Abstract
Methanoculleus bourgensis strain MAB1 has been identified as the hydrogenotrophic partner of mesophilic acetate-oxidising bacteria, a syntrophic relationship operating close to the thermodynamic equilibrium and of considerable importance in ammonia-rich engineered biogas processes. Methanoculleus bourgensis strain MAB1 belongs to the order Methanomicrobiales, family Methanomicrobiaceae, within the phylum Euryarchaeota. The genome shows a total size of 2,859,299 bp encoding 3450 predicted protein-encoding genes, of which only 1472 (43 %) have been assigned tentative functions. The genome encodes further 44 tRNA genes and three rRNA genes (5S, 16S and 23S rRNA). This study presents assembling and annotation features as well as genomic traits related to ammonia tolerance and methanogenesis.
Electronic supplementary material
The online version of this article (doi:10.1186/s40793-016-0199-x) contains supplementary material, which is available to authorized users.
Keywords: Syntrophy, Methanogens, Methane production, Biogas process, Syntrophic acetate-oxidising bacteria
Introduction
In anaerobic digestion processes, syntrophy is a particular important interspecies relationship that is of benefit to all contributing partners and is essential for the methanogenesis of organic matter [1, 2]. Syntrophic interaction operates close to the thermodynamic equilibrium, whereby both partners have to share the limited energy released in the overall reactions [2]. Syntrophic acetate-oxidation (SAO) releases a very small amount of energy (ΔGo` = −35 kJ per mol rct), just enough to support microbial growth. The two-step reaction starts with the oxidation of acetate to CO2 and hydrogen/formate performed by so-called syntrophic acetate-oxidising bacteria. This can only proceed when, in a second step, the hydrogen/formate is immediately consumed by a hydrogenotrophic methanogenic archaea reducing CO2 to methane, which makes the overall acetate oxidation thermodynamically favourable [1]. In a mesophilic co-culture, the hydrogen partial pressure has been observed to be as low as 1.6–6.8 Pa [3] and in thermophilic co-cultures as low as 10–50 Pa [4].
Hydrogenotrophic methanogens, mainly belonging to the order Methanomicrobiales and Methanobacteriales, have been shown to be present in high abundances in thermophilic and mesophilic high ammonia biogas digesters [5–8]. Methanothermobacter thermoautrophicus affiliating to the order Methanobacterales has been isolated as a methanogenic partner in thermophilic SAO [9, 10]. Within the order Methanomicrobiales, members of the genus Methanoculleus have been reported to be the prevailing species in ammonia-enriched processes dominated by SAO [6, 11–13]. In total, four methanogenic strains have been isolated from ammonia-rich mesophilic biogas processes [11, 14, 15], named MAB1, MAB2, MAB3 and BA1, which are phylogenetically affiliated to the species Methanoculleus bourgensis. MAB1 and BA1 have proven to be a suitable methanogenic partner for mesophilic syntrophic acetate-oxidising bacteria Clostridium ultunense, “Tepidanaerobacter acetatoxydans ” and Syntrophaceticus schinkii [16–18]. One of the major characteristics of SAO communities is that they can tolerate ammonia levels up to 1 g/L, giving them a selective advantage over aceticlastic methanogens, which convert acetate directly to methane and cannot tolerate such high concentrations [6, 8, 11, 19–22].
This study reports the genome sequencing, assembly and annotation of the methanogenic SAOB partner Methanoculleus bourgensis strain MAB1, a key organism in methane production from ammonia-rich feed stocks in anaerobic digestion processes.
Organism information
Classification and features
Methanoculleus bourgensis MAB1 is an obligate anaerobic archaea that has been isolated from a mesophilic methanogenic reactor operating with swine manure at 6 g NH4 + −N/L and a pH of 7.5. The isolated cells were between 1.0 and 3.0 μm in diameter, irregular and coccoid in shape (Fig. 1) and surrounded by a protein S-layer [11]. The strain forms methane from H2/CO2, formate, 2-propanol and 1,2-propanol, but not from acetate, which is required for growth. A more detailed description can be found in [11]. Although isolated from mesophilic reactors, the optimal methane production rate has been observed at hyper-mesophilic temperatures of between 44 and 45 °C [23]. It can probably tolerate ammonia concentrations up to 1 g/L [12]. Minimum information about the genome sequence (MIGS) of M. bourgensis strain MAB1 is given in Table 1 and Table S1 (Additional file 1).
Fig. 1.
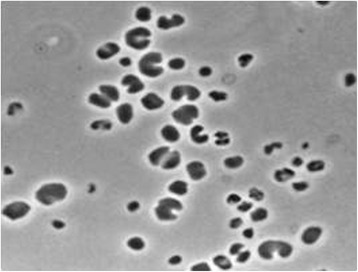
Image. Micrograph of Methanoculleus bourgensis strain MAB1
Table 1.
