Abstract
Evolving interest in meningioma, the most common primary brain tumor, has refined contemporary management of these tumors. Problematic, however, is the paucity of prospective clinical trials that provide an evidence-based algorithm for managing meningioma. The current review summarizes the published literature regarding the treatment of newly diagnosed and recurrent meningioma, with an emphasis on outcomes stratified by World Health Organization (WHO) tumor grade. In particular this review focuses on patient outcomes following treatment (either adjuvant or at recurrence) with surgery or radiation therapy inclusive of radiosurgery and fractionated irradiation.
Phase II trials for patients with meningioma have recently completed accrual within the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) consortia, and phase III studies are being developed. However, at present, there are no completed prospective, randomized trials assessing the role of either surgery or radiotherapy. Successful completion of future studies will require a multidisciplinary effort, dissemination of the current knowledge base, improved implementation of WHO grading criteria, standardization of response criteria and other outcome endpoints, and concerted efforts to address weaknesses in present treatment paradigms, particularly for patients with progressive or recurrent low grade meningioma, or with high-grade meningioma. In parallel efforts, Response Assessment in Neuro-Oncology (RANO) subcommittees are developing a manuscript on systemic therapies for meningioma, and a separate article proposing standardized endpoint and response criteria for meningioma.
Keywords: Meningioma, Outcomes, Surgery, Radiotherapy
Introduction
Harvey Cushing first used the term “meningioma” in a 1922 publication describing tumors that originate from the meningeal, i.e. dural, coverings of the brain and spinal cord.20 Since then, considerable progress has been made, including improved methods of treatment, better characterization of histology with the development of grading systems that provide more accurate prognostic information, use of proliferative markers such as MIB-1, and gains in translational research that have improved understanding of the molecular genetics of these tumors.
With reference to molecular genetics, meningiomas occur with greater frequency in genetic conditions such as type 2 neurofibromatosis (NF2),108,109 or multiple endocrine neoplasia type 1 (MEN1).4 Nearly all NF2-associated meningiomas, and many sporadic meningiomas, have mutations of the NF2 gene.121 Nevertheless, phenotypic NF2 accounts for only a small minority. MEN1 has also been reported to carry an increased risk for meningioma, although with less likelihood of aberration at the NF2 gene locus.4 However, there is no clear documentation that NF2 or MEN1 associated meningiomas behave more aggressively than their sporadic counterparts.
Incidental, asymptomatic, radiographically presumed meningiomas appear to behave less aggressively,12,145 may be observed, and treatment withheld until symptoms develop, sustained growth occurs, or concerns of encroachment on sensitive structures arise.94 The focus of this manuscript is on larger, symptomatic meningiomas that undergo surgery or other definitive management options stratified by tumor grade, and not a detailed review of incidental, untreated meningiomas. Indeed the grade of an incidental, observed meningioma is unknown, and its natural history may differ considerably from the larger, symptomatic tumors selected for definitive treatment. Studies have been undertaken to define the natural history of incidental meningiomas, and their results have been described in other papers.12,46,91,92,101,145 Further systematic investigations are warranted to delineate which patients are best served by observation, how such observation should be tailored, which subgroups are at higher risk for tumor growth or symptom development, and whether long-term patient outcomes differ between surveillance and early definitive treatment.
Many questions remain regarding the selection and timing of treatment especially in cases of recurrent meningioma or newly diagnosed high-grade meningioma (WHO Grade 2 [atypical] or Grade 3 [malignant] meningioma). For patients undergoing definitive therapy, complete surgical resection has been the standard for meningioma, however, there is a significant subset of patients who are not successfully managed by surgery alone, or in whom a complete resection is not possible due the relationship of tumor to eloquent anatomy. The potential for recurrence, whether following subtotal resection (STR) or gross total resection (GTR), is well recognized in the literature.19,87,118,136,138,148 Limitations associated with an initial treatment strategy of surgical resection alone are even more apparent for patients with recurrent or high-grade meningioma. 2,78 The current WHO criteria110 have improved the prediction of risk of tumor recurrence, but there remains significant uncertainty. Moreover, the relevance of the original (pre-MRI) Simpson classification based upon the extent of resection has been questioned in the MRI era.19,100,144,146 In particular the surgeon’s observations at the time of surgery are critical toward defining the difference, for example, between a Simpson Grade 1 and Grade 2 excision. Consequently, there needs to be updated agreement regarding how to report the extent of meningioma resection.
