provided written informed consent in Spanish or Aymara.
Laboratory analysis and definitions of inflammation.
Venous blood was collected by trained phlebotomists using sterile, disposable equipment. Blood was drawn into zinc-free microtainers using butterfly needles, and stored at 2–8°C before transport later that day to our partner laboratory in La Paz, Bolivia. Samples were then frozen before shipping to Emory, where they were aliquoted and shipped for further analyses. Plasma was analyzed by sandwich enzyme-linked immunosorbent assay for CRP (a marker of inflammation; limit of detection [LOD] = 0.5 mg/L) and AGP (a marker of inflammation; LOD = 0.1 g/L).30 Quality control values for CRP and AGP indicated high quality at lower (CRP < 0.1 mg/L, AGP < 0.1 g/L) and higher (CRP up to 45 mg/L, AGP up to 2.5 g/L) ranges.
Data collection.
Sociodemographic data were collected by trained Bolivian interviewers at the first study visit via questionnaire. At each visit, interviewers collected data on maternal report of infant morbidities and feeding practices over the 2 weeks before the interview. Anthropometry was conducted by a two-person team of trained interviewers. Infants were weighed nude or with light clothing (no diaper) on a Seca scale (weight measured to the nearest 0.1kg), and measured on a ShorrBoard® (length measured to the nearest 0.1 cm). Weight-for-length and length-for-age Z-scores (WLZ and LAZ, respectively) were calculated based on World Health Organization (WHO) references andusing the WHO SAS macro.31 In the field, stunted (LAZ < −2) and wasted (WLZ < −2) infants were identified by study staff according to WHO growth charts and were referred as appropriate.
Variable definitions for outcomes, exposures, and covariates.
Inflammation was defined as CRP > 5 mg/L or AGP > 1 g/L.9,32 As described above, acute illness, anthropometry, and WASH resources were considered as primary exposures of interest. Acute illnesses were defined as positive 2-week maternal recall of infant symptoms; diarrhea, fever, and respiratory symptoms were included in each model as indicator variables. Anthropometric variables included overweight or obesity (WLZ > 1) and stunting (LAZ < −2).33 Several measures of WASH resources (a proxy of pathogenic exposure) were considered: sewer type (piped versus other, e.g., pit latrine), private toilet (versus shared or no access), water source (piped indoors versus other, e.g., piped outdoors), water treatment (any versus none), trash disposal (picked up versus other, e.g., thrown onto patio), crowding (> 2 people sleeping per bedroom). Potential covariates included preterm birth (< 37 completed gestational weeks), caesarian section (versus vaginal) birth, months of exclusive breastfeeding completed at the time of visit (defined as months during which infant had received only breast milk, with no other liquids or solids), maternal employment, maternal education (categorized as primary or less, at least some secondary, or at least some superior [reference]), and sample-specific wealth index (created via principal components analysis of assets and house materials, divided into quintiles with the highest quintile as reference34). Cow's milk intake was considered for the models, but could not be included due to low prevalence (0% at 2 months, though 14–30% at later visits). Concurrent or recent vaccination was not considered as a confounder, given its potential role as an intermediate between age and inflammation.
Statistical methods.
Given that the same infants were followed over time and contributed multiple measurements, methods appropriate for clustered data were applied. AGP and CRP were considered first separately and as continuous variables, and then as categorical variables (elevated/nonelevated), and finally together (dichotomized inflammation defined as either elevated AGP or CRP). Potential confounders of the relationships of acute illnesses, sanitation, and obesity, to AGP and CRP were selected a priori based on literature review and directed acyclic graph analysis, and were then included in the initial models based on significant bivariate association with the exposure or the outcome.
Continuous AGP and CRP were log transformed (base 10) to meet normality assumptions, and linear models were used to test relationships of predictors to the outcome; percent relative changes in the outcome are reported for each predictor. Percent relative change was calculated as 100 × (10β − 1), where β is the coefficient associated with predictor X and thus 10β is equal to the ratio of E(Y | X = 1) to E(Y | X = 0). For categorized AGP and CRP, logistic regression was used. In each case, mixed models were first applied, and a random intercept was included if significant (approximate likelihood ratio test). If nonsignificant, generalized estimating equations (GEE) were fit using an appropriate distribution (binomial for dichotomized outcomes) and an exchangeable correlation structure. Interactions of acute illnesses with time were tested using likelihood ratio tests (with maximum likelihood estimates) for mixed models and Wald tests for GEE models, and P < 0.05 was considered significant. Collinearity was assessed for each model using condition indices and variance decomposition proportions, and models reduced as necessary until collinearity was no longer present.35 Final linear mixed models used restricted maximum likelihood estimation and were fit using the lme4 package in the R Environment for Statistical Computing (R Core Team, Vienna, Austria).36,37 Marginal R2 (reflecting the proportion of variance explained by fixed factors) and conditional R2 (reflecting the proportion of variance explained by fixed and random factors) were calculated for linear mixed models using the piecewise SEM R package (http://arxiv.org/abs/1509.01845).38,39 Fixed effects were tested using F tests with the Satterthwaite approximation for denominator degrees of freedom. GEE models (http://CRAN.R-project.org/package=gee) were fit using the R gee package,40 and Wald tests based on robust variances are presented for fixed effects. Data were cleaned and analyzed using SAS v9.4 (Cary, NC) and the R Environment for Statistical Computing.36
Results
Characteristics of the study sample.