Classification and general features Methanoculleus bourgensis strain MAB1 according to the MIGS specification [42]
| MIGS ID | Property | Term | Evidence codea |
|---|---|---|---|
| Classification | Domain Archaea | TAS [43] | |
| Phylum Euryarchaeotes | TAS [44] | ||
| Class Methanomicrobia | TAS [45, 46] | ||
| Order Methanomicrobiales | TAS [47, 48] | ||
| Family Methanomicrobiaceae | TAS [49] | ||
| Genus Methanoculleus | TAS [50] | ||
| Species Methanoculleus bourgensis | TAS [18, 51] | ||
| Strain MAB1 | TAS [11] | ||
| Gram stain | Negative | TAS [11] | |
| Cell shape | Irregular coccus | TAS [11] | |
| Motility | Not observed | TAS [11] | |
| Sporulation | Not observed | TAS [11] | |
| Temperature range | 15–50 °C | TAS [23] | |
| Optimum temperature | 44–45 °C | TAS [23] | |
| Carbon source | CO2 | TAS [11] | |
| MIGS-6 | Habitat | Anaerobic digester | TAS [11] |
| MIGS-6.3 | Salinity | 0.0–0.220 M NH4Cl | TAS [11] |
| MIGS-22 | Oxygen requirement | Anaerobe | TAS [11] |
| MIGS-15 | Biotic relationship | Syntrophy (beneficial), free living | TAS [11] |
| MIGS-14 | Pathogenicity | Not reported | NAS |
| MIGS-4 | Geographic location | Biogas reactor, Uppsala, Sweden | NAS |
| MIGS-5 | Sample collection | 1989 | NAS |
| MIGS-4.1 | Latitude | 59.8581° N | NAS |
| MIGS-4.2 | Longitude | 17.6447° E | NAS |
| MIGS-4.4 | Altitude | not applicable | NAS |
aEvidence codes - IDA Inferred from Direct Assay, TAS Traceable Author Statement (i.e., a direct report exists in the literature); NAS Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence) These evidence codes are from the Gene Ontology project [52]
Phylogenetic analysis of the single 16 s rRNA gene copy affiliates M. bourgensis MAB1 to the Methanomicrobia class within the phylum Euryarchaeota and therein to the family Methanomicrobiaceae (RDP Naive Bayesian rRNA Classifier Version 2.10, October 2014). The comparison of the 16S rRNA gene with the latest available databases from GenBank (2016-01-29) using BLAST under default settings have revealed Methanoculleus marisnigri JR1 (NC_009051.1) to be the closest current relative, sharing 97 % identity (Fig. 2). The type strain is Methanoculleus bourgensis MS2 (T), whose 16 s rRNA gene is 99 % identical to strain MAB1 and which was isolated from a tannery by-product enrichment culture inoculated with sewage sludge [24]. Methanoculleus olentangyi and Methanoculleus oldenburgensis are subjective synonyms [25]. Cells of M. bourgensis strain MAB1 show a polyamine pattern that is distinctly different from the type strain MS2 [11].
Fig. 2.
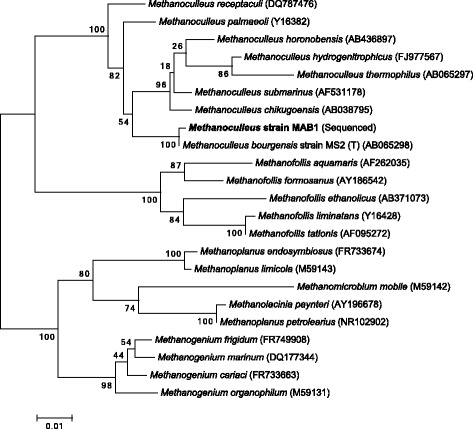
Phylogentic tree. Maximum likelihood tree highlighting the phylogenetic position of Methanoculleus bourgensis strain MAB1 within the family Methanomicrobiacaea. The 16S rRNA-based alignment was carried out using MUSCLE [53] and the phylogenetic tree was inferred from 1521 aligned characteristics of the 16S rRNA gene sequence using the maximum-likelihood (ML) algorithm [54] with MEGA 6.06 [55, 56]. Bootstrap analysis [57] with 100 replicates was performed to assess the support of the clusters
Genome sequencing information
Genome project history
Methanoculleus bourgensis MAB1 was sequenced and annotated by the SLU-Global Bioinformatics Centre at the Swedish University of Agricultural Sciences, Uppsala, Sweden. The genome project is deposited in the Genomes OnLine Database [26] with GOLD id Gb0126792, and the complete genome is deposited in the European Nucleotide Archive database with accession number ERS1044365. This methanogenic partner of SAOB was selected for sequencing on the basis of environmental relevance to issues in global carbon cycling, alternative energy production and geochemical importance. Table 2 contains a summary of the project information.
Table 2.
