Abstract
Background
A substantial proportion of HIV-infected adults starting antiretroviral therapy (ART) in sub-Saharan Africa are malnourished. We aimed to increase understanding of the factors affecting their high mortality, particularly in the high-risk period before ART initiation.
Methods
We analysed potential risk factors for mortality of Zambian and Tanzanian participants enrolled in the NUSTART clinical trial. Malnourished adults (n = 1815; body mass index [BMI] <18.5 kg/m2) were recruited at referral to ART and randomised to receive different nutritional supplements. Demographics, measures of body composition, blood electrolytes and clinical conditions were investigated as potential risk factors using Poisson regression models.
Results
The mortality rate was higher in the period from referral to starting ART (121 deaths/100 person-years; 95 % CI 103, 142) than during the first 12 weeks of ART (66; 95 % CI 57, 76) and was not affected by trial study arm. In adjusted analyses, lower CD4 count, BMI and mid-arm circumference and raised C-reactive protein were associated with an increased risk of mortality throughout the study. Male sex and lower hand-grip strength carried an increased risk in the pre-ART period. Participants on tuberculosis treatment at referral had a lower mortality rate (adjusted Rate Ratio 0.44; 95 % CI 0.31, 0.63).
Conclusion
Among malnourished ART-eligible adults, pre-ART mortality was twice that in the early post-ART period, suggesting many early ART deaths represent advanced HIV disease rather than treatment-related events. Therefore, more efforts are needed to promote earlier diagnosis and immediate initiation of ART, as recently recommended by WHO for all persons with HIV worldwide. The positive effect of tuberculosis treatment suggests undiagnosed tuberculosis is a contributor to mortality in this population.
Trial registration
Pan African Clinical Trials Registry, PACTR201106000300631; registered on 1st June 2011.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-016-1894-3) contains supplementary material, which is available to authorized users.
Keywords: Mortality risk factors, Malnutrition, ART, Africa
Background
The last decade has seen great advances in expanding access to antiretroviral therapy (ART) for HIV-infected Africans. However, high mortality immediately before and in the first few months after starting ART remains a major concern [1, 2]. Consistent risk factors for early mortality include delayed ART initiation, indicated by very low CD4 count or advanced WHO disease stage, the presence of opportunistic infections such as tuberculosis (TB), and low body mass index (BMI) [1–3]. Male sex, low haemoglobin, low serum phosphate and older or younger age are additional risk factors for mortality in some cohorts [4–9].
In our recent Nutritional Support for African Adults Starting Antiretroviral Therapy (NUSTART) trial we addressed malnutrition as a risk factor for mortality by randomizing underweight African adults initiating ART to a lipid-based nutritional supplement (LNS) containing multiple vitamins and minerals compared to LNS alone. While we observed no difference in mortality between treatment arms, the trial design provided a unique opportunity for detailed clinical assessments of malnourished, HIV-infected persons both prior to ART-initiation and during the early months of treatment [10, 11]. We noted that the mortality rate between referral for ART and 12 weeks after starting ART was considerably higher, 83/100-person-years (95 % confidence interval (CI) 75, 92), than in most other reports of early mortality in patients starting ART in low-income settings [1–3, 6, 12].
Many studies have reported mortality risk factors among patients starting ART but, as far as we know, none has included the high-risk period between referral for ART and actually starting ART in a large cohort of malnourished patients. Given the very high mortality observed, we felt it important to analyse further the extensive NUSTART database, including clinical conditions, blood electrolytes and body composition, to determine risk factors that could be used to improve clinical understanding and management of this vulnerable population.
Methods
Design and intervention
The NUSTART trial was a randomised, controlled phase III trial with the primary outcome of mortality from recruitment at referral to ART until 12 weeks after starting ART and registered as PACTR201106000300631; details have been described previously [10]. Briefly, the study was conducted between August 2011 and December 2013 at two sites: the National Institute for Medical Research (NIMR), Mwanza, Tanzania and the University Teaching Hospital (UTH), Lusaka, Zambia.
The intervention was designed to mimic protocols for nutritional management of severely malnourished children [13] and was a lipid-based nutritional supplement, made for the trial by Nutriset, Malaunay, France, either with high levels of vitamins and minerals (LNS-VM) or without (LNS). From recruitment at referral for ART to 2 weeks after starting ART, the LNS-VM or LNS was given with minimal calories, i.e. 30 g/day, ~150 kcal/day. From 2 to 6 weeks after initiating ART the LNS-VM or LNS were given in 250 g/day, comprising ~1400 kcal/day. Patients were followed to 12 weeks of ART.
Participant recruitment and follow-up
Adults attending free HIV testing services at both sites, eligible for ART and fulfilling the study inclusion criteria were deemed eligible for the trial. Inclusion criteria were: at least 18 years old, ART-naive (except for previous short-term regimens to prevent maternal-to-child HIV transmission), BMI < 18.5 kg/m2, requiring ART as determined by CD4 count <350 cells/μl or stage 3 or 4 disease, willing to undertake intensive follow-up in the study clinic and providing written (or thumbprint) informed consent. Exclusion criteria were participation in a potentially conflicting research protocol, or pregnancy by self-report.
Medical care, including ART, was provided primarily by local health services though study staff treated minor illnesses and referred as necessary during patients’ study visits. National treatment guidelines for ART regimen differed in the two countries: 74 % Zambians were prescribed efavirenz, tenofovir, and emtricitabine, whereas in Tanzania a wider range of regimens was used (all comprising two nucleoside/nucleotide reverse transcriptase inhibitors and a non-nucleoside reverse transcriptase inhibitor).
Patients were seen weekly from recruitment until they initiated ART and then at 2, 4, 6, 8, and 12 weeks after starting ART. Patients who were ill were invited to come for unscheduled visits at any time. Patients who missed scheduled visits were actively followed up through phone-calls to patients and relatives, contact with their ART clinic and additionally in Mwanza by visiting patients at home. We therefore ascertained the primary outcome of mortality before 12 weeks ART in 91 % of the 1815 participants.
Data collection
Anthropometry, body composition, grip strength, and haemoglobin
Patient weight and mid-upper arm circumference were measured at all scheduled visits, and height at recruitment. Measurements were taken in triplicate and the median used in analyses. Staff were trained according to standard protocols [14]. Technical errors of measurement were within acceptable limits [15]. Body fat and lean proportions were measured by bioelectrical impedance (Tanita BC-418, Tokyo, Japan) and calibrated against air displacement plethysmography results from a sample of Lusaka patients [16]. Values were divided by patient’s height squared to create an index. Grip strength was measured using a digital handgrip dynamometer (Takei TKK 5401, Chasmor, UK); two measurements were taken from each hand and the machine takes the average of the two maximum readings. Haemoglobin was measured in the clinic by Hemocue (Angelholm, Sweden).