Another commonly used treatment for meningioma is radiotherapy (RT), including single session stereotactic radiosurgery (SRS), hypofractionated stereotactic radiotherapy (FSRT), and conventionally fractionated external beam radiotherapy (EBRT). A growing number of series have evaluated the use of SRS or EBRT as an adjuvant to surgery after STR, for treatment of recurrent low-grade or high-grade meningioma, or as an alternative to surgery. When RT is used as an alternative to surgery, however, there is no tissue available for grading, or ability to assign a proliferative index, or otherwise assess prognosis by histopathologic or molecular measures. Recognizing that these studies are largely retrospective or single arm in design, as will be reviewed herewith, they have suggested improved tumor control compared to surgery alone or to observation. At present the most appropriate patients, tumor target volumes, radiation dose, and fractionation schemes are still undefined by prospective trials.
At 5 years WHO grade II and III meningiomas carry a 5 to 10 fold greater progression risk than their initially diagnosed WHO grade I counterparts.107 These tumors can readily become refractory to treatment, and entail considerably higher rates of cause-specific mortality. WHO grade III (anaplastic) meningiomas have short recurrence-free intervals and high mortality rates. Pharmacologic approaches, whether adjuvant or primary, are desirable, but have met with limited results. Consequently, considerable opportunity exists for the development of systemic or targeted agents for the treatment of high-grade meningiomas.
As a prelude to discussing outcomes of meningioma by WHO grade, it is important to note that the currently used grading criteria were developed and amended over the course of the last 2 decades. In 1993, the WHO attempted to codify and standardize meningioma grading; previously many differing grading systems were in use.37,44,83,107 The 1993 standards were an important advance, but were subject to considerable subjectivity. The 2000 and 2007 WHO iterations are less vague and more reliably applicable, but much of the pertinent literature is based upon prior grading schemes. This renders comparisons among many published difficult and tenuous.
It is also important to recognize that the reported incidence of all grades of meningioma has varied substantially over time and by the method of meningioma identification, from 1 to 8.4 per 100,000.79 Considering both microscopically confirmed and presumed tumors, a recent analysis reported an incidence of 3 to 3.5 per 100,000.50 Adjusting for increases in population in the United States (USA), approximately 150,000 persons are currently diagnosed with meningioma.15,22 Outcomes may vary according to histologic, genetic, tumor size and location, presenting clinical characteristics, and even by the method of identification.
Recognition of the limitations of existing methods to evaluate outcomes of neuro-oncology patients led to the initiation of an international effort to develop consensus response and outcome evaluation criteria, particularly in the setting of prospective clinical research. This Response Assessment in Neuro-Oncology (RANO) Working Group consists of a multidisciplinary group of experienced clinical researchers including neuro-oncologists, neurosurgeons, radiation oncologists, neuro-radiologists, neuropsychologists and experts in quality of life measures. Open meetings of RANO have included representatives from government, funding and regulatory bodies, and members of the drug and device industry. Recommendations made by the RANO Working Group are based on expert consensus opinion rather than level 1 or 2 evidence. The primary purpose of this expert opinion process is to recommend a common set of definitions to be used in the conduct of clinical research in neuro-oncology, in this case meningiomas. Previous reviews conducted by the RANO Working Group have focused on high and low grade gliomas, brain metastases, clinical trial design, and surgical applications of novel outcomes measures. 69,70,120,153,154,157,160
Appreciating these important qualifications, this overview will examine published treatment outcomes, underscore deficiencies in our meningioma-related knowledge base, provide a foundation for response assessment (for which a future RANO publication is in progress) and suggest opportunities for future research. This manuscript focuses on surgery and radiation therapy. A companion article will appraise developments and opportunities with systemic therapies.