Project information
| MIGS ID | Property | Term |
|---|---|---|
| MIGS-31 | Finishing quality | Complete |
| MIGD-28 | Libraries used | Ion Torrent single end reads |
| MIGS-29 | Sequencing platform | Ion Torrent PGM Systems |
| MIGS-31.2 | Sequencing coverage | 35× |
| MIGS-30 | Assemblers | Newbler 2.8 and MIRA 4.0 |
| MIGS-32 | Gene calling method | PRODIGAL and AMIGene |
| Locus Tag | MMAB1 | |
| Genbank ID | LT158599.1 | |
| GenBank Data of release | 12-FEB-2016 | |
| GOLD ID | Gb0126792 | |
| BIOPROJECT | PRJEB12532 | |
| MIGS 13 | Source Material Identifier | Biogas digester sludge |
| Project relevance | Biogas production |
Growth conditions and genomic DNA preparation
The strain had been stored as liquid cultures since its isolation in the laboratory. For DNA isolation, batch cultures were grown in basal medium as described by Zehnder et al. [27] and modified by Schnürer et al., [28] supplemented with 5 mM acetate and 0.3 M NH4Cl2. The headspace was filled with H2/CO2 (80:20, v/v). Cells were grown over 2 months at 37 °C without shaking, and harvested at 5000 X g. DNA was isolated using the Blood & Tissue Kit from Qiagen (Hilden, Germany) according to the standard protocol, but omitting the lysozyme step. The quality was visualised by agarose gel electrophoresis and the quantity determined by fluorometric measurements using Qubit (Thermo Fisher Scientific, Waltham, MA, USA).
Genome sequencing and assembly
The genome of Methanoculleus bourgensis strain MAB1 was sequenced at the SciLifeLab Uppsala, Sweden using Ion Torrent PM systems with a mean length of 206 bp, a longest read length 392 bp and a total of final library reads of 2,985,963 for single end reads. General aspects about the sequencing performed can be found on the SciLifeLab website [29]. The FastQC software package [30] was used for read quality assessment. After preassembly quality checking, the reads were assembled with MIRA 4.0 and Newbler 2.8 assemblers. Possible miss-assemblies were corrected manually using Tablet, a graphical viewer for visualisation of assemblies and read mappings [31]. Whole-genome assembly of the M. bourgensis strain MAB1 genome was accomplished using a comparative genome assembly method [32], which combines de novo and mapping assemblies. The filtered reads were fed into MIRA version 4.0 [33] for both mapping and de novo assembly, and the same read data were also provided to Newbler 2.8 de novo assembler. Mapping assembly was undertaken against the available genome of Methanoculleus marisnigri JR1 (accession no. NC_009051.1). Contigs produced through de novo assembly of read data from both assemblers were sorted and oriented along the reference genome and then aligned to the mapping assembly using Mauve genome alignment software [34]. Alignment of contigs to mapping assembly indels covered all the gaps in the genome. These covered gaps were all verified through PCR amplification using a Hot Start High-fidelity DNA polymerase (Phusion, Thermo Fisher Scientific, Waltham, MA, USA) and subsequent Sanger sequencing (Macrogen Corporation, Geumcheon District, South Korea). The complete genome sequence of Methanoculleus bourgensis strain MAB1 contained 2,859,299 bp based on the analysis performed using the tools summarised above.
Genome annotation
Automated gene modelling was completed by MaGe [35], a bacterial genome annotation system. Genes were identified using Prodigal [36] and AMIGene [37] as part of the MaGe genome annotation pipeline. The predicted CDSs were translated and used to search the NCBI non-redundant database and UniProt, TIGRFam, Pfam, PRIAM, KEGG, COG and InterPro databases using BLASTP. Predicted coding sequences were subjected to manual analysis using the MaGe web-based platform, which also provides functional information about proteins and was used to assess and correct genes predicted through the automated pipeline. The predicted functions were also further analysed by the MaGe annotation system (Fig. 4).
Fig. 4.
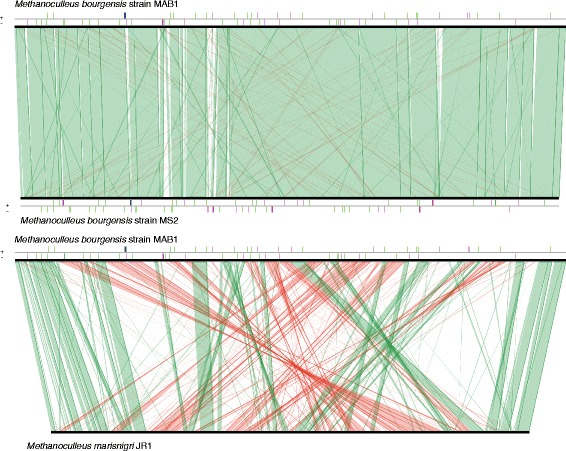
Synteny comparison. Synteny comparison of Methanoculleus bourgensis strain MAB1 genome with the closely related genome of Methanoculleus marisnigri strain JR1 and the type strain Methanoculleus bourgensis strain MS2. Linear comparisons of all predicted gene loci from Methanoculleus bourgensis strain MAB1 with Methanoculleus marisnigri strain JR1 and Methanoculleus bourgensis strain MS2, respectively, were performed using the built-in tool in MaGe platform with the synton size of > = three genes. The lines indicate syntons between two genomes. Red lines show inversions around the origin of replication. Vertical bars on the border line indicate different elements in genomes such as pink: transposases or insertion sequences: blue: rRNA and green: tRNA
Genome properties
The complete genome comprised a single contig with a total size of 2,859,299 bp and a calculated GC content of 60.26 %. The genome showed a protein coding density of 84.59 % with an average intergenic length of 162.92 bp. The genome encoded a further 44 tRNA genes and three rRNA genes (5S, 16S and 23S rRNA) (Table 3, Fig. 3).