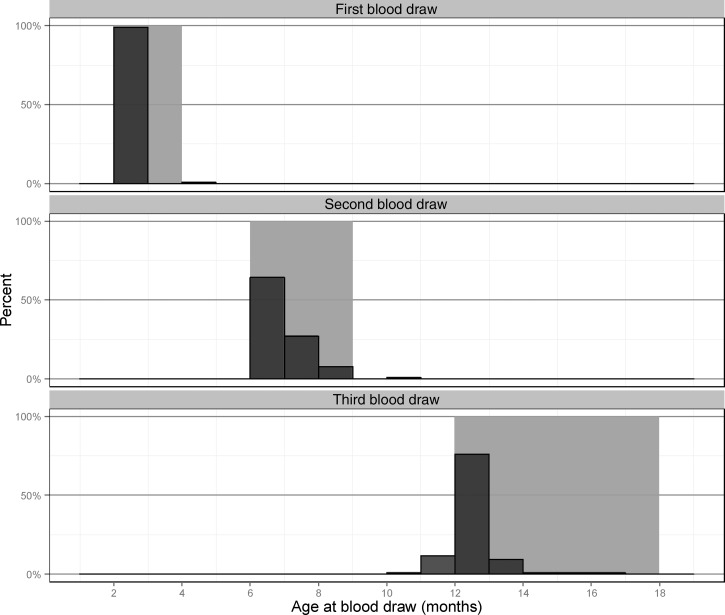
Of 451 enrolled singletons, 365 infants (80.9%) had at least the first blood draw (target schedule 2 months), and 272 of these (74.5%) had nonmissing data for exposures and outcomes. The most common missing data were for wealth index (16% missing) and type of sewer (8% missing). Of these 272, 239 (87.8%) also completed a second blood draw (at a target schedule of 6–8 months), whereas 126 infants (46.3%) completed a blood draw at the final study visit (target schedule of 12–18 months), and 121 had all three blood draws. Although some blood draws took place outside the target age range, the majority were within the target ranges (Figure 2 ).
Figure 2.
Most of the blood draws (dark gray bars) fell within the target age ranges (light gray bands), with the highest success in the first blood draw.
Infants were fairly evenly distributed in terms of gender, nearly one-third were born via caesarian section, and one-fifth were born preterm (Table 1). At least 60% of the sample had access to improved WASH for at least one measure, though only 40% had water that was piped inside their home (the majority of the rest had access to water that was piped to a point outside their home but close by, often in the same lot). Mothers had a mean age of 26 years, one-quarter were employed, and most had at least some secondary education. About one-quarter of households owned a refrigerator, and one-third had high-quality flooring material. The prevalence of exclusive breastfeeding was 60% at 1–5 months and declined with age (Table 2). Stunting varied between 14% and 18%, whereas overweight varied from 32% at the first blood draw to 19% at the third blood draw.
Table 1.
Characteristics of the study sample (N = 272)
| Frequency* or mean (±SD) | % | |
|---|---|---|
| Infant characteristics | ||
| Age (days) at enrollment | 34 ± 8 | – |
| Male | 144 | 52.9 |
| C-section | 81 | 29.8 |
| Preterm (< 37 weeks gestational age) | 48 | 17.6 |
| Water and sanitation | ||
| Water piped indoors | 108 | 39.7 |
| Treats water before drinking | 176 | 64.7 |
| Private toilet (vs. shared or none) | 170 | 62.5 |
| Piped sewer (vs. pit latrine) | 226 | 83.1 |
| Trash picked up (vs. thrown onto patio, other) | 226 | 83.1 |
| Crowding (> 2 people per bedroom) | 200 | 73.5 |
| Other sociodemographics | ||
| Maternal age (years) | 25.5 ± 6.3 | – |
| Maternal employment | 70 | 25.8 |
| Household owns refrigerator | 77 | 28.3 |
| Higher quality floor material (hardwood, carpet, or tile vs. cement) | 98 | 36.0 |
| Maternal education | ||
| Primary or less | 35 | 12.9 |
| At least some secondary | 167 | 61.4 |
| At least some superior | 70 | 25.7 |
SD = standard deviation.
Of singleton infants with plasma available for at least the 2-month visit and no missing data for exposures or outcomes.
Table 2.
Sample characteristics by age
| First assessment (range = 1–5 months) | Second assessment (range = 6–10 months)† | Third assessment (range = 10–19 months)† | ||||
|---|---|---|---|---|---|---|
| Frequency or mean (±SD) | Percent | Frequency or mean (±SD) | Percent | Frequency or mean (±SD) | Percent | |
| N | 272 | – | 243 | – | 127 | |
| Age and nutrition | ||||||
| Age (months) | 2.1 ± 0.3 | – | 6.7 ± 0.9 | – | 14.1 ± 2.3 | – |
| Exclusively breastfed | 162 | 59.8 | 21 | 8.6 | 0 | 0.0 |
| Months of exclusive breastfeeding | 1.9 ± 1.3 | – | 3.1 ± 2.3 | – | 3.0 ± 2.4 | – |
| Any breastfeeding | 267 | 98.2 | 235 | 96.7 | 107 | 84.3 |
| Stunted (LAZ < −2) | 44 | 16.2 | 36 | 14.2 | 22 | 17.5 |
| Overweight (WLZ > 2) | 86 | 31.6 | 70 | 29.3 | 24 | 19.0 |
| Inflammation and morbidities | ||||||
| AGP (g/L) | ||||||
| Elevated (> 1 g/L) | 4 | 1.5 | 30 | 12.3 | 14 | 11.0 |
| Continuous (median, first quartile, third quartile) | 0.3 (0.2, 0.4) | 0.6 (0.4, 0.7) | (0.5, 0.4, 0.8) | |||
| CRP (mg/L) | ||||||
| Elevated (> 5 mg/L) | 7 | 2.6 | 40 | 16.5 | 16 | 12.6 |
| Continuous (median, first quartile, third quartile) | 0.3 (0.2, 0.6) | 0.6 (0.3, 2.1) | 0.6 (0.3, 2.2) | |||
| Any inflammation* | 7 | 2.6 | 54 | 22.2 | 19 | 15.0 |
| Recent (2-week) diarrhea | 38 | 14.0 | 38 | 15.6 | 30 | 23.6 |
| Recent (2-week) cough | 110 | 40.4 | 136 | 56.0 | 60 | 47.2 |
| Recent (2-week) fever | 56 | 20.6 | 107 | 44.0 | 49 | 38.6 |
AGP = alpha(1)-acid-glycoprotein; CRP = C-reactive protein; LAZ = length-for-age Z-score; SD = standard deviation; WLZ = weight-for-length Z-score.
Inflammation: CRP > 5 mg/L or AGP > 1 g/L.
Table includes all infants in analysis (all infants with at least first blood draw and no missing data on key predictors or outcomes).
Presence of inflammation and recent illness.