Methods
A PubMed literature search encompassing the years 2000 through 2013 for all English language publications reporting clinical outcomes for patients with surgically or radiotherapeutically treated meningioma was undertaken. Terms employed in the search were meningioma in multiple combinations that included surgery, radiation therapy, radiosurgery, survival, disease-free survival, progression-free survival, local control, tumor or WHO grade, pathology, atypical, anaplastic, malignant, and derivatives or synonyms of these terms. Bibliographies from the publications identified within PubMed were reviewed to identify further applicable articles. For outcome measures, surgery articles were included if extent of resection and tumor grade were specified. Radiation therapy publications were included if radiation dose and technical details were described; radiosurgery publications were subject to these same constraints.
Reports were tabulated by year, number of patients, treatment technique, tumor location, mean or median follow-up, histologic grade, and outcome measures. For surgery patients, the extent of resection was collected, and for patients receiving radiation therapy or radiosurgery, dose and, when available, target volume definitions were recorded. Applicable outcome measures were recorded, along with their respective time points. The most consistently reported measure was progression-free survival at 5 years, and when possible this was used as a unifying endpoint.
Results
WHO Grade I (Benign) Meningioma
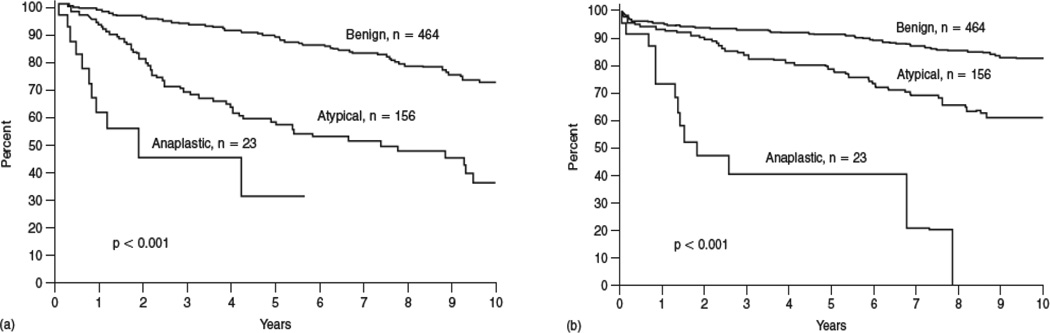
Meningioma has long been recognized as the most common non-glial intracranial tumor.10 Recent data reveal that they are, in fact, the most frequently reported primary intracranial neoplasm,15 accounting for 33.8% of all such tumors.11 The majority of meningiomas are benign. With more uniform adoption of the current WHO 2007 standards, approximately 65% to 80% are grade I (see Figure 1).107,162
Figure 1.
Recurrence-free (a) and overall (b) survival for 643 patients with meningioma stratified by WHO grade. Among the 643 patients studied, 464 (72.2%) had a grade I meningioma, 156 (24.3%) grade II, and 23 (3.5%) grade III].100
Surgery
Since the publication of the seminal work of Simpson, maximal resection has been the objective of surgical management for meningiomas. Simpson correlated the extent resection of tumor, associated dural attachments, and any hyperostotic bone to local recurrence risk, and defined 5 grades of resection, which were associated with distinct rates of recurrence. These so-called “Simpson Grades” and their respective recurrence rates are summarized in Table 1.134 The completeness of surgical removal has consistently been identified as an important prognostic feature, 19,23,115,138 and the majority of centers continue to use Simpson’s criteria.
Table 1.
Simpson grades of resection, as derived from a series of 265 patients. [Simpson 1957].
| Extent of Resection Simpson’s Grade | ||
|---|---|---|
| Resection Grade |
Definition | Recurrence (%) |
| 1 | GTR of tumor, dural attachments and abnormal bone | 9% |
| 2 | GTR of tumor, coagulation of dural attachments | 19% |
| 3 | GTR of tumor without resection or coagulation of dural attachments or extradural extensions (e.g invaded or hyperostotic bone) | 29% |
| 4 | Partial resection of tumor | 44% |
| 5 | Simple decompression (biopsy) | - |
GTR: gross total resection
Sughrue challenged the applicability of the Simpson classification in the present era. With 373 WHO grade I meningioma patients followed for a median of 3.7 years, they found no significant difference in 5-year progression-free survival (PFS) following Simpson grade I through IV resections, with respective 5-year PFS results of 95%, 85%, 88%, and 81% (p=ns).144,146 Similar findings were reported previously by Condra,19 and more recently by Oya.100 These studies, while identifying no difference in local control after Simpson grade I-III resections, did reveal shorter PFS following Simpson grade IV surgery.19,100 A large series by Hasseleid of 391 patients with convexity meningioma, studied expressly to address modern challenges to the predictive value of the Simpson resection grading system, identified significant outcome differences between Simpson grade 1, grades 2 +3, and grade 4+547 serving in support of the continued applicablility of Simpson’s crieteria.