Laboratory analyses
CD4 count was measured by local central clinical services. Serum C-reactive protein (CRP) was measured by ELISA (Assaypro, St Charles, MO, USA) in the site laboratories. At both sites values for external quality control (QC) sera (Assaypro) were within expected ranges but both the sites had high inter-assay coefficients of variation (CV): 32 % in Lusaka and 37 % in Mwanza. In Lusaka, serum phosphate was measured on a Pointe 180 analyser (Pointe Scientific, Ann Arbor, MI, USA). Sample results were only accepted from runs for which the external QC was within the expected range. Inter-assay CV was 7 % for this external QC. In Mwanza, serum phosphate was measured by Roche COBAS Integra 400 analyser in an external laboratory (Bugando Medical Centre).
Appetite
Appetite was measured by a 4-question questionnaire, combined to produce an appetite score using polychoric correlation [17]. Lower scores represent lower appetite. Preliminary analysis [18] indicated that the test results were clustered in Lusaka and the score correlated best with subsequent weight change in Tanzanian patients. Therefore effect of appetite was only analysed for Tanzanian patients.
Clinical signs and treatment
At each visit, patients were asked if they were taking medications, including anti-TB drugs and cotrimoxazole. All patients were examined at recruitment and assessed for signs of oedema. When a patient died in hospital the diagnosis recorded in the hospital records was taken as the cause of death. Many patients died at home and the cause of death was considered to be unknown.
Sample size
The planned sample size of 2300 participants was based on the primary outcome of mortality. Recruitment was stopped early because of a higher than expected mortality rate as previously described [10]. The final sample size of 1815 was sufficient to detect, at 90 % power, a mortality difference of 30 % between trial arms.
Data management and statistics
Data were double entered, checked and analyses were conducted in STATA version 13.1. Risk factors at baseline which were analysed for their associations with mortality during the study were sex, age, socioeconomic status (SES), CD4 count, BMI, haemoglobin, mid-upper arm circumference (MUAC), fat-free mass index, fat-mass index, oedema, TB treatment, cotrimoxazole treatment, serum phosphate and CRP, grip strength, and appetite score (for Tanzanians only). Principal components analysis (PCA), used to describe the SES of the population, [19] was conducted separately for each country since there were notable differences between sites; variables offered into the PCA were housing characteristics, sanitation, water source, and ownership of electrical goods, animals and modes of transport.
BMI, haemoglobin, fat-free mass index, fat mass index and grip strength were used as continuous variables in regression analysis, after testing for linearity using fractional polynomials. Age, CD4 count and CRP did not have linear relationships and were categorised using standard cut-offs for CD4 and in 4 groups for age (18–29, 30–39, 40–49, ≥50) and CRP (<10 mg/l, 10–49 mg/l, 50–159 mg/l, ≥160 mg/l including the maximum cut-off for most plates of 160 mg/l). Appetite score was divided into tertiles. Serum phosphate was analysed as restricted cubic splines with 4 knots to reflect the increased mortality at high and low values. Low values were defined by US National Institutes of Health Division of AIDS criteria [20] and high values were considered any above the normal range [21]. Continuous variables were also categorised according to standard cut-offs or tertiles for presentation of mortality rates.
Poisson regression was used to estimate hazard ratios. Follow-up time was censored at 10 weeks if patients had not yet started ART (n = 115) and these patients were excluded from all post-ART analysis to avoid the distorting effect of a prolonged time from risk-factor measurement to post-ART period. Factors associated with mortality in univariable analysis were investigated in multivariable models controlling for age, sex, CD4 count, BMI, TB treatment, oedema, grip strength, treatment arm, study site and follow-up time. BMI was omitted from the model when analysing fat-free mass index and MUAC due to collinearity and when analysing appetite as it could be considered a mediator. Statistical significance was assessed using Wald tests. After analysis of the total follow-up time, the study was split into pre- and post-ART periods and baseline factors assessed for association with mortality in each period. Thirty-one patients were excluded due to missing information on ART start date, including 19 patients who died. Interactions between each risk factor and study period were tested in univariable analysis. Length of time spent pre-ART was controlled for in post-ART analyses.
Results
Table 1 describes the population at recruitment. Characteristics were similar at baseline between treatment arms [10]. Half the cohort was male. At baseline a quarter of participants had CD4 count < 50 cells/μl and mean BMI was 16.7 kg/m2. Two-thirds of participants had moderate or severe anaemia, 11 % had low serum phosphate and 17 % reported taking TB treatment at their recruitment visit (Table 1).
Table 1.
Baseline characteristics of the study populationa
| Variable | Level | n (%) |
|---|---|---|
| N (%) | 1815 (100) | |
| Lusaka | 1111 (61.2) | |
| Age (years), median (IQR) | 35 (29–41) | |
| Female | 900 (49.6) | |
| BMI (kg/m2), median (IQR) | 16.7 (15.6–17.5) | |
| BMI <17 kg/m2 | 1074 (59.2) | |
| CD4 count (cells/μl), median (IQR) | 120 (51–211) | |
| CD4 count group | > = 200 | 494 (27.2) |
| 100–199 | 531 (29.3) | |
| 50–99 | 347 (19.1) | |
| <50 | 443 (24.4) | |
| Haemoglobin (g/L), median (IQR) | 96 (80–111) | |
| Haemoglobin groupb | Normal | 177 (9.8) |
| Mild anaemia | 285 (15.7) | |
| Moderate anaemia | 810 (44.6) | |
| Severe anaemia | 398 (21.9) | |
| Missing | 145 (8.0) | |
| Phosphate <0.87 mmol/L | <0.87 | 196 (10.8) |
| Missing | 51 (2.8) | |
| Marital status | Married/Cohabiting | 858 (47.3) |
| Widowed | 203 (11.2) | |
| Divorced-Separated | 510 (28.1) | |
| Single | 243 (13.4) | |
| Missing | 1 (<0.1) | |
| Occupation | Salaried | 271 (14.9) |
| Self-employed | 945 (52.1) | |
| Housewife | 183 (10.1) | |
| Student | 18 (1.0) | |
| Unemployed | 397 (21.9) | |
| Missing | 1 (<0.1) | |
| Education | None | 343 (18.9) |
| Primary | 1045 (57.6) | |
| Secondary | 377 (20.8) | |
| Tertiary | 49 (2.7) | |
| Missing | 1 (<0.1) | |
| TB treatment at baseline | 304 (16.9) | |
| Oedema at baseline | 66 (3.6) | |
| Initial ART regimen (% of 1474 patients who started ART) | AZT/3TC/EFV | 135 (9.2) |
| AZT/FTC/NVP | 236 (16.0) | |
| TDF/FTC/EFV | 816 (55.4) | |
| TDF/FTC/NVP | 64 (4.3) | |
| Other | 54 (3.7) | |
| Missing | 169 (11.5) |
a BMI body mass index, CI confidence interval, LNS lipid-based nutritional supplement without added vitamins and minerals, LNS-VM lipid nutritional supplement with added vitamins and minerals, AZT Zidovudine, 3TC Lamivudine, NVP nevirapine, TDF tenofovir, EFV efavirenz; FTC emtricitabine
bHaemoglobin categories were selected based on usual nutritional cut-offs, not as defined for specific adverse events; adequate haemoglobin was defined as 130 g/L for men and 120 g/L for women; mild anaemia was 110 g/L to the adequate cut-off; moderate anaemia was 80–109 g/L; severe anaemia was < 80 g/L
Participants spent a median of 14.3 weeks in the study (IQR 10.4 to 15.7) and a median of 3 weeks (IQR 2.1 to 4.7) in the pre-ART period. The mortality rate from recruitment until 12 weeks after starting ART was 83.1 deaths/100 person-years (95 % CI 75.0 to 92.1). In the pre-ART period it was particularly high at 120.8 (95 % CI 103.0 to 141.7) and the rate increased as participants spent longer before starting ART, whereas it dropped to 66.1 (95 % CI 57.4 to 76.1) after ART initiation (Table 2).