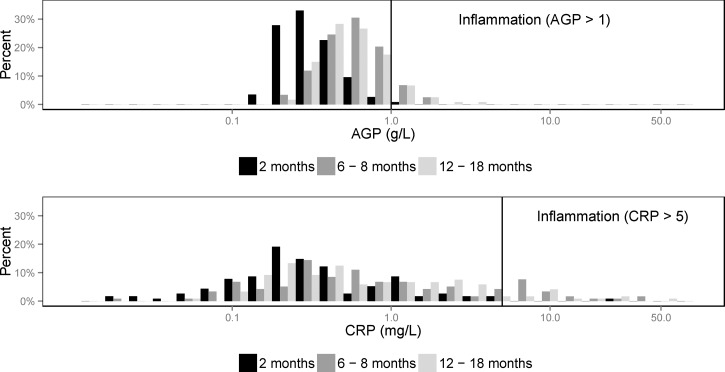
Both CRP and AGP were right skewed at all time points, with both also right shifted at the second and third blood draws (at approximately 6–10 and 10–18 months of age) as compared with the first blood draw (at approximately 2 months of age; Figure 3 ). Almost no inflammation (elevated CRP or AGP) was detected at the first blood draw when infants were 1–5 months of age (3%), but the prevalence of inflammation rose to 22% among infants assessed at 6–10 months of age and 15% among the infants assessed at 10–18 months. The prevalence of recent illness among infants varied by age group, with younger infants tending to have lower prevalence of recent illness (Table 2). Specifically, the prevalence of diarrhea increased with increasing age from 14% among infants 1–5 months to 24% among infants 10–18 months of age. Cough or respiratory illness was highly prevalent, with 40% of the youngest infants affected and around 50% of the older age groups affected. The prevalence of fever was also high, with 21% among the youngest infants and around 40% for older infants and toddlers.
Figure 3.
The distribution of infant AGP and CRP was right shifted for ages older than 2 months. Both biomarkers of inflammation were right skewed. The x axes are shown on the log scale. The y axes indicate the percent of observations within each visit at each level of AGP and CRP. AGP = alpha(1)-acid glycoprotein; CRP = C-reactive protein.
Results of regression models.
Alpha(1)-acid-glycoprotein.
Continuous (log-transformed) infant AGP was significantly associated with recent cough (relative increase of 18%, P = 0.0001), recent fever (relative increase of 23%, P < 0.0001), and age in months (relative increase of 5% per month, P < 0.0001; Table 3). Marginal and conditional R2, representing the proportion of variance explained by fixed, and fixed plus random effects, respectively, were 27% (marginal) and 35% (conditional). Elevated AGP (> 1 g/L) was also significantly associated with recent cough (odds ratio [OR] = 3.9, P = 0.001), recent fever (OR = 2.8, P = 0.013), and age in months (OR = 1.1, P < 0.0001, for a 1-month increase; Table 4). Interactions between acute illnesses and age were tested for each model and found to be nonsignificant (data not shown).
Table 3.
Mixed linear regression models of associations of predictors and (log-transformed) AGP and CRP
| AGP* (N = 626 observations of 272 infants) | CRP† (N = 626 observations of 272 infants) | |||||
|---|---|---|---|---|---|---|
| Percent change‡ | 95% CI | P value§ | Percent change‡ | 95% CI | P value§ | |
| Recent illness (2-week recall) and age | ||||||
| Diarrhea | 7.6 | (−2.9–19.1) | 0.16 | 47.7 | (10.0–98.2) | 0.01 |
| Cough or respiratory illness | 17.7 | (8.5–27.8) | 0.0001 | 44.2 | (13.7–82.7) | 0.003 |
| Fever | 23.3 | (13.0–34.6) | < 0.0001 | 34.6 | (4.7–73.1) | 0.021 |
| Age (1-month increase) | 4.9 | (4.0–5.7) | < 0.0001 | 7.3 | (4.8–9.8) | < 0.0001 |
| Anthropometry | ||||||
| Stunted (LAZ < −2) | −2.0 | (−12.0–9.0) | 0.70 | 6.7 | (−21.9–45.8) | 0.68 |
| Overweight (WLZ > 2) | 1.3 | (−6.9–10.2) | 0.77 | −11.5 | (−30.9–13.2) | 0.33 |
| Water and sanitation | ||||||
| Water piped indoors (vs. other source) | 7.1 | (−2.2–17.3) | 0.14 | 8.5 | (−17.8–43.2) | 0.56 |
| Treats water | 5.5 | (−3.5–15.3) | 0.24 | 10.3 | (−15.8–44.4) | 0.48 |
| Private toilet (vs. shared with other households or none) | 6.1 | (−3.2–16.3) | 0.21 | 20.0 | (−9.2–58.6) | 0.20 |
| Flush toilet (vs. other) | 7.5 | (−4.5–20.9) | 0.23 | 28.1 | (−10.4–83.3) | 0.18 |
| Trash picked up (vs. burned or otherwise disposed of) | −4.6 | (−14.9–6.9) | 0.42 | −14.4 | (−39.4–20.8) | 0.38 |
| Crowding (> 2 people per bedroom) | −2.5 | (−12.3–8.5) | 0.65 | 3.8 | (−24.9–43.5) | 0.82 |
| Other sociodemographics | ||||||
| Preterm birth (< 37 weeks gestational age) | 7.8 | (−3.6–20.4) | 0.19 | 28.9 | (−7.9–80.4) | 0.14 |
| Months of exclusive breastfeeding (1-month increase)¶ | −0.6 | (−2.6–1.4) | 0.55 | −1.3 | (−7.1–4.8) | 0.67 |
| Maternal education | ||||||
| Primary or less | 0.7 | (−12.5–16.0) | 0.95 | 23.1 | (−19.8–88.9) | 0.28 |
| At least some secondary | 1.5 | (−7.9–11.9) | – | 27.4 | (−5.4–71.4) | – |
| At least some superior (reference) | 0.0 | – | – | 0.0 | – | – |
| Wealth index** | ||||||
| First (lowest) quintile | 9.8 | (−6.2–28.5) | 0.71 | 31.7 | (−18.4–112.6) | 0.20 |
| Second quintile | 6.3 | (−8.4–23.4) | – | 25.0 | (−20.6–96.6) | – |
| Third quintile | 10.0 | (−3.9–26.0) | – | 64.8 | (9.0–149.1) | – |
| Fourth quintile | 4.5 | (−8.1–19.0) | – | 30.4 | (−12.0–93.2) | – |
| Fifth (highest) quintile (reference) | 0.0 | – | – | 0.0 | – | – |
AGP = alpha(1)-acid-glycoprotein; CI = confidence interval; CRP = C-reactive protein; LAZ = length-for-age Z-score; WLZ = weight-for-length Z-score.
All available observations with nonmissing data included for all infants with at least first blood draw. Random intercept with variance 0.004 was significant with P = 0.013 (approximate likelihood ratio test). The marginal R2, reflecting variance explained by fixed effects, was 0.27, and the conditional R2, reflecting variance explained by fixed and random effects, was 0.35.
All available observations with nonmissing data included for all infants with at least first blood draw. Random intercept with variance 0.065 was significant with P = 0.0004 (approximate likelihood ratio test). The marginal R2, reflecting variance explained by fixed factors, was 0.13, and the conditional R2, reflecting variance explained by fixed and random factors, was 0.29.
Percent relative change calculated as 100 × (10β − 1), where β is the coefficient associated with predictor X and thus 10β is equal to the ratio of E(Y | X = 1) to E(Y | X = 0).
F tests with Satterthwaite approximation for degrees of freedom.
Months of exclusive breastfeeding defined as total months, at time of assessment, that infant was fed only with breast milk, with no other liquids or food. One month of formula was allowed as long as breastfeeding was concurrent.
Constructed using principal component analysis of household assets and construction materials.
Table 4.
GEE models of associations of predictors and elevated AGP and CRP
| Elevated AGP* (N = 626 observations of 272 infants) | Elevated CRP* (N = 626 observations of 272 infants) | Elevated AGP or CRP* (N = 626 observations of 272 infants) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value† | OR | 95% CI | P value† | OR | 95% CI | P value† | |
| Recent illness (2-week recall) and age | |||||||||
| Diarrhea | 1.24 | (0.56–2.75) | 0.59 | 2.18 | (1.16–4.10) | 0.015 | 1.78 | (0.99–3.20) | 0.053 |
| Cough or respiratory illness | 3.86 | (1.71–8.72) | 0.001 | 1.58 | (0.80–3.14) | 0.19 | 1.78 | (0.97–3.25) | 0.062 |
| Fever | 2.78 | (1.24–6.23) | 0.013 | 1.67 | (0.86–3.24) | 0.13 | 2.05 | (1.13–3.73) | 0.019 |
| Age (1-month increase) | 1.15 | (1.08–1.22) | < 0.0001 | 1.09 | (1.03–1.15) | 0.001 | 1.10 | (1.05–1.15) | < 0.0001 |
| Anthropometry | |||||||||
| Stunted (LAZ < −2) | 1.79 | (0.75–4.28) | 0.19 | 0.79 | (0.36–1.75) | 0.56 | 1.28 | (0.66–2.50) | 0.47 |
| Overweight (WLZ > 1) | 1.17 | (0.55–2.48) | 0.68 | 0.89 | (0.46–1.69) | 0.71 | 1.12 | (0.64–1.95) | 0.70 |
| Water and sanitation | |||||||||
| Water piped indoors (vs. other source) | 0.57 | (0.27–1.19) | 0.14 | 1.00 | (0.52–1.93) | 1.00 | 0.80 | (0.45–1.43) | 0.45 |
| Treats water | 0.88 | (0.41–1.90) | 0.75 | 1.45 | (0.80–2.62) | 0.22 | 1.06 | (0.62–1.80) | 0.83 |
| Private toilet (vs. shared with other households or none) | 0.85 | (0.39–1.82) | 0.67 | 1.21 | (0.64–2.28) | 0.55 | 1.02 | (0.58–1.82) | 0.93 |
| Flush toilet (vs. other) | 1.30 | (0.46–3.72) | 0.62 | 2.12 | (0.79–5.66) | 0.13 | 1.56 | (0.73–3.35) | 0.25 |
| Trash picked up (vs. burned or otherwise disposed of) | 0.84 | (0.34–2.06) | 0.71 | 1.10 | (0.46–2.63) | 0.83 | 0.92 | (0.46–1.85) | 0.81 |
| Crowding (> 2 people per bedroom) | 0.75 | (0.33–1.70) | 0.49 | 0.85 | (0.42–1.73) | 0.65 | 0.72 | (0.39–1.35) | 0.31 |
| Other sociodemographics | |||||||||
| Preterm birth (< 37 weeks gestational age) | 1.05 | (0.46–2.43) | 0.90 | 1.76 | (0.92–3.35) | 0.088 | 1.44 | (0.79–2.62) | 0.23 |
| Months of exclusive breastfeeding (1-month increase)‡ | 0.92 | (0.79–1.07) | 0.27 | 1.03 | (0.90–1.19) | 0.65 | 1.03 | (0.91–1.16) | 0.67 |
| Maternal education | |||||||||
| Primary or less | 0.78 | (0.36–1.67) | 0.68 | 2.13 | (0.88–5.16) | 0.12 | 1.28 | (0.59–2.74) | 0.57 |
| At least some secondary | 0.64 | (0.22–1.83) | – | 2.16 | (1.02–4.55) | – | 1.37 | (0.76–2.47) | – |
| At least some superior (reference) | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – |
| Wealth index§ | |||||||||
| First (lowest) quintile | 0.36 | (0.09–1.44) | 0.56 | 1.50 | (0.48–4.67) | 0.11 | 0.82 | (0.30–2.24) | 0.14 |
| Second quintile | 0.66 | (0.23–1.93) | – | 1.95 | (0.70–5.44) | – | 1.26 | (0.54–2.95) | – |
| Third quintile | 1.04 | (0.35–3.06) | – | 3.23 | (1.24–8.42) | – | 2.17 | (0.98–4.82) | – |
| Fourth quintile | 1.03 | (0.41–2.60) | – | 2.43 | (1.05–5.66) | – | 1.66 | (0.81–3.42) | – |
| Fifth quintile (reference) | 1.00 | – | – | 1.00 | – | – | 1.00 | – | – |
AGP = alpha(1)-acid-glycoprotein; CI = confidence interval; CRP = C-reactive protein; GEE = generalized estimating equation; LAZ = length-for-age Z-score; OR = odds ratio; WLZ = weight-for-length Z-score.
All available observations included for all infants with at least first blood draw. Elevated AGP defined as AGP > 1 g/L. Elevated CRP defined as CRP > 5 mg/L. GEE models used, with binomial link function and exchangeable correlation structure.
Wald tests used.
Months of exclusive breastfeeding defined as total months, at time of assessment, that infant was fed only with breast milk, with no other liquids or food. One month of formula was allowed as long as breastfeeding was concurrent.
Constructed using principal components analysis of household assets and construction materials.
C-reactive protein.
Continuous (log-transformed) infant CRP was significantly associated with recent diarrhea (relative increase of 48%, P = 0.01), recent cough (relative increase of 44%, P = 0.003), recent fever (relative increase of 35%, P = 0.021), and age in months (relative increase of 7% per month, P < 0.0001; Table 3). Marginal and conditional R2 were 13% and 29%, respectively. Elevated CRP (> 5 mg/L) was significantly associated with recent diarrhea (OR = 2.2, P = 0.015) and age in months (OR = 1.1, P = 0.001, for a1-month increase), but not with other recent illnesses (Table 4). Interactions between acute illnesses and age were tested for both models and found to be nonsignificant (data not shown).
Inflammation.
Dichotomized infant inflammation (elevated AGP or CRP) was significantly associated with recent fever (OR = 2.1, P = 0.019) and age in months (OR = 1.1, P < 0.0001, for a 1-month increase; Table 4). Interactions between acute illnesses and age were tested and found to be nonsignificant (data not shown).
Discussion
In this prospective cohort study of Bolivian infants, the prevalence of any inflammation increased from 3% at the first assessment (approximately 2 months of age) to 15–22% at later time points (approximately 6–8 and 12–18 months of age for most infants). Although this is much lower than the prevalence of elevated CRP (> 5 mg/L) in a cohort of Tanzanian infants 6–35 months of age,16 the overall geometric mean CRP in our older infants (0.80 mg/L at the third blood draw, ∼12–18 months of age) is comparable to, though somewhat higher than, that in a cohort of European and south Asian British infants at 12 months of age (0.69 mg/L for European-origin infants, 0.51 mg/L for south Asian–origin infants).25 These comparisons potentially reflect population differences in socioeconomic status, rurality, age, and other correlates or potential modifiers of inflammation. Our study also measured AGP and found it to be elevated in only 2% of infants at the first blood draw, but > 10% of infants at later blood draws. This is a new finding, as previous studies of inflammation in healthy infants have not measured AGP.
In our study of Bolivian infants, inflammation was significantly associated with maternal recall of recent illnesses and increased age in months regardless of biomarker. The association of recent illness with inflammation and the larger magnitude of the effect in linear CRP models as opposed to linear AGP models are consistent with the biology of the APR. Specifically, CRP experiences a much more dramatic relative increase as compared with AGP during the APR.9 We did not see significant interactions of acute illness and age, which may suggest that the infant inflammatory reaction to infectious illness does not change significantly between 2and 18 months of age. Although two other identified studies demonstrated a significant effect of age in the opposite (negative) direction, these studies included older children: one compared children at 6–35 months to those at 36–59 months,16 whereas the other included only children 2–15 years of age.18 It is possible that age is not linearly related to inflammation. Possibly, inflammation in developing-country settings peaks between 12 and 24 months of age, and then declines as children's immune systems are trained. Another study in British infants more similar in age to our study population did not show any significant effect of age, but took place in a low-inflammation, high-resource, developed-country setting.25 We hypothesize that our finding of increasing inflammation with age may be related to the fact that older infants and toddlers will have much more opportunity for inflammatory exposures (e.g., pathogens) given their increased mobility as well as the incorporation of new foods and potentially unsafe liquids during weaning. Although it is possible that the prevalence of inflammation could be affected by the receipt ofvaccines concurrent with blood draws, we did not see asignificant relationship between receipt of Rotarix® and inflammation at the first blood draw (data not shown). Furthermore, while older infants (∼12 months) were more likely than younger infants (∼6 months) to receive more vaccines near the time of the blood draw (due to the vaccine schedule), the prevalence of inflammation was similar between these two groups. Therefore, we hypothesize that our findings of increased inflammation at older ages are unlikely to be explained by variations in vaccination. Although our models did not demonstrate a significant effect of cumulative months of exclusive breastfeeding, a sensitivity analysis assessing current breastfeeding practices within each age group did show a trend toward a protective effect of exclusive breastfeeding among younger infants (data not shown). A study in Tanzanian children also suggested a “protective” effect of breastfeeding.16
Neither marker of anthropometry (stunting nor overweight, both at low-to-moderate levels in the population) was significantly associated with inflammation in this cohort. Although an association of adiposity and inflammation has been well established in adults,19,20,24,41 and other authors have found similar associations in children,17,20,42–52 we did not see a significant association of WLZ with inflammation in our cohort. Alternative definitions of adiposity (using body mass index–for-age Z-score as well as cutoffs for obesity versus normal–overweight) also failed to yield significant results in our models, suggesting that differences in adiposity definition were not the explanation for our results. Our results may be due to a lack of power or to the lower prevalence of overweight in our cohort as compared with developed-country children. Alternatively, it may be that our infants were too young for the inflammatory effect of adiposity to have presented; the studies showing significant associations were all in children of at least preschool age. Although a study of Ecuadorian children found a negative association between CRP and attained growth in children 2–7 years ofage,53 two other studies of inflammation that included infants (one in British infants 3–24 months of age, one in Tanzanian children 6–59 months of age) did not find significant associations between body size measures, including length, and CRP.16,25
Markers of WASH resources were also not significantly associated with individual markers of inflammation in bivariate or multivariable analyses. Though this was contrary to expectations, as we hypothesized that lack of access to WASH resources would reflect increased pathogen exposure, it is consistent with results from Hadley and others and Thompson and others,16,20 who also failed to see significant associations of WASH resources (e.g., private toilet, water quality) in multivariable models of child inflammation. It may be that the available measures of WASH were not granular or specific enough to capture true pathogen exposure, or that young infants are more protected from these effects given their lower mobility, restricted eating, and close maternal supervision. It may also be that WASH measures are more important to a longer-term process of immune development as opposed to short-term markers of inflammation in young infants, whose immune systems may still be undeveloped.