GTR (Simpson I-III) remains the prevalent objective of surgery for meningioma, and is achieved in approximately one-half to two-thirds of patients in surgical series inclusive of meningiomas located in a variety of intracranial sites ,87,115 and in over 95% of convexity meningiomas.90 For benign meningioma, GTR is considered definitive therapy.19,87,115,138 However, with extended follow-up, recurrences in this setting are not infrequent.1,19,87,136,138,148 In 5 separate series, rates of local recurrence after GTR ranged from 7–23% at 5 years, 20–39% at 10 years, and 24–60% at 15 years (Table 2). The higher rates documented in the most recent of these analyses likely reflect the current use of serial evaluation with modern neuroimaging such as MRI.136
Table 2.
Five single institution series with prolonged follow-up, documenting rates of recurrence following gross total resection alone.
| Local Recurrence After Gross Total Resection Alone | ||||||
|---|---|---|---|---|---|---|
| Local Recurrence Rate | ||||||
| Author | Institution | Year | n | 5-year | 10-year | 15-year |
| Mirimanoff | MGH | 1985 | 145 | 7% | 20% | 32% |
| Taylor | U of Florida | 1988 | 90 | 13%* | 25%* | 33%* |
| Condra | U of Florida | 1997 | 175 | 7% | 20% | 24% |
| Stafford | Mayo Clinic | 1998 | 465 | 12% | 25% | - |
| Soyuer | MD Anderson | 2004 | 48 | 23% | 39% | 60%* |
| Total: | 923 | 7–23% | 20–39% | 24–60% | ||
n: number of patients
MGH: Massachusetts General Hospital; U of Florida: University of Florida
data extracted from graph
STR (e.g. Simpson IV-V) carries substantially higher rates of progression in many studies, even in benign meningioma. As shown by the 7 studies summarized in Table 3, local progression rates following STR vary from 37–47% at 5 years, to 55 to 63% at 10 years, and to 70 to 91% at 15 years. Condra also found that STR impacted cause-specific survival. Their patients with STR alone experienced a 15-year cause-specific survival of 51%, significantly inferior to 88% after GTR, and 86% after STR+RT (p=.0003).19 In a recent evaluation of clinical and molecular prognostic features of meningioma, Jensen reported that STR was associated with both poorer progression-free and overall survival.57 In spite of these reports, observation remains commonplace following STR. A Mayo Clinic series detailed 581 patients, 116 (20%) of whom had STR. Only 10 (9%) of these patients received adjuvant radiation therapy.138
Table 3.
Seven single institution series with prolonged follow-up, assessing rates of recurrence following sub-total resection alone.
| Local Progression After Sub-Total Resection Alone | ||||||
|---|---|---|---|---|---|---|
| Local Progression Rate | ||||||
| Author | Institution | Year | n | 5-year | 10-year | 15-year |
| Wara | UCSF | 1975 | 58 | 47% | 62% | - |
| Barbaro | UCSF | 1987 | 30 | 40%* | 100%* | - |
| Mirimanoff | MGH | 1985 | 80 | 37% | 55% | 91% |
| Condra | U of Florida | 1997 | 55 | 47% | 60% | 70% |
| Mirabell | MGH | 1992 | 79 | 40% | 52%§ | - |
| Stafford | Mayo Clinic | 1998 | 116 | 39% | 61% | - |
| Soyuer | MD Anderson | 2004 | 32 | 62% | 82%* | 87%* |
| Total: | 450 | 37–62% | 52–100% | 70–91% | ||
n: number of patients
UCSF: University of California San Francisco; MGH: Massachusetts General Hospital;
U of Florