Abstract
Adult workers in honeybee (Apis mellifera) colonies exhibit plasticity in hormonally regulated, age-based division of labor by altering their pattern of behavioral development in response to changes in colony conditions. One form of this plasticity is precocious development: levels of juvenile hormone increase prematurely and bees begin foraging as much as 2 weeks earlier than average. We used two experimental paradigms inspired by developmental biology to study how bees obtain information on changing colony needs that results in precocious foraging. An analog of "cell culture," with bees reared outside of colonies in different sized groups, revealed that worker-worker interactions exert quantitative effects on endocrine and behavioral development. "Transplants" of older bees to colonies otherwise lacking foragers demonstrated that worker-worker interactions also affect behavioral development in whole colonies. These results provide insights to a long-standing problem in the biology of social insects and further highlight similarities in the integration of activity that exist between individuals in insect colonies and cells in metazoans.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blau H. M., Baltimore D. Differentiation requires continuous regulation. J Cell Biol. 1991 Mar;112(5):781–783. doi: 10.1083/jcb.112.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin J. T., Warchol M. E. Auditory hair cells: structure, function, development, and regeneration. Annu Rev Neurosci. 1991;14:301–333. doi: 10.1146/annurev.ne.14.030191.001505. [DOI] [PubMed] [Google Scholar]
- Doe C. Q., Goodman C. S. Early events in insect neurogenesis. II. The role of cell interactions and cell lineage in the determination of neuronal precursor cells. Dev Biol. 1985 Sep;111(1):206–219. doi: 10.1016/0012-1606(85)90446-4. [DOI] [PubMed] [Google Scholar]
- Feyereisen R., Tobe S. S. A rapid partition assay for routine analysis of juvenile hormone release by insect corpora allata. Anal Biochem. 1981 Mar 1;111(2):372–375. doi: 10.1016/0003-2697(81)90575-3. [DOI] [PubMed] [Google Scholar]
- Grant D. S., Tashiro K., Segui-Real B., Yamada Y., Martin G. R., Kleinman H. K. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like structures in vitro. Cell. 1989 Sep 8;58(5):933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Heitzler P., Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell. 1991 Mar 22;64(6):1083–1092. doi: 10.1016/0092-8674(91)90263-x. [DOI] [PubMed] [Google Scholar]
- Pratt G. E., Tobe S. S. Juvenile hormones radiobiosynthesised by corpora allata of adult female locusts in vitro. Life Sci. 1974 Feb 1;14(3):575–586. doi: 10.1016/0024-3205(74)90372-5. [DOI] [PubMed] [Google Scholar]
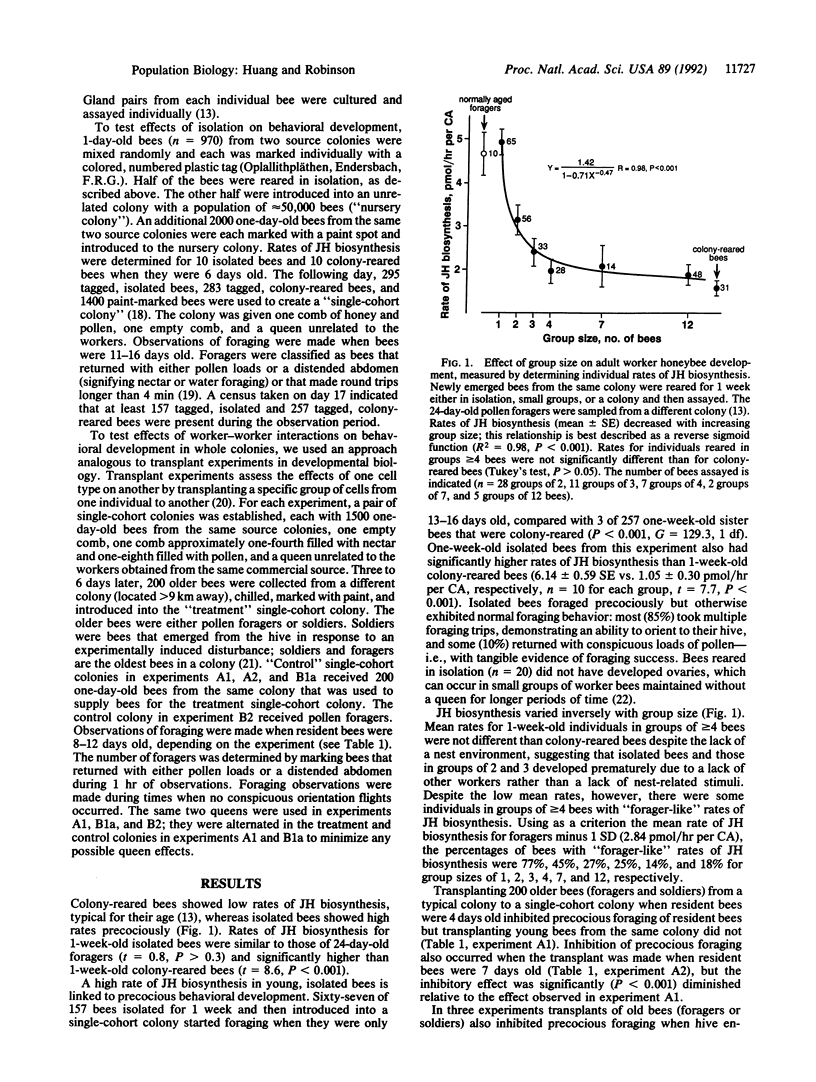
- Robinson G. E., Page R. E., Jr, Strambi C., Strambi A. Hormonal and genetic control of behavioral integration in honey bee colonies. Science. 1989 Oct 6;246(4926):109–112. doi: 10.1126/science.246.4926.109. [DOI] [PubMed] [Google Scholar]
- Robinson G. E. Regulation of division of labor in insect societies. Annu Rev Entomol. 1992;37:637–665. doi: 10.1146/annurev.en.37.010192.003225. [DOI] [PubMed] [Google Scholar]
- Robinson G. E., Strambi A., Strambi C., Paulino-Simões Z. L., Tozeto S. O., Barbosa J. M. Juvenile hormone titers in European and Africanized honey bees in Brazil. Gen Comp Endocrinol. 1987 Jun;66(3):457–459. doi: 10.1016/0016-6480(87)90258-9. [DOI] [PubMed] [Google Scholar]
- Tobe S. S., Pratt G. E. The influence of substrate concentrations on the rate of insect juvenile hormone biosynthesis by corpora allata of the desert locust in vitro. Biochem J. 1974 Oct;144(1):107–113. doi: 10.1042/bj1440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel G. M., Zhang W., Tomlinson C. R., Lennarz W. J., Klein W. H. Transcription of the Spec 1-like gene of Lytechinus is selectively inhibited in response to disruption of the extracellular matrix. Development. 1989 Jun;106(2):355–365. doi: 10.1242/dev.106.2.355. [DOI] [PubMed] [Google Scholar]
- Wilson E. O. The sociogenesis of insect colonies. Science. 1985 Jun 28;228(4707):1489–1495. doi: 10.1126/science.228.4707.1489. [DOI] [PubMed] [Google Scholar]