Table 3.
Genomic statistics
| Attribute | Value | % of total |
|---|---|---|
| Genome size (bp) | 2,859,299 | 100.00 |
| DNA Coding (bp) | 2,430,404 | 85.00 |
| DNA G + C (bp) | 1,723,014 | 60.26 |
| DNA scaffolds | 1 | - |
| Total genes | 3507 | 100.00 |
| Protein coding genes | 3450 | 98.37 |
| RNA genes | 44 | 1.25 |
| Pseudo gene | 57 | 1.62 |
| Genes in internal clusters | 1580 | 45.00 |
| Genes with function prediction | 1472 | 43.00 |
| Genes assigned to COGs | 2323 | 66.24 |
| Genes with Pfam domains | 2801 | 79.84 |
| Genes with signal peptides | 332 | 9.46 |
| Genes with transmembrane helices | 650 | 18.53 |
| CRISPR repeats | 3 | .08 |
Fig. 3.
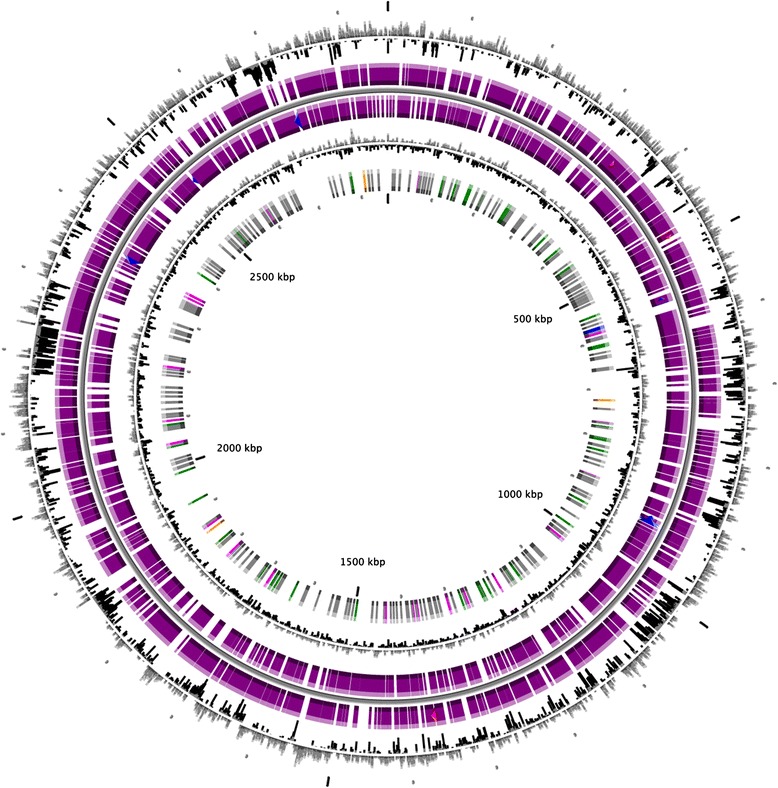
Circular map. Circular map of the Methanoculleus bourgensis strain MAB1 genome (from the outside to the centre): (1) GC percent deviation (GC window - mean GC) in a 1000-bp window; (2) predicted CDSs transcribed in a clockwise direction; (3) predicted CDSs transcribed in a counterclockwise direction; (4) GC skew (G + C/G-C) in a 1000-bp window; (5) rRNA (blue), tRNA (green), misc_RNA (orange), transposable elements (pink) and pseudogenes (grey)
The genome of Methanoculleus bourgensis strain MAB1 genome contained 3450 predicted protein-encoding genes, of which 1472 (43 %) have been assigned tentative functions. The remaining 1978 ORFs were hypothetical/unknown proteins. 2323 (app. 66 %) of all predicted protein-encoding genes could be allocated to the 22 functional COGs. Analysis of COGs revealed that ~21 % of all protein-encoding genes fell into four main categories: energy metabolism (6.4 %), amino acid transport and metabolism (5.9 %), coenzyme transport and metabolism (4.6 %) replication, and recombination and repair (4.2 %) (Table 4).
Table 4.