Calculated R2 for linear models indicated that fixed effects explained a larger proportion of AGP variance as compared with CRP variance, but that no more than a third of variance was explained by the parameters in either model. This suggests that factors outside of our measured morbidities, anthropometry, and WASH resources may be important to the development of inflammation in infants. The similar R2 (marginal and conditional) for the model of AGP implies that within-subject correlations are less important (supported by the small magnitude of the variance of the random intercept term), and that other environmental factors or behavioral factors may be important. Conversely, the large jump from the marginal to the conditional R2 for the model of CRP suggests that within-subject factors are important for CRP levels, potentially implying constitutional differences or other factors present from birth. To our knowledge, this is the first that this type of analysis has been applied to the study of inflammation in healthy infants.
Although the inflammation cutoff of 5 mg/L for CRP is widely used,12 it has been suggested that this cutoff is inappropriate for healthy or pediatric populations.12,47,54 Therefore, we reran the model of elevated CRP with a cutoff of >3 mg/L, which has previously been suggested and used as a “high-risk” cutoff.12,55,56 This analysis demonstrated similar results in terms of significance to the model with the original cutoff (5 mg/L), although the effect of recent diarrhea became nonsignificant, whereas recent cough became significant (OR = 2.2 [1.2–4.0], P = 0.012). We also tested a cutoff of > 1.1 mg/L as suggested by Wander and others' analysis of Tanzanian children.54 This model also showed similar results to the models with cutoffs of 3 and 5 mg/L. However, recent fever (OR = 1.8 [1.2–2.7], P = 0.005) and trash picked up (OR = 0.6 [0.3–1.0], P = 0.043) became significant (recent diarrhea was again nonsignificant). We could not perform an analysis using a cutoff of CRP > 10 mg/L (often suggested as a marker of acute inflammation or infection57), because not enough infants at all time points exhibited CRP values this high.
This study has several strengths. First, we were able to follow infants over the course of their 1st year of life, gathering data on inflammation and its potential correlates at multiple time points. This enabled us to test whether the effects of acute illness varied over time as infants' immune systems matured. We also had access to a rich variety of data, including not only recent illness recall, but also anthropometry, birth characteristics, WASH resources, and other sociodemographics. Furthermore, we were able to test two separate markers of inflammation—CRP and AGP—that reflect different time points in the APR. However, our study is limited by our decreased sample size at the third visit, which negatively impacts our power to test associations with many predictors at once. Comparison of the full analytical sample with the subset with data at all three visits revealed similar prevalence of elevated inflammatory biomarkers (though cough and diarrhea were slightly less prevalent among younger infants) and few differences in characteristics—the prevalence of preterm infants was slightly lower in the subset (13% versus 18%), whereas the prevalence of overweight among 2-month-olds was slightly lower (24% versus 32%) and mothers were slightly more educated (32% with superior education versus 26%). Linear and logistic models for both AGP and CRP gave similar conclusions with the subset as compared with the analytic dataset, though the significance of some results varied (the effects of flushing toilet and preterm birth became significant, but with a wide confidence interval, in several models, whereas the effects of wealth index and water treatment became significant in the logistic model of CRP, and the effect of fever became nonsignificant in two models; data not shown). Although this may point to a mild selection bias resulting from dropout at the third visit, it is reassuring that our reported results are in the same direction and toward the null as compared with those in the subset. Another possible limitation was that the reported LOD for the CRP assay used was higher than that of some high-sensitivity CRP assays, and may not fully capture low levels of inflammation. However, this higher LOD does not affect the main conclusions of this study. Another limitation is that we did not have information on other potential markers of pathogen exposure, such as the presence of feces around the home, and that morbidities were captured via maternal recall and not verified by examination in all cases.
Conclusions
In this study, we found that inflammation increased from early infancy to later infancy and early toddlerhood, potentially reflecting increased exposure to pathogens and other inflammatory agents in this cohort of Bolivian infants. We also found that recent illnesses were significantly associated with CRP and AGP, but did not fully explain differences in either acute phase protein. This cohort of infants did not demonstrate significant associations between inflammation and WASH or anthropometry, suggesting that these exposures may be more relevant to older children. Overall, the results underscore the importance of biochemical measures of inflammation in infants, given that inflammation cannot be identified by sociodemographic or morbidity information alone, and may manifest only subclinically.
ACKNOWLEDGMENTS
First, we thank our study participants and their families. We also thank our study personnel, colleagues at the Universidad Mayor de San Andrés and Centro de Atención Integral para Adolescentes (CAIA), and participating Hospitals “Infantil Los Andes” and “Modelo Corea” in La Paz and El Alto, Bolivia. We additionally thank Juergen Erhardt and Donnie Whitehead for assistance with sample analysis. We also gratefully acknowledge the assistance and work of the late Lic. Rosario Calderón, Executive Director of CAIA, for her support of this study.
Disclaimer: The findings and conclusions in this report are those ofthe authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Financial support: This work was supported in part by NIH-NIAID K01 grant (1K01AI087724-01) grant; PHS Grant UL1 TR000454 from the Clinical and Translational Science Award Program, National Institutes of Health, National Center for Research Resource; the Emory + Children's Pediatric Center Seed Grant Program; the National Institutes of Health/NIAID grant U19-AI057266; the International Collaborative Award for Research from the International Pediatric Research Foundation; the Laney Graduate School of Emory University; NIH T32 training grant in reproductive, pediatric and perinatal epidemiology (HD052460-01); Burroughs Wellcome Fund's Molecules to Mankind Program (M2M); and the NIH T32 Vaccinology Training Program (T32AI074492).
Authors' addresses: Rachel M. Burke, Paulina A. Rebolledo, Anna M. Fabiszewski de Aceituno, Mitchel Klein, Carolyn Drews-Botsch, and Juan S. Leon, Emory University, Atlanta, GA, E-mails: rachel.m.burke@gmail.com, preboll@emory.edu, anna.m.aceituno@gmail.com, mklein@emory.edu, cdrews@emory.edu, and juan.leon@emory.edu. Parminder S. Suchdev, Nutrition Branch, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: psuchde@emory.edu. Rita Revollo, Servicio Departamental de Salud, La Paz, Bolivia, E-mail: ritarevollom@hotmail.com. Volga Iñiguez, Instituto de Biología Molecular y Biotecnología, Universidad Mayor de San Andrés, La Paz, Bolivia, E-mail: volgavir@yahoo.com.
References
- 1.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 3.Koenig W. High-sensitivity C-reactive protein and atherosclerotic disease: from improved risk prediction to risk-guided therapy. Int J Cardiol. 2013;168:5126–5134. doi: 10.1016/j.ijcard.2013.07.113. [DOI] [PubMed] [Google Scholar]
- 4.Reus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, Kapczinski F, Quevedo J. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience. 2015;300:141–154. doi: 10.1016/j.neuroscience.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Furtado M, Katzman MA. Examining the role of neuroinflammation in major depression. Psychiatry Res. 2015;229:27–36. doi: 10.1016/j.psychres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Kosek M, Haque R, Lima A, Babji S, Shrestha S, Qureshi S, Amidou S, Mduma E, Lee G, Yori PP, Guerrant RL, Bhutta Z, Mason C, Kang G, Kabir M, Amour C, Bessong P, Turab A, Seidman J, Olortegui MP, Quetz J, Lang D, Gratz J, Miller M, Gottlieb M. MAL-ED Network Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88:390–396. doi: 10.4269/ajtmh.2012.12-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Anguita A, Kakourou A, Tsilidis KK. Biomarkers of inflammation and immune function and risk of colorectal cancer. Curr Colorectal Cancer Rep. 2015;11:250–258. doi: 10.1007/s11888-015-0282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivas-Fuentes S, Salgado-Aguayo A, Pertuz Belloso S, Gorocica Rosete P, Alvarado-Vasquez N, Aquino-Jarquin G. Role of chemokines in non-small cell lung cancer: angiogenesis and inflammation. J Cancer. 2015;6:938–952. doi: 10.7150/jca.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thurnham D, McCabe G. Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. In: World Health Organization, editor. Report: Priorities in the Assessment of Vitamin A and Iron Status in Populations, Panama City, Panama, 15–17 September 2010. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 10.Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB. The immune system in children with malnutrition: a systematic review. PLoS One. 2014;9:e105017. doi: 10.1371/journal.pone.0105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagundes CP, Glaser R, Kiecolt-Glaser JK. Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav Immun. 2013;27:8–12. doi: 10.1016/j.bbi.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raiten DJ, Sakr Ashour FA, Ross AC, Meydani SN, Dawson HD, Stephensen CB, Brabin BJ, Suchdev PS, van Ommen B. Group IC Inflammation and nutritional science for programs/policies and interpretation of research evidence (INSPIRE) J Nutr. 2015;145:1039S–1108S. doi: 10.3945/jn.114.194571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 14.Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14:232–244. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- 15.Warnberg J, Marcos A. Low-grade inflammation and the metabolic syndrome in children and adolescents. Curr Opin Lipidol. 2008;19:11–15. doi: 10.1097/MOL.0b013e3282f4096b. [DOI] [PubMed] [Google Scholar]
- 16.Hadley C, Decaro JA. Testing hypothesized predictors of immune activation in Tanzanian infants and children: community, household, caretaker, and child effects. Am J Hum Biol. 2014;26:523–529. doi: 10.1002/ajhb.22558. [DOI] [PubMed] [Google Scholar]
- 17.Dowd JB, Zajacova A, Aiello AE. Predictors of inflammation in U.S. children aged 3–16 years. Am J Prev Med. 2010;39:314–320. doi: 10.1016/j.amepre.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDade TW, Leonard WR, Burhop J, Reyes-Garcia V, Vadez V, Huanca T, Godoy RA. Predictors of C-reactive protein in Tsimane' 2 to 15 year-olds in lowland Bolivia. Am J Phys Anthropol. 2005;128:906–913. doi: 10.1002/ajpa.20222. [DOI] [PubMed] [Google Scholar]
- 19.McDade TW, Rutherford JN, Adair L, Kuzawa C. Population differences in associations between C-reactive protein concentration and adiposity: comparison of young adults in the Philippines and the United States. Am J Clin Nutr. 2009;89:1237–1245. doi: 10.3945/ajcn.2008.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson AL, Houck KM, Adair L, Gordon-Larsen P, Du S, Zhang B, Popkin B. Pathogenic and obesogenic factors associated with inflammation in Chinese children, adolescents and adults. Am J Hum Biol. 2014;26:18–28. doi: 10.1002/ajhb.22462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203:311–319. doi: 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Shima M. Air pollution and serum C-reactive protein concentration in children. J Epidemiol. 2007;17:169–176. doi: 10.2188/jea.17.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita M, Brindle E, Lo YJ, Castro P, Cameroamortegui F. Nutrient intakes associated with elevated serum C-reactive protein concentrations in normal to underweight breastfeeding women in northern Kenya. Am J Hum Biol. 2014;26:796–802. doi: 10.1002/ajhb.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nazmi A, Oliveira IO, Victora CG. Correlates of C-reactive protein levels in young adults: a population-based cohort study of 3827 subjects in Brazil. Braz J Med Biol Res. 2008;41:357–367. doi: 10.1590/s0100-879x2008000500003. [DOI] [PubMed] [Google Scholar]
- 25.Oldroyd JC, Heald A, Bansal N, Vyas A, Siddals K, Gibson M, Clayton P, Cruickshank JK. Inflammatory markers and growth in south Asian and European origin infants in Britain: the Manchester Children's Growth and Vascular Health Study. Atherosclerosis. 2009;207:227–231. doi: 10.1016/j.atherosclerosis.2009.03.045. [DOI] [PubMed] [Google Scholar]
- 26.Blackwell C, Moscovis S, Hall S, Burns C, Scott RJ. Exploring the risk factors for sudden infant deaths and their role in inflammatory responses to infection. Front Immunol. 2015;6:44. doi: 10.3389/fimmu.2015.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prescott SL. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J Allergy Clin Immunol. 2013;131:23–30. doi: 10.1016/j.jaci.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Measure DHS. In: Encuesta Nacional de Demografiìa y Salud 2008. Estadistica INd, editor. La Paz, Bolivia: Ministerio de Salud y Deportes, Instituto Nacional de Estadística; 2009. [Google Scholar]
- 29.Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30:105–112. doi: 10.1055/s-0032-1333412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134:3127–3132. doi: 10.1093/jn/134.11.3127. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization (WHO) WHO Growth Charts. 2010. http://www.cdc.gov/growthcharts/who_charts.htm# Available at. Accessed May 29, 2014.
- 32.Thurnham DI, Mburu AS, Mwaniki DL, Muniu EM, Alumasa F, de Wagt A. Using plasma acute-phase protein concentrations to interpret nutritional biomarkers in apparently healthy HIV-1-seropositive Kenyan adults. Br J Nutr. 2008;100:174–182. doi: 10.1017/S0007114507883012. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization (WHO) Training Course on Child Growth Assessment. Geneva, Switzerland: WHO; 2008. [Google Scholar]
- 34.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–468. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 35.Kleinbaum DG, Klein M, Pryor ER. Logistic Regression: A Self-Learning Text. New York, NY: Springer; 2002. [Google Scholar]
- 36.Team RC . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 37.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 38.Johnson PC. Extension of Nakagawa and Schielzeth's R2GLMM to random slopes models. Methods Ecol Evol. 2014;5:944–946. doi: 10.1111/2041-210X.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. [Google Scholar]
- 40.Carey VJ. gee: Generalized Estimation Equation Solver. 2015.
- 41.Bertran N, Camps J, Fernandez-Ballart J, Arija V, Ferre N, Tous M, Simo D, Murphy MM, Vilella E, Joven J. Diet and lifestyle are associated with serum C-reactive protein concentrations in a population-based study. J Lab Clin Med. 2005;145:41–46. doi: 10.1016/j.lab.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Aeberli I, Molinari L, Spinas G, Lehmann R, l'Allemand D, Zimmermann MB. Dietary intakes of fat and antioxidant vitamins are predictors of subclinical inflammation in overweight Swiss children. Am J Clin Nutr. 2006;84:748–755. doi: 10.1093/ajcn/84.4.748. [DOI] [PubMed] [Google Scholar]
- 43.Dayal D, Jain H, Attri SV, Bharti B, Bhalla AK. Relationship of high sensitivity C-reactive protein levels to anthropometric and other metabolic parameters in Indian children with simple overweight and obesity. J Clin Diagn Res. 2014;8:PC05–PC08. doi: 10.7860/JCDR/2014/8191.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambert M, Delvin EE, Paradis G, O'Loughlin J, Hanley JA, Levy E. C-reactive protein and features of the metabolic syndrome in a population-based sample of children and adolescents. Clin Chem. 2004;50:1762–1768. doi: 10.1373/clinchem.2004.036418. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Alcaraz F, Del Toro-Equihua M, Orta-Duarte M, Flores-Ruelas Y, Sanchez-Ramirez CA. Higher levels of C-reactive protein associated with higher adiposity in Mexican schoolchildren. Nutr Hosp. 2014;29:531–536. doi: 10.3305/nh.2014.29.3.7158. [DOI] [PubMed] [Google Scholar]
- 46.Singer K, Eng DS, Lumeng CN, Gebremariam A, Lee MJ. The relationship between body fat mass percentiles and inflammation in children. Obesity (Silver Spring) 2014;22:1332–1336. doi: 10.1002/oby.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: cross-sectional analyses throughout childhood. Pediatrics. 2010;125:e801–e809. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Low-grade systemic inflammation in overweight children. Pediatrics. 2001;107:E13. doi: 10.1542/peds.107.1.e13. [DOI] [PubMed] [Google Scholar]
- 49.Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, Miller GJ, Strachan DP. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149:139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- 50.Lopez-Jaramillo P, Herrera E, Garcia RG, Camacho PA, Castillo VR. Inter-relationships between body mass index, C-reactive protein and blood pressure in a Hispanic pediatric population. Am J Hypertens. 2008;21:527–532. doi: 10.1038/ajh.2007.86. [DOI] [PubMed] [Google Scholar]
- 51.Nappo A, Iacoviello L, Fraterman A, Gonzalez-Gil EM, Hadjigeorgiou C, Marild S, Molnar D, Moreno LA, Peplies J, Sioen I, Veidebaum T, Siani A, Russo P. High-sensitivity C-reactive protein is a predictive factor of adiposity in children: results of the identification and prevention of dietary- and lifestyle-induced health effects in children and infants (IDEFICS) study. J Am Heart Assoc. 2013;2:e000101. doi: 10.1161/JAHA.113.000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parrett AL, Valentine RJ, Arngrimsson SA, Castelli DM, Evans EM. Adiposity, activity, fitness, and C-reactive protein in children. Med Sci Sports Exerc. 2010;42:1981–1986. doi: 10.1249/MSS.0b013e3181e0355e. [DOI] [PubMed] [Google Scholar]
- 53.Blackwell AD, Snodgrass JJ, Madimenos FC, Sugiyama LS. Life history, immune function, and intestinal helminths: trade-offs among immunoglobulin E, C-reactive protein, and growth in an Amazonian population. Am J Hum Biol. 2010;22:836–848. doi: 10.1002/ajhb.21092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wander K, Brindle E, O'Connor KA. Sensitivity and specificity of C-reactive protein and alpha(1)-acid glycoprotein for episodes of acute infection among children in Kilimanjaro, Tanzania. Am J Hum Biol. 2012;24:565–568. doi: 10.1002/ajhb.22261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia OP, Ronquillo D, del Carmen Caamano M, Martinez G, Camacho M, Lopez V, Rosado JL. Zinc, iron and vitamins A, C and E are associated with obesity, inflammation, lipid profile and insulin resistance in Mexican school-aged children. Nutrients. 2013;5:5012–5030. doi: 10.3390/nu5125012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Myers GL, Rifai N, Tracy RP, Roberts WL, Alexander RW, Biasucci LM, Catravas JD, Cole TG, Cooper GR, Khan BV, Kimberly MM, Stein EA, Taubert KA, Warnick GR, Waymack PP. CDC; AHA CDC/AHA workshop on markers of inflammation and cardiovascular disease: application to clinical and public health practice: report from the laboratory science discussion group. Circulation. 2004;110:e545–e549. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- 57.Smith SC, Jr, Anderson JL, Cannon RO, 3rd, Fadl YY, Koenig W, Libby P, Lipshultz SE, Mensah GA, Ridker PM, Rosenson R. CDC; AHA CDC/AHA workshop on markers of inflammation and cardiovascular disease: application to clinical and public health practice: report from the clinical practice discussion group. Circulation. 2004;110:e550–e553. doi: 10.1161/01.CIR.0000148981.71644.C7. [DOI] [PubMed] [Google Scholar]