Table 2.
Mortality rates by potential baseline risk factorsa
| n | deaths | Mortality rate per 100 person-years (95 % CI) | ||
|---|---|---|---|---|
| Total population | 1815 | 365 | 83.1 (75.0 to 92.1) | |
| Study period | Pre-ART | 1784e | 151 | 120.8 (103.0 to 141.7) |
| Post-ART | 1441 | 195 | 66.1 (57.4 to 76.1) | |
| Days Pre-ART | 0–13 | 1784 | 46 | 73.6 (55.1 to 98.3) |
| 14–20 | 1401 | 25 | 119.0 (80.4 to 176.2) | |
| 21–27 | 867 | 20 | 151.4 (97.6 to 234.6) | |
| 28–70 | 554 | 60 | 211.9 (164.5 to 272. 9) | |
| Days Post-ART | 0–13 | 1441 | 44 | 81.3 (60.5 to 109.2) |
| 14–27 | 1380 | 39 | 75.5 (55.1 to 103.3) | |
| 28–41 | 1326 | 35 | 70.4 (50.5 to 98.0) | |
| 42–55 | 1277 | 32 | 66.9 (47.3 to 94.6) | |
| 56–69 | 1266 | 24 | 52.2 (35.0 to 77.9) | |
| ≥70 | 1184 | 21 | 46.0 (30.0 to 70.5) | |
| Site | Lusaka | 1111 | 170 | 64.4 (55.4 to 74.9) |
| Mwanza | 704 | 195 | 122.3 (106.3 to 140.8) | |
| Sex | Women | 900 | 156 | 72.6 (62.0 to 84.9) |
| Men | 915 | 209 | 100.3 (87.6 to 114.9) | |
| Age group (years) | 18–29 | 463 | 85 | 80.1 (64.7 to 99.1) |
| 30–39 | 788 | 159 | 87.5 (74.9 to 102.2) | |
| 40–49 | 411 | 81 | 82.2 (66.1 to 102.1) | |
| > = 50 | 153 | 40 | 108.3 (79.4 to 147.6) | |
| Socioeconomic quintiles | Highest | 360 | 73 | 85.4 (67.9 to 107.5) |
| High | 355 | 77 | 93.0 (74.3 to 116.2) | |
| Middle | 362 | 72 | 84.7 (67.2 to 106.7) | |
| Low | 374 | 81 | 93.9 (75.5 to 116.8) | |
| Lowest | 364 | 62 | 74.0 (57.7 to 94.9) | |
| CD4 group (cells/μl) | > = 200 | 494 | 59 | 48.1 (37.3 to 62.1) |
| 100–199 | 531 | 82 | 64.5 (51.9 to 80.1) | |
| 50–99 | 347 | 88 | 112.0 (90.9 to 138.0) | |
| <50 | 443 | 136 | 143.3 (121.1 to 169.5) | |
| BMI group (kg/m2) | 17.0–18.49 | 741 | 106 | 57.7 (47.7 to 69.8) |
| 16.0–16.9 | 468 | 93 | 85.5 (69.8 to 104.8) | |
| <16.0 | 606 | 166 | 126.9 (109.0 to 147.7) | |
| Haemoglobin (g/L) b | > = 120/130 | 177 | 15 | 33.2 (20.0 to 55.0) |
| 110–119/129 | 285 | 39 | 53.7 (39.2 to 73.4) | |
| 80–190 | 810 | 158 | 83.5 (71.4 to 97.6) | |
| <80 | 398 | 127 | 153.9 (129.3 to 183.1) | |
| Oedema | No | 1749 | 335 | 81.5 (73.2 to 90.7) |
| Yes | 66 | 30 | 245.5 (171.7 to 351.1) | |
| On Tuberculosis Treatment | No | 1511 | 320 | 93.0 (83.3 to 103.7) |
| Yes | 304 | 38 | 50.2 (36.5 to 69.0) | |
| On cotrimoxazole | No | 319 | 59 | 77.7 (60.2 to 100.3) |
| Yes | 1486 | 302 | 87.5 (78.2 to 98.0) | |
| Serum phosphatec | Grades 3–4 low, <0.65 mmol/L | 54 | 16 | 134.0 (82.1 to 218.8) |
| Grades 1–2 low, 0.65–0.87 mmol/L | 142 | 28 | 83.5 (57.7 to 120.9) | |
| Normal, 0.87–1.45 mmol/L | 1241 | 232 | 79.6 (70.0 to 90.5) | |
| Above normal, >1.45 mmol/L | 327 | 74 | 98.3 (78.3 to 123.5) | |
| CRP group (mg/l) | <10 | 358 | 27 | 28.7 (19.7 to 41.8) |
| 10–49 | 450 | 57 | 50.7 (39.1 to 65.8) | |
| 50–159 | 491 | 118 | 109.2 (91.1 to 130.7) | |
| ≥160 | 463 | 151 | 155.8 (132.8 to 182.7) | |
| Fat-free mass indexc Tertiles (kg/m2) | ≥14.5 | 486 | 65 | 55.6 (43.6 to 70.9) |
| 13.7 to 14.49 | 503 | 81 | 65.2 (52.4 to 81.0) | |
| <13.7 | 471 | 92 | 81.4 (66.4 to 99.9) | |
| Fat mass indexc Tertiles (kg/m2) | ≥3.0 | 466 | 62 | 52.2 (40.7 to 67.0) |
| 2.2 to 2.99 | 520 | 80 | 62.3 (50.0 to 77.5) | |
| <2.2 | 474 | 96 | 89.8 (73.5 to 109.7) | |
| Grip strengthc Tertiles (kg) | >22.2 | 592 | 79 | 53.6 (43.0 to 66.8) |
| 16.2 to 22.2 | 588 | 119 | 85.9 (71.8 to 102.8) | |
| <16.2 | 572 | 155 | 125.7 (107.4 to 147.1) | |
| MUACc Tertiles (cm) | >22.3 | 603 | 63 | 41.4 (32.3 to 52.9) |
| 20.5–22.3 | 633 | 123 | 82.8 (69.4 to 98.8) | |
| <20.5 | 566 | 179 | 150.3 (129.8 to 174.0) | |
| Appetitec, d Tertiles | > − 0.5 | 229 | 48 | 86.5 (65.2 to 114.8) |
| −1.48 to −0.55 | 227 | 59 | 112.8 (87.4 to 145.6) | |
| <−1.5 | 228 | 76 | 158.1 (126.3 to 197.9) | |
aAbbreviations: BMI body mass index, MUAC mid-upper arm circumference, CRP C-reactive protein
bNormal haemoglobin cut-offs used were 120 g/L for women and 130 g/L for men
cValues were divided into tertiles for factors without established cut-offs
dAppetite score from polychoric correlation
e31 patients excluded due to missing information on ART
Higher mortality rates were observed in participants who were male, Tanzanian, or at recruitment had very low CD4, BMI, haemoglobin, grip strength or appetite scores, had very high CRP, had severely low or high phosphate or oedema (Table 2). Grip strength and MUAC showed strong independent associations with mortality; rate of death decreased 6 % for every 1 kg increase in grip strength and 13 % for every 1 cm gain in MUAC. Patients receiving TB treatment at baseline had less than half the mortality rate of those not on treatment, after adjusting for other factors including CD4 count. Age, SES and treatment with co-trimoxazole at recruitment had no association with mortality. Appetite score, phosphate and fat-mass index showed crude associations but these did not persist after adjusting for other factors (Table 3).
Table 3.
Poisson regression models for baseline factors associated with overall mortality
| Factor | Level | Unadjusted HR (95 % CI) b | p-value | Adjusted HR (95 % CI) c n = 1685c | Adjusted p-value |
|---|---|---|---|---|---|
| Site | Zambia | 1 | <0.001 | 1 | <0.001 |
| Tanzania | 1.90 (1.55 to 2.33) | 1.60 (1.27 to 2.03) | |||
| Sex | Female | 1 | 0.002 | 1 | <0.001 |
| Male | 1.38 (1.12 to 1.70) | 2.05 (1.60 to 2.64) | |||
| Age group (years) | 18–29 | 1 | 0.43 | 1 | 0.79 |
| 30–39 | 1.09 (0.84 to 1.42) | 1.01 (0.77 to 1.34) | |||
| 40–49 | 1.03 (0.76 to 1.39) | 0.94 (0.67 to 1.30) | |||
| > = 50 | 1.35 (0.93 to 1.97) | 0.84 (0.55 to 1.28) | |||
| Socioeconomic quintiles | Highest | 1 | 0.65 | ||
| High | 1.09 (0.79 to 1.50) | ||||
| Middle | 0.99 (0.72 to 1.37) | ||||
| Low | 1.10 (0.80 to 1.51) | ||||
| Lowest | 0.87 (0.62 to 1.22) | ||||
| CD4 group (cells/μl) | > = 200 | 1 | <0.001 | 1 | <0.001 |
| 100–199 | 1.34 (0.96 to 1.87) | 1.19 (0.84 to 1.69) | |||
| 50–99 | 2.33 (1.67 to 3.24) | 1.69 (1.19 to 2.40) | |||
| <50 | 2.98 (2.19 to 4.04) | 2.10 (1.52 to 2.91) | |||
| BMIa | Per 1 kg/m2 increase | 0.76 (0.71 to 0.81) | <0.001 | 0.85 (0.78 to 0.91) | <0.001 |
| Haemoglobin | Per 10 g/L increase | 0.82 (0.78 to 0.86) | <0.001 | ||
| Oedema | No | 1 | <0.001 | 1 | 0.004 |
| Yes | 3.01 (2.07 to 4.38) | 1.84 (1.21 to 2.78) | |||
| Baseline TBa treatment | No | 1 | <0.001 | 1 | <0.001 |
| Yes | 0.54 (0.39 to 0.76) | 0.44 (0.31 to 0.63) | |||
| Baseline cotrimoxazole treatment | No | 1 | 0.41 | ||
| Yes | 1.13 (0.85 to 1.49) | ||||
| CRPa (mg/l) | <10 | 1 | <0.001 | 1 | <0.001 |
| 10–49 | 1.77 (1.12 to 2.80) | 1.61 (1.00 to 2.58) | |||
| 50–159 | 3.80 (2.50 to 5.78) | 3.03 (1.97 to 4.66) | |||
| ≥160 | 5.43 (3.61 to 8.18) | 3.67 (2.40 to 5.62) | |||
| Fat-free mass index | Per 1 kg/m2 increase | 0.76 (0.66 to 0.88) | <0.001 | 0.80 (0.67 to 0.95) | 0.01 |
| Fat mass index | Per 1 kg/m2 increase | 0.75 (0.64 to 0.88) | 0.001 | 0.86 (0.70 to 1.05) | 0.15 |
| Grip strength | Per 1 kg increase | 0.94 (0.93 to 0.96) | <0.001 | 0.94 (0.92 to 0.96) | <0.001 |
| MUACa | Per 1 cm increase | 0.78 (0.74 to 0.82) | <0.001 | 0.87 (0.82 to 0.92) | <0.001 |
| Appetite score | > − 0.5 | 1 | 0.004 | 1 | 0.65 |
| −1.49 to−0.55 | 1.30 (0.89 to 1.91) | 1.20 (0.81 to 1.78) | |||
| <−1.5 | 1.83 (1.27 to 2.62 | 1.08 (0.72 to 1.61) | |||
| Phosphate (mmol/l) | Cubic spline | 0.001 | 0.40 |
aAbbreviations: BMI body mass index, MUAC mid-upper arm circumference, CRP C-reactive protein, TB tuberculosis
b n = 1815 except haemoglobin n = 1670, CRP n = 1762, fat-free mass n = 1461, grip strength n = 1752, MUAC n = 1802, appetite n = 684 (Tanzanian patients only), phosphate n = 1764
cMultivariable analysis adjusted for age, sex, CD4, BMI, oedema, TB treatment, CRP, grip strength, site, follow-up time in 3 week bands (association with mortality p < 0.002 except in appetite analysis p = 0.24) and study arm. Association with fat-free mass, MUAC and appetite not adjusted for BMI. n = 1685 except fat-free mass n = 1349, MUAC n = 1672, appetite n = 630, (Tanzanian patients only), Phosphate n = 1644
In unadjusted analysis there is good evidence that the association between CRP and mortality changes with study period (p = 0.01) and in adjusted analysis the effect of CRP on mortality appears larger after starting ART than before (Table 4). There is at least weak evidence of interactions between study period and each of study site, sex and grip strength. In each case there is a strong association with mortality in the pre-ART period with Tanzanians, men and those with low grip strength having an increased mortality rate. However, these association do not appear to persist after starting ART. BMI, fat-free mass index and MUAC measured at recruitment have similar hazard ratios before and after starting ART and no evidence of interaction with study period.
Table 4.
Poisson regression models for baseline factors associated with mortality during the pre- and post-ART periodsa
| Pre-ART period (n = 1656) c | After starting ART (n = 1334) d | ||||
|---|---|---|---|---|---|
| Factor | Level | Adjusted HRb (95 % CI) | p-value | Adjusted HRb (95 % CI) | p-value |
| Site | Zambia | 1 | <0.001 | 1 | 0.28 |
| Tanzania | 2.65 (1.82 to 3.84) | 1.20 (0.86 to 1.66) | |||
| Sex | Female | 1 | <0.001 | 1 | 0.16 |
| Male | 3.44 (2.31 to 5.14) | 1.29 (0.90 to 1.84) | |||
| Age group (years) | 18–29 | 1 | 0.65 | 1 | 0.71 |
| 30–39 | 1.18 (0.77 to 1.83) | 0.81 (0.55 to 1.20) | |||
| 40–49 | 1.04 (0.62 to 1.75) | 0.87 (0.56 to 1.35) | |||
| > = 50 | 0.83 (0.43 to 1.60) | 1.00 (0.56 to 1.76) | |||
| CD4 group (cells/μl) | > = 200 | 1 | 0.004 | 1 | 0.001 |
| 100–199 | 1.23 (0.69 to 2.19) | 1.34 (0.83 to 2.14) | |||
| 50–99 | 2.08 (1.21 to 3.59) | 1.60 (0.97 to 2.64) | |||
| <50 | 2.25 (1.35 to 3.59) | 2.33 (1.49 to 3.66) | |||
| BMIa | Per 1 kg/m2 increase | 0.93 (0.82 to 1.05) | 0.23 | 0.80 (0.72 to 0.89) | <0.001 |
| Oedema | No | 1 | 0.13 | 1 | 0.03 |
| Yes | 1.57 (0.88 to 2.82) | 2.03 (1.09 to 3.79) | |||
| Baseline TBa treatment | No | 1 | <0.001 | 1 | 0.003 |
| Yes | 0.32 (0.18 to 0.58) | 0.49 (0.30 to 0.79) | |||
| CRPa (mg/l) | <10 | 1 | 0.001 | 1 | <0.001 |
| 10–49 | 0.69 (0.32 to 1.49) | 3.00 (1.49 to 6.05) | |||
| 50–159 | 2.02 (1.13 to 3.62) | 4.79 (2.43 to 9.43) | |||
| ≥160 | 1.94 (1.09 to 3.45) | 5.94 (3.02 to 11.67) | |||
| Fat-free mass index | Per 1 kg/m2 increase | 0.97 (0.74 to 1.26) | 0.78 | 0.70 (0.56 to 0.89) | 0.003 |
| Grip strength | Per 1 kg increase | 0.90 (0.87 to 0.93) | <0.001 | 0.98 (0.95 to 1.01) | 0.12 |
| MUACa | Per 1 cm increase | 0.93 (0.85 to 1.02) | 0.11 | 0.84 (0.78 to 0.91) | <0.001 |
| Appetite score | > − 0.5 | 1 | 0.34 | 1 | 0.85 |
| −1.49 to−0.55 | 0.90 (0.50 to 1.61) | 1.00 (0.54 to 1.82) | |||
| <−1.5 | 1.31 (0.76 to 2.28) | 1.15 (0.65 to 2.05) | |||
| Phosphate (mmol/l) | Cubic spline | 0.06 | 0.50 | ||
aAbbreviations: ART antiretroviral therapy, BMI body mass index, MUAC mid-upper arm circumference, CRP C-reactive protein, TB tuberculosis
bMultivariable analysis adjusted for age, sex, CD4, BMI, oedema, TB treatment, CRP, grip strength, site, follow-up period and study arm. Post-ART analysis also adjusted for weeks spent pre-ART. Association with fat-free mass, MUAC and appetite not adjusted for BMI. Post-ART analysis also adjusted for weeks spent pre-ART
cPre-ART analysis; Fat-free mass n = 1331, MUAC n = 1643, Appetite n = 623, Phosphate n = 1615
dPost-ART analysis; Fat-free mass n = 1113, MUAC n = 1323, Appetite n = 478, Phosphate n = 1304
Baseline TB treatment was protective against mortality in both time periods. A secondary analysis of 1190 patients not on TB treatment at recruitment also shows a lower post-ART mortality rate among 117 patients who started TB treatment prior to initiating ART (HR 0.37 95 % CI 0.19, 0.72 p-value 0.004).
Risk of death before starting ART appears higher among patients with very high phosphate values (Fig. 1), however, there is no evidence of an association in the study overall (Table 3). There were few patients with extremely abnormal values (Table 2), so power to detect an association is low.
Fig. 1.
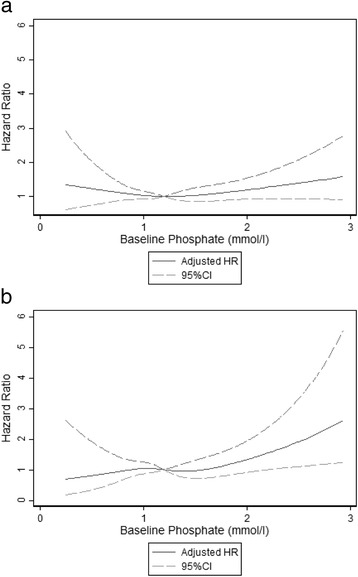
Adjusted hazard ratio* for baseline phosphate compared to median value of 1.2 mmol/l (using cubic splines with 4 knots). a. Hazard ratio throughout the study period. b. Hazard ratio in the pre-ART period. *Multivariable analysis adjusted for age sex, CD4, Hb, BMI, oedema, TB treatment, Cotrimoxazole, CRP, grip strength, site, follow-up period and study arm. Results shown for baseline phosphate <3mmol/l
One fifth of patients died from confirmed or suspected TB. Respiratory tract infections and diarrhoea were also common diagnoses for patients who died. Cause of death was not confirmed for one third of patients, the majority of whom died at home (Additional file 1: Table S1).
Discussion
The high mortality observed around the initiation of ART is a major concern for programme managers and clinicians in Africa. To our knowledge, this is one of the first studies to assess risk factors for mortality in both the period from referral for ART and in the early post-ART period among malnourished adults, a group comprising a third of new patients in some low-resource settings [2]. Well-established risk factors we found consistent with previous studies [4, 6, 9] include low BMI, low fat-free mass, low CD4, high CRP, and male sex. Less-frequently investigated factors associated with an increase in mortality include oedema, low grip strength and low MUAC. More surprising results were seen with TB treatment and phosphate level.
Overall mortality was high in our population but, when stratified by BMI (results not shown), was similar in the post-ART period to that found in a large Tanzanian study [3]. This suggests our patients were not unusual in their characteristics or medical care compared to other malnourished HIV-positive adults in Africa, and supports the validity of the extremely high mortality rates seen in the pre-ART period. Other studies also show higher mortality before, compared to the first months after, starting ART [1, 22]. When examined in more detail, the mortality rate in our study increased through the pre-ART period, highlighting the extreme vulnerability of these patients and further supporting the theory that much early ART mortality results from advanced disease. This confirms the value of immediate ART initiation, as recently recommended by WHO for all persons with HIV worldwide [23].
We expected receipt of TB treatment, as a proxy for TB infection, to be associated with higher risk of mortality [24] but it was actually strongly protective. This could suggest a substantial burden of undiagnosed TB is present in this population. A study conducted in Zambia and Tanzania at the same time as ours but among a population with median BMI of 19.3 found a similar proportion of patients (16 %) were on TB treatment at baseline [25]. However, previous work in Tanzania has shown that TB explains most of the weight deficit seen in TB/HIV co-infected patients [26] so we would expect to see more TB in our cohort of malnourished adults than in a better-nourished population. It is also possible that TB treatment was effective against other pathogens, including those causing diarrhoea [27], which might otherwise have contributed to mortality. Patients already on TB treatment when first assessed for ART may be better care-seekers and therefore healthier. However the protective effect of TB treatment on post-ART mortality remained when only studying patients who started TB treatment after recruitment. These findings call for greater efforts to be made to diagnose and treat TB, especially among malnourished patients for whom algorithms and investigations focused on respiratory symptoms and signs may be less sensitive. They may also support empiric treatment for TB, especially in undernourished individuals; however, prioritising TB treatment for HIV positive South Africans with high probability of TB, including BMI<18.5, did not reduce mortality [28].
Our prior studies, which formed the foundation for NUSTART’s design, found low pre-ART serum phosphate levels to be independently associated with early ART mortality [5, 29]. In contrast, in our population, serum phosphate did not show evidence of an association with overall mortality and high phosphate appeared to carry a greater risk in the pre-ART period. Our attempt to treat patients having low electrolyte levels with oral electrolyte supplementation may have reduced any impact of low phosphate on mortality. Serum phosphate levels are known to change over time and we are planning more detailed analysis of electrolytes as time-varying variables.
In this population, oedema carried a high risk of death. Very few patients presented with oedema, so the risk estimate lacked precision; however, oedema can be a sign of advanced malnutrition, so it is not a surprising finding. MUAC, which is routinely used in the diagnosis and monitoring of malnourished children but rarely for adults, was also strongly associated with mortality. It is inexpensive and simple to measure, even in very ill patients unable to stand, and may therefore have a role in resource-constrained settings and for severely ill patients where measuring BMI is not an option.
While some risk factors were significantly associated with mortality in both the pre-and post-ART periods, there are some interesting exceptions. Male sex was a risk only in the pre-ART period, possibly reflecting later care-seeking by men but an equivalently good response to ART as in women.
Grip strength is often considered a functional indicator of lean mass, and there is also evidence of an association with lipodystrophy and inflammation in HIV patients [30]. However, in this population low grip strength carried a risk for mortality independent of BMI and serum CRP. Grip strength of HIV positive adults starting ART in Ethiopia was improved by a lipid-based nutritional supplement [31] and it has been used as a component of ‘frailty’ in monitoring African patients on ART [32]. Therefore, further research is warranted into what affects grip strength, how it interacts with body composition, fat distribution, and inflammation, and how it performs as an adjunct to clinical care in populations at different stages of HIV or ART treatment.
Mortality was higher in Mwanza than Lusaka even in adjusted analyses that controlled for the slightly lower CD4 counts in Mwanza. This could be partly explained by the tracing of non-attendees to their home in Mwanza, but only by phone in Lusaka, therefore identifying more patients who died during the study. There may also have been additional, unmeasured factors indicative of more severe disease in Mwanza patients and the effect of localised conditions such as the higher incidence of malaria in Mwanza. Of note, once the Tanzanian patients were on ART treatment, they did as well as those in Zambia.
Our study benefited from a large sample size, excellent follow-up for the mortality outcome, and rich database of interview and anthropometric measures and biomarkers. It had several limitations as well. Due to financial constraints, we were unable to measure HIV viral load. Data on cause of death were often missing or unreliable, so we could not investigate how risk factors were associated with particular causes. Appetite could be analysed only for Mwanza patients because the appetite score was clustered and possibly unreliable in Lusaka, [18] so this analysis was under-powered. TB data relied on patient self-report which could be unreliable, however, this is unlikely to explain the large effect seen, especially in a population with high TB awareness and understanding. We could not analyse associations of particular ART regimens with mortality because there was a large difference in regimens between sites and the sites themselves differed in mortality risk.
Conclusions
Our results extend knowledge of risk factors for mortality of HIV patients to the high risk period between referral for ART and ART initiation which in most African clinics lasts several weeks. The extremely high mortality that occurred during this period provides further support for minimising delays in diagnosis and treatment initiation, especially among malnourished patients. The strong association between TB treatment and reduced mortality supports more commitment to diagnosis of TB of underweight patients commencing ART.
Acknowledgments
The authors are grateful to the European and Developing Countries Clinical Trials Partnership for funding the study, and to Nutriset, Malaunay, France for preparing the trial intervention supplements. We thank the Data Safety and Monitoring Board-Nicholas Paton (chair), Kathy Baisley (statistician), Muhammad Bakari (Tanzanian representative), Sekelani Banda (Zambian representative)-for their support and advice, and the on-site monitors, Jim Todd and Max Katubulushi, for their assistance. We are grateful to the study participants for their involvement.
The work was conducted by the NUSTART study team which includes: Principal Investigator: Suzanne Filteau; Senior investigators: Aase Bengaard Andersen, John Changalucha, Henrik Friis, Douglas C. Heimburger, Lackson Kasonka, Paul Kelly; Statisticians and other senior research fellows: John R. Koethe, Daniela Manno, Natasha Larke, Andrea M. Rehman, Susannah Woodd; Steering group: David Thurnham, Andrew Tomkins; Mwanza trial manager: George PrayGod; Lusaka trial managers: Molly Chisenga, Joshua Siame; Mwanza senior clinic team: Jeremiah Kidola, Denna Michael, Kelvin Musa, Charles Masilingi, Elizabeth Fue, Eva Masesa, Neema Mpandachalo; Lusaka senior clinic team: Anne Kanunga, Likando Munalula, Brenda Kapinda, Nellie Sikanyika; Laboratory technicians: Julius Mngara, George Ogweno, Piu Ikigo, Mutinta Muchimba, Memory Samwinga, Leo Beacroft, Ellen Besa, Harry Black, Celeste Gregg Smith; Administrators and data managers: Yolanda Fernandez, Gunda Wandore, Aswile Jonas, Hildah Banda Mabuda, Wakwoya Adugna; Pharmacists: Stephen Makandilo, Mwangana Mubita, Jessy Mulenga. We are grateful also to nurses, data entry clerks, drivers and other support staff at both NUSTART sites.
Funding
European and Developing Countries Clinical Trials Partnership funded the study. Nutriset France provided support in-kind through the development of the nutrition supplement for the main clinical trial.
They were not involved in study design or the collection, analysis and interpretation of data. Salary support for AMR was provided by the UK Medical Research Council through a grant to the LSHTM Tropical Epidemiology Group; grant code MR/K012126/1.
Availability of data and materials
The Zambian Government does not allow data from studies conducted in Zambia to be made publicly available. Anonymised data is available on request from the author for bone fide research purposes, as agreed with patients during the consent process.
Authors’ contributions
All authors were involved in conducting the NUSTART clinical trial, contributed to the interpretation of the results and commented on multiple versions of the paper. SW prepared the data and designed and conducted the analysis. SF was Principal Investigator; PK, DH and HF were senior investigators; GP (Tanzania), MC (Zambia) and JS (Zambia) were trial managers. AR and JK contributed towards the statistical analysis. All read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethical approval was obtained from the Research Ethics Committee of the London School of Hygiene and Tropical Medicine (5876), the University of Zambia Biomedical Research Ethics Committee (009-01-11) and the Medical Research Coordinating Committee of NIMR, Tanzania (NIMR/HQ/R.8 a/vol. IXI1102). All participants provided written or thumbprint informed consent. Medical care of patients was according to national guidelines and provided through the local health services.
Abbreviations
- 3TC
Lamivudine
- ART
Anti-retroviral therapy
- AZT
Zidovudine
- BMI
Body Mass Index
- CI
Confidence Interval
- CRP
C-reactive protein
- CV
Coefficient of variance
- EFV
Efavirenz
- FTC
Emtricitabine
- HR
Hazard ratio
- IQR
Inter-quartile range
- LNS
Lipid-based nutritional supplement
- LNS-VM
LNS with high level vitamins and minerals
- MUAC
Mid-upper arm circumference
- NIMR
National Institute for Medical Research (Tanzania)
- NUSTART
Nutritional Support for African Adults Starting Antiretroviral Therapy
- NVP
Nevirapine
- PCA
Principal component analysis
- QC
Quality control
- SES
Socio-economic status
- TB
Tuberculosis
- TDF
Tenofovir
- UTH
University Teaching Hospital (Zambia)
Additional file
Cause of death during the pre and post-ART periods. Cause of death obtained from hospital records for patients who died during the study, presented separately for deaths during the pre-ART and post-ART time periods. (DOCX 14 kb)
Contributor Information
Susannah L Woodd, Email: susannah.woodd@lshtm.ac.uk.
Paul Kelly, Email: m.p.kelly@qmul.ac.uk.
John R. Koethe, Email: john.r.koethe@Vanderbilt.Edu
George Praygod, Email: gpraygod@gmail.com.
Andrea M. Rehman, Email: andrea.rehman@lshtm.ac.uk
Molly Chisenga, Email: mchisenga04@yahoo.co.uk.
Joshua Siame, Email: josiame@yahoo.com.
Douglas C. Heimburger, Email: douglas.heimburger@Vanderbilt.Edu
Henrik Friis, Email: hfr@nexs.ku.dk.
Suzanne Filteau, Email: suzanne.filteau@lshtm.ac.uk.
References
- 1.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koethe JR, Lukusa A, Giganti MJ, Chi BH, Nyirenda CK, Limbada MI, et al. Association between Weight Gain and Clinical Outcomes Among Malnourished Adults Initiating Antiretroviral Therapy in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2010;53:507–513. doi: 10.1097/QAI.0b013e3181b32baf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu E, Spiegelman D, Semu H, Hawkins C, Chalamilla G, Aveika A, et al. Nutritional status and mortality among HIV-infected patients receiving antiretroviral therapy in Tanzania. J Infect Dis. 2011;204(2):282–290. doi: 10.1093/infdis/jir246. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A, Nadkarni G, Yang WT, Chandrasekhar A, Gupte N, Bisson GP, et al. Early Mortality in Adults Initiating Antiretroviral Therapy (ART) in Low-and Middle-Income Countries (LMIC): A Systematic Review and Meta-Analysis. PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heimburger DC, Koethe JR, Nyirenda C, Bosire C, Chiasera JM, Blevins M, et al. Serum phosphate predicts early mortality in adults starting antiretroviral therapy in Lusaka, Zambia: a prospective cohort study. PLoS ONE. 2010;5(5) doi: 10.1371/journal.pone.0010687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marazzi MC, Liotta G, Germano P, Guidotti G, Altan AD, Ceffa S, et al. Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res Hum Retroviruses. 2008;24(4):555–560. doi: 10.1089/aid.2007.0217. [DOI] [PubMed] [Google Scholar]
- 7.Dao CN, Peters PJ, Kiarie JN, Zulu I, Muiruri P, Ong’ech J, et al. Hyponatremia, hypochloremia, and hypoalbuminemia predict an increased risk of mortality during the first year of antiretroviral therapy among HIV-infected Zambian and Kenyan women. AIDS Res Hum Retroviruses. 2011;27(11):1149–1155. doi: 10.1089/aid.2010.0345. [DOI] [PubMed] [Google Scholar]
- 8.Worodria W, Massinga-Loembe M, Mazakpwe D, Luzinda K, Menten J, Van Leth F, et al. Incidence and predictors of mortality and the effect of tuberculosis immune reconstitution inflammatory syndrome in a cohort of TB/HIV patients commencing antiretroviral therapy. J Acquir Immune Defic Syndr. 2011;58(1):32–37. doi: 10.1097/QAI.0b013e3182255dc2. [DOI] [PubMed] [Google Scholar]
- 9.Argemi X, Dara S, You S, Mattei JF, Courpotin C, Simon B, et al. Impact of malnutrition and social determinants on survival of HIV-infected adults starting antiretroviral therapy in resource-limited settings. AIDS. 2012;26(9):1161–1166. doi: 10.1097/QAD.0b013e328353f363. [DOI] [PubMed] [Google Scholar]
- 10.Filteau S, PrayGod G, Kasonka L, Woodd S, Rehman AM, Chisenga M, et al. Effects on mortality of a nutritional intervention for malnourished HIV-infected adults referred for antiretroviral therapy: a randomised controlled trial. BMC Med. 2015;13(1):17. doi: 10.1186/s12916-014-0253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rehman AM, Woodd S, PrayGod G, Chisenga M, Siame J, Koethe JR, et al. Effects on Anthropometry and Appetite of Vitamins and Minerals Given in Lipid Nutritional Supplements for Malnourished HIV-Infected Adults Referred for Antiretroviral Therapy: Results From the NUSTART Randomized Controlled Trial. J Acquir Immune Defic Syndr. 2015;68(4):405. doi: 10.1097/QAI.0000000000000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 13.Ashworth A, Khanum S, Jackson A, Schofield C. Guidelines for the inpatient treatment of severely malnourished children2003. Available from: http://www.who.int/nutrition/publications/severemalnutrition/9241546093/en/index.html.
- 14.Lohman T, Roche A, Martorell R. Anthropometric standardization reference manual. Champaign: Human Kinetics Publications; 1988. [Google Scholar]
- 15.Ulijaszek S, Kerr D. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr. 1999;82:165–177. doi: 10.1017/S0007114599001348. [DOI] [PubMed] [Google Scholar]
- 16.Wibaek R, Kaestel P, Skov S, Christensen D, Girma T, Wells J, et al. Calibration of bioelectrical impedance analysis for body composition assessment in Ethiopian infacnts using air-displacement plethysmography. Eur J Clin Nutr. 2015. [DOI] [PubMed]
- 17.Kolenikov SAG. The Use of discrete Data in PCA: Theory, simulations, and Application to Socioeconomic Indices. In: Manly BFJ, editor. Multivariate Statistical Methods: A Primer. 3. Boca Raton: CRC Press; 2004. [Google Scholar]
- 18.Rehman A, Woodd S, Chisenga M, Siame J, Sampson G, PrayGod G, et al. Appetite testing in HIV-infected African adults recovering from malnutrition and given antiretroviral therapy. Pub Health Nutr. 2014;17. [DOI] [PMC free article] [PubMed]
- 19.Filmer D, Pritchett L. Estimating wealth effects without expenditure data-or tears: an application to educational enrolments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 20.Division of AIDS. Division of AIDS table for grading the severity of adult and pediatric adverse events 2009. Available from: http://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf.
- 21.Tietz N. Clinical Guide to Laboratory Tests. 3. Philadelphia: WB Saunders; 1995. [Google Scholar]
- 22.Hoffmann CJ, Lewis JJ, Dowdy DW, Fielding KL, Grant AD, Martinson NA, et al. Mortality associated with delays between clinic entry and ART initiation in resource-limited settings: results of a transition-state model. J Acquir Immune Defic Syndr. 2013;63(1):105–111. doi: 10.1097/QAI.0b013e3182893fb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV: World Health Organisation; 2015. Available from: http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/. [PubMed]
- 24.Gupta A, Wood R, Kaplan R, Bekker L-G, Lawn SD. Prevalent and incident tuberculosis are independent risk factors for mortality among patients accessing antiretroviral therapy in South Africa. PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0055824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet. 2015;385:2173–2182. doi: 10.1016/S0140-6736(15)60164-7. [DOI] [PubMed] [Google Scholar]
- 26.Praygod GA. The role of micronutrient and energy-protein supplementation during tuberculosis treatment: determinants of body composition and effects of supplementation. Copenhagen: University of Copenhagen; 2010. [Google Scholar]
- 27.Mwinga A, Hosp M, Zulu I, Farthing MJ, Mulambo S, Kelly P. Tuberculosis preventative treatment also prevented diarrhoea in HIV-infected patients in Zambia. AIDS. 2002;16(5):806–808. doi: 10.1097/00002030-200203290-00026. [DOI] [PubMed] [Google Scholar]
- 28.Grant A, Charalambous S, Tlali M, Johnson S, Dorman S, Hoffmann C. Empirical TB Treatment in Advanced HIV Disease: Results of the TB Fast Track Trial. Conference on Retroviruses and Opportunistic Infections. 2016; Abstract 155.
- 29.Koethe JR, Blevins M, Nyirenda CK, Kabagambe EK, Chiasera JM, Shepherd BE, et al. Serum phosphate predicts early mortality among underweight adults starting ART in Zambia: a novel context for refeeding syndrome? Journal of nutrition and metabolism. 2013. [DOI] [PMC free article] [PubMed]
- 30.Crawford KW, Li X, Xu X, Abraham AG, Dobs AS, Margolick JB, et al. Lipodystrophy and inflammation predict later grip strength in HIV-infected men: the MACS Body Composition substudy. AIDS Res Hum Retroviruses. 2013;29(8):1138–1145. doi: 10.1089/aid.2013.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen MF, Abdissa A, Kæstel P, Tesfaye M, Yilma D, Girma T, et al. Effects of nutritional supplementation for HIV patients starting antiretroviral treatment: randomised controlled trial in Ethiopia. BMJ. 2014;348:g3187. doi: 10.1136/bmj.g3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathai S, Gilbert C, Weiss HA, Cook C, Wood R, Bekker LG, et al. Frailty in HIV-infected adults in South Africa. J Acquir Immune Defic Syndr. 2013;62(1):43–51. doi: 10.1097/QAI.0b013e318273b631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Zambian Government does not allow data from studies conducted in Zambia to be made publicly available. Anonymised data is available on request from the author for bone fide research purposes, as agreed with patients during the consent process.