Number of genes associated with the general COG functional categories
| Code | Value | % age | Description |
|---|---|---|---|
| J | 180 | 5.13 | Translation, ribosomal structure and biogenesis |
| A | 2 | 0.05 | RNA processing and modification |
| K | 119 | 3.39 | Transcription |
| L | 146 | 4.16 | Replication, recombination and repair |
| B | 5 | 0.14 | Chromatin structure and dynamics |
| D | 32 | 0.91 | Cell cycle control, cell division, chromosome partitioning |
| Y | 0 | 0.00 | Nuclear structure |
| V | 51 | 1.45 | Defence mechanisms |
| T | 82 | 2.33 | Signal transduction mechanisms |
| M | 114 | 3.25 | Cell wall/membrane/envelope biogenesis |
| N | 30 | 0.85 | Cell motility |
| Z | 0 | 0.00 | Cytoskeleton |
| W | 0 | 0.00 | Extracellular structures |
| U | 29 | 0.82 | Intracellular trafficking, secretion and vesicular transport |
| O | 120 | 3.42 | Posttranslational modification, protein turnover, chaperones |
| C | 224 | 6.38 | Energy production and conversion |
| G | 128 | 3.64 | Carbohydrate transport and metabolism |
| E | 208 | 5.93 | Amino acid transport and metabolism |
| F | 70 | 1.99 | Nucleotide transport and metabolism |
| H | 160 | 4.56 | Coenzyme transport and metabolism |
| I | 42 | 1.19 | Lipid transport and metabolism |
| P | 174 | 4.96 | Inorganic ion transport and metabolism |
| Q | 34 | 0.96 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 429 | 12.23 | General function prediction only |
| S | 333 | 9.49 | Function unknown |
| 1184 | 34.00 | Not in COGs |
Insights from the genome sequence
Synteny-based analysis revealed that Methanoculleus bourgensis strain MAB1 had approximately 55 % of the total genome size in synteny with its closest relative Methanoculleus marisnigri JR1 (Fig. 4). The type strain Methanoculleus bourgensis strain MS2 had approximately 70 % of the total genome size in synteny with Methanoculleus bourgensis strain MAB1 (Fig. 4). A comparison of all inferred proteins of M. bourgensis strain MAB1 with all proteins collected in the NCBI RefSeq database revealed the highest number of orthologous (2800: 79.75 %) with M. bourgensis strain MS2 and next to M. marisnigri JR1 (2163: 61.61 %).
Analysis of COGs revealed that 2323 (app. 66 %) of all predicted protein-encoding genes of M. bourgensis strain MAB1 could be allocated to the 22 functional COGs, which is slightly lower than that predicted for M. bourgensis strain MS2 (2072 genes; 69 %) and for M. marisnigri JR1 (2016 genes; 75 %), where the protein-encoding genes of both could be allocated to 23 functional GOGs.
Although Methanoculleus bourgensis strain MAB1 has not yet been observed to express a flagellum, a cluster (MMAB1_2416, MMAB1_2434;MMAB1_0328) encoding flagellum (flaB,H,J,K) and chemotaxis (cheW,A,D,C) related genes (MMAB1_2416-2434) indicate chemotactic capabilities [38].
The genome does not contain genes related to ammonium transport systems, which have been found to be encoded by the genome of its close relative Methanoculleus marisnigri (BlastP search using ammonium transporter of M. marisnigri as protein query), and might therefore be considered an adaptation to high osmolarity environments and to high ammonium levels in particular. The same genotype has also described for the type strain MS2 [39] and for its acetate-oxidising syntrophic partner organism “T. acetatoxydans ” [40] and S. schinkii (unpublished). As also predicted for the type strain MS2 [39], a putative potassium ABC transport system (MMBA1_2581,MMAB1_2585) with an adjacent two-component regulatory system (MMAB1_2586, MMAB1_2587), two cation transporter (MMAB1_0409, MMAB1_1566), two potassium antiporter (MMBA1_1794, MMAB1_2374), a choline/carnitine/betaine transporter and one glycine/betaine ABC transport system might be involved in osmoregulation.
The genes encoding the methanogenesis pathway from H2 and CO2, including formylmethanofuran dehydrogenase (MMAB1_2225-MMAB1_2227), formylmethanofuran-tetrahydromethanopterin fromyltransferase (MMAB1_2217), methenyltetrahydromethanopterin cyclohydrolase (MMAB1_2292), methylenetetrahydromethanopterin dehydrogenase (MMAB1_2159), methylenetetrahydromethanopterin reductase (MMAB1_2155), tetrahydromethanopterin S-methyltransferase (MMAB1_2236-MMAB1_2244), methyl-CoM reductase (MMAB1_2231-2235) and CoB-CoM heterodisulfide reductase (MMAB1_2220), were found clustering together. The genome further revealed duplicates in the case of formylmethanofuran dehydrogenase (MMAB1_1584-1586, MMAB1_1958-1962) and methyl-CoM reductase (MMAB1_1952-MMAB1_1956). The expression levels of the two methyl-CoM reductases encoded by the genome of M. thermoautotrophicus were shown to be dependent on syntrophic or autotrophic growth conditions [41].
Moreover, the genome of M. bourgensis strain MAB1 codes for three formate dehydrogenases (MMBA1_1689-1690, MMAB1_1105-1106, MMAB1_2913-2914), as has also been described for strain MS2 [39] as well as a putative formate/nitrite transporter (MMAB1_1101/2). These proteins might be involved in efficient uptake and utilisation of formate by donating electrons from formate via formate dehydrogenases to the heterodisulfide reductase, an electron-bifurcating mechanism recently suggested for Methanococcus maripaludis [40].
Conclusions
Methanoculleus bourgensis strain MAB1 has been identified as a syntrophic partner for acetate-oxidising bacteria in biogas processes operating with high ammonia levels. Initial genome surveillance indicates an adaption of strain MAB1 to the high osmolarity of this particular environment, as has also been observed for its syntrophic partner organisms. It reveals further gene sets likely to mediate efficient formate uptake and conversion, a possible end product of acetate oxidation. There is a remarkable discrepancy between Methanoculleus bourgensis strain MAB1 and the type strain Methanoculleus bourgensis strain MS2, as indicated by the number of orthologous and synteny percentages. Follow-up genome analysis and –omics approaches will investigate these differences further and elucidate what is specific about strain MAB1 that makes it the preferred partner organism of this particular syntrophy.
Acknowledgements
This work was supported by the University of the Punjab, Lahore, Pakistan. Uppsala Genome Center performed sequencing supported by the Science for Life Laboratory (Uppsala), the Swedish Bioinformatics Infrastructure for Life Sciences (BILS) supporting the SGBC bioinformatics platform at SLU and Uppsala Multidisciplinary Center for Advanced Computational Science (UPPMAX), Uppsala, Sweden. The contribution of SM and EB-R was supported by EU-COST action BM1006-SeqAhead. EB-R was also partially supported by EU FP7 ALLBIO project, grant number 289452. AS and BM were supported by the Swedish Energy Agency (project no P36651-1). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Authors’ contributions
SM, BM, EB and AS contributed to the conception and design of this project. SM and EB were involved in the acquisition and initial analysis of the data. SM, BM and AS were involved in the interpretation of the data. SM and BM prepared the manuscript. AS and EB provided financial support. All the authors have been involved in the critical revision of the manuscript, have given final approval of the version to be published and agree to be accountable for all aspects of the work.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- SAOB
syntrophic acetate-oxidising bacteria
- MIRA
Mimicking Intelligent Read Assembly, MaGe, Magnifying Genomes
- BLASTP
Basic local alignment search tool for proteins
- NCBI
National Center for Biotechnology Information
Additional file
Associated MIGS record. (DOCX 124 kb)
References
- 1.Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–80. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morris BEL, Henneberger R, Huber H, Moissl-Eichinger C. Microbial syntrophy: interaction for the common good. FEMS Microbiol Rev. 2013;37:384–406. doi: 10.1111/1574-6976.12019. [DOI] [PubMed] [Google Scholar]
- 3.Schnürer A, Svensson BH, Schink B. Enzyme activities in and energetics of acetate metabolism by the mesophilic syntrophically acetate-oxidizing anaerobe Clostridium ultunense. FEMS Microbiol Lett. 1997;154:331–6. doi: 10.1016/S0378-1097(97)00350-9. [DOI] [Google Scholar]
- 4.Hattori S, Luo H, Shoun H, Kamagata Y. Involvement of formate as an interspecies electron carrier in a syntrophic acetate-oxidizing anaerobic microorganism in coculture with methanogens. J Biosci Bioeng. 2001;91:294–8. doi: 10.1016/S1389-1723(01)80137-7. [DOI] [PubMed] [Google Scholar]
- 5.Hao L-P, Lü F, He P-J, Li L, Shao L-M. Predominant contribution of syntrophic acetate oxidation to thermophilic methane formation at high acetate concentrations. Environ Sci Technol. 2011;45:508–13. doi: 10.1021/es102228v. [DOI] [PubMed] [Google Scholar]
- 6.Westerholm M, Leven L, Schnurer A. Bioaugmentation of Syntrophic Acetate-Oxidizing Culture in Biogas Reactors Exposed to Increasing Levels of Ammonia. Appl Environ Microbiol. 2012;78:7619–25. [DOI] [PMC free article] [PubMed]
- 7.Sun L, Müller B, Westerholm M, Schnürer A. Syntrophic acetate oxidation in industrial CSTR biogas digesters. J Biotechnol. 2014;171:39–44. [DOI] [PubMed]
- 8.Angenent LT, Sung S, Raskin L. Methanogenic population dynamics during startup of a full-scale anaerobic sequencing batch reactor treating swine waste. Water Res. 2002;36:4648–54. doi: 10.1016/S0043-1354(02)00199-9. [DOI] [PubMed] [Google Scholar]
- 9.Balk M, Weijma J, Stams AJM. Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor. Int J Syst Evol Microbiol. 2002;52:1361–8. doi: 10.1099/00207713-52-4-1361. [DOI] [PubMed] [Google Scholar]
- 10.Hattori S, Kamagata Y, Hanada S, Shoun H. Thermacetogenium phaeum gen. nov., sp. nov., a strictly anaerobic, thermophilic, syntrophic acetate-oxidizing bacterium. Int J Syst Evol Microbiol. 2000;50:1601–9. doi: 10.1099/00207713-50-4-1601. [DOI] [PubMed] [Google Scholar]
- 11.Schnürer A, Zellner G, Svensson BH. Mesophilic syntrophic acetate oxidation during methane formation in biogas reactors. 1999;29:249–61.
- 12.Moestedt J, Müller B, Westerholm M, Schnürer A. Ammonia threshold for inhibition of anaerobic digestion of thin stillage and the importance of organic loading rate. Microb Biotechnol. 2015 doi: 10.1111/1751-7915.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki D, Hori T, Haruta S, Ueno Y, Ishii M, Igarashi Y. Methanogenic pathway and community structure in a thermophilic anaerobic digestion process of organic solid waste. J Biosci Bioeng. 2011;111:41–6. doi: 10.1016/j.jbiosc.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Blomgren A, Hansen A, Svensson BH. Enrichment of A Mesophilic, Syntrophic Bacterial Consortium Converting Acetate to Methane at High Ammonium Concentrations. In: Bélaich J-P, Bruschi M, Garcia J-L, editors. Microbiology and Biochemistry of Strict Anaerobes Involved in Interspecies Hydrogen Transfer. US: Springer; 1990. pp. 225–34. [Google Scholar]
- 15.Bélaich J-P, Bruschi M, Garcia J-L, editors. Microbiology and Biochemistry of Strict Anaerobes Involved in Interspecies Hydrogen Transfer. Boston: Springer US; 1990. [Google Scholar]
- 16.Schnürer A, Schink B, Svensson BH. Clostridium ultunense sp. nov., a mesophilic bacterium oxidizing acetate in syntrophic association with a hydrogenotrophic methanogenic bacterium. Int J Syst Bacteriol. 1996;46:1145–52. doi: 10.1099/00207713-46-4-1145. [DOI] [PubMed] [Google Scholar]
- 17.Westerholm M, Roos S, Schnürer A. Tepidanaerobacter acetatoxydans sp. nov., an anaerobic, syntrophic acetate-oxidizing bacterium isolated from two ammonium-enriched mesophilic methanogenic processes. Syst Appl Microbiol. 2011;34:260–6. doi: 10.1016/j.syapm.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Westerholm M, Roos S, Schnürer A. Syntrophaceticus schinkii gen. nov., sp. nov., an anaerobic, syntrophic acetate-oxidizing bacterium isolated from a mesophilic anaerobic filter. FEMS Microbiol Lett. 2010;309:100–4. doi: 10.1111/j.1574-6968.2010.02023.x. [DOI] [PubMed] [Google Scholar]
- 19.Sprott GD, Patel GB. Ammonia toxicity in pure cultures of methanogenic bacteria. Syst Appl Microbiol. 1986;7:358–63. doi: 10.1016/S0723-2020(86)80034-0. [DOI] [Google Scholar]
- 20.Steinhaus B, Garcia ML, Shen AQ, Angenent LT. A portable anaerobic microbioreactor reveals optimum growth conditions for the methanogen Methanosaeta concilii. Appl Environ Microbiol. 2007;73:1653–8. doi: 10.1128/AEM.01827-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnürer A, Nordberg A. Ammonia, a selective agent for methane production by syntrophic acetate oxidation at mesophilic temperature. Water Sci Technol. 2008;57:735–40. doi: 10.2166/wst.2008.097. [DOI] [PubMed] [Google Scholar]
- 22.Shimada T, Morgenroth E, Tandukar M, Pavlostathis SG, Smith A, Raskin L, et al. Syntrophic acetate oxidation in two-phase (acid-methane) anaerobic digesters. Water Sci Technol. 2011;64:1812–20. doi: 10.2166/wst.2011.748. [DOI] [PubMed] [Google Scholar]
- 23.Westerholm M. Biogas production through the syntrophic acetate-oxidising pathway characterisation and detection of syntrophic acetate-oxidising bacteria. 2012. http://urn.kb.se/resolve?urn=urn:nbn:se:slu:epsilon-e-532. Accessed 28 May 2012.
- 24.Ollivier BM, Mah RA, Garcia JL, Boone DR. Isolation and characterization of methanogenium bourgense sp. nov. Int J Syst Evol Microbiol. 1986;36:297–301. [Google Scholar]
- 25.Asakawa S, Nagaoka K. Methanoculleus bourgensis, Methanoculleus olentangyi and Methanoculleus oldenburgensis are subjective synonyms. Int J Syst Evol Microbiol. 2003;53:1551–2. doi: 10.1099/ijs.0.02508-0. [DOI] [PubMed] [Google Scholar]
- 26.Reddy TBK, Thomas AD, Stamatis D, Bertsch J, Isbandi M, Jansson J, et al. The Genomes OnLine Database (GOLD) v.5: a metadata management system based on a four level (meta)genome project classification. Nucleic Acids Res. 2015;43:D1099–106. [DOI] [PMC free article] [PubMed]
- 27.Zehnder AJ, Huser BA, Brock TD, Wuhrmann K. Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium. Arch Microbiol. 1980;124:1–11. doi: 10.1007/BF00407022. [DOI] [PubMed] [Google Scholar]
- 28.Schnürer A, Houwen FP, Svensson BH. Mesophilic syntrophic acetate oxidation during methane formation by a triculture at high ammonium concentration. Arch Microbiol. 1994;162:70–4. doi: 10.1007/BF00264375. [DOI] [Google Scholar]
- 29.SciLifeLab [Internet]. SciLifeLab. 2013 [cited 2016 Aug 19]. Available from: https://www.scilifelab.se/.
- 30.Andrews S. FastQC: a quality control tool for high throughput sequence data. 2010. [Google Scholar]
- 31.Milne I, Stephen G, Bayer M, Cock PJA, Pritchard L, Cardle L, et al. Using Tablet for visual exploration of second-generation sequencing data. Brief Bioinform. 2013;14:193–202. doi: 10.1093/bib/bbs012. [DOI] [PubMed] [Google Scholar]
- 32.Nishito Y, Osana Y, Hachiya T, Popendorf K, Toyoda A, Fujiyama A, et al. Whole genome assembly of a natto production strain Bacillus subtilis natto from very short read data. BMC Genomics. 2010;11:243. doi: 10.1186/1471-2164-11-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chevreux B, Wetter T, Suhai S. Genome sequence assembly using trace signals and additional sequence information. Comput Sci Biol Proc German Conf Bioinf. 1999;99. [http://www.bioinfo.de/isb/gcb99/talks/chevreux/main.html]
- 34.Darling ACE, Mau B, Blattner FR, Perna NT. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14:1394–403. [DOI] [PMC free article] [PubMed]
- 35.Vallenet D, Labarre L, Rouy Z, Barbe V, Bocs S, Cruveiller S, et al. MaGe: a microbial genome annotation system supported by synteny results. Nucleic Acids Res. 2006;34:53–65. doi: 10.1093/nar/gkj406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyatt D, Chen G-L, Locascio PF, Land ML, Larimer FW, Hauser LJ. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics. 2010;11:119. doi: 10.1186/1471-2105-11-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bocs S, Cruveiller S, Vallenet D, Nuel G, Medigue C. AMIGene: annotation of MIcrobial genes. Nucleic Acids Res. 2003;31:3723–6. doi: 10.1093/nar/gkg590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlesner M, Miller A, Streif S, Staudinger WF, Müller J, Scheffer B, et al. Identification of Archaea-specific chemotaxis proteins which interact with the flagellar apparatus. BMC Microbiol. 2009;9:56. doi: 10.1186/1471-2180-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maus I, Wibberg D, Stantscheff R, Stolze Y, Blom J, Eikmeyer F-G, et al. Insights into the annotated genome sequence of Methanoculleus bourgensis MS2(T), related to dominant methanogens in biogas-producing plants. J Biotechnol. 2015;201:43–53. doi: 10.1016/j.jbiotec.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 40.Costa KC, Wong PM, Wang T, Lie TJ, Dodsworth JA, Swanson I, et al. Protein complexing in a methanogen suggests electron bifurcation and electron delivery from formate to heterodisulfide reductase. Proc Natl Acad Sci U S A. 2010;107:11050–5. doi: 10.1073/pnas.1003653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo H-W, Zhang H, Suzuki T, Hattori S, Kamagata Y. Differential expression of methanogenesis genes of Methanothermobacter thermoautotrophicus (formerly Methanobacterium thermoautotrophicum) in pure culture and in cocultures with fatty acid-oxidizing syntrophs. Appl Environ Microbiol. 2002;68:1173–9. doi: 10.1128/AEM.68.3.1173-1179.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol. 2008;26:541–7. doi: 10.1038/nbt1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains archaea, bacteria, and eucarya. Proc Natl Acad Sci U S A. 1990;87:4576–9. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garrity GM, Holt JG, Whitman WB, Keswani J, Boone DR, Koga Y, et al. Phylum All. Euryarchaeota phy. nov. In: Boone DR, Castenholz RW, Garrity GM, et al., editors. Bergey’s Manual of Systematic Bacteriology. New York: Springer; 2001. pp. 211–355. [Google Scholar]
- 45.Oren A, Garrity GM. List of new names and new combinations previously effectively, but not validly, published. International Journal Of Systematic And Evolutionary Microbiology. 2015;65:1–4. doi: 10.1099/ijs.0.000008-0. [DOI] [PubMed] [Google Scholar]
- 46.Garrity, GM, Bell, JA, Lilburn, TG. Taxonomic outline of the Procaryotes. 2004. http://www.bergeys.org/outlines/bergeysoutline_5_2004.pdf. Accessed 1 May 2004.
- 47.Euzéby JP, Tindall BJ. Nomenclatural type of orders: corrections necessary according to Rules 15 and 21a of the Bacteriological Code (1990 Revision), and designation of appropriate nomenclatural types of classes and subclasses. Request for an opinion. Int J Syst Evol Microbiol. 2001;51:725–7. doi: 10.1099/00207713-51-2-725. [DOI] [PubMed] [Google Scholar]
- 48.List editor. Validation of the publication of new names and new combinations previously effectively published outside the IJSB. Int. J. Syst. Bacteriol. 1996;46:625–6. [DOI] [PubMed]
- 49.Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979;43:260–96. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maestrojuan GM, Boone DR, Xun L, Mah RA, Zhang L. Transfer of Methanogenium bourgense, Methanogenium marisnigri, Methanogenium olentangyi, and Methanogenium thermophilicum to the Genus Methanoculleus gen. nov., Emendation of Methanoculleus marisnigri and Methanogenium, and Description of New Strains of Methanoculleus bourgense and Methanoculleus marisnigri. Int J Syst Bacteriol. 1990;40:117–22. doi: 10.1099/00207713-40-2-117. [DOI] [Google Scholar]
- 51.Oren A, Garrity GM. List of new names and new combinations previously effectively, but not validly, published. Int J Syst Evol Microbiol. 2016;66:2463–6. doi: 10.1099/ijsem.0.001149. [DOI] [PubMed] [Google Scholar]
- 52.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–7. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17:368–76. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- 55.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 56.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]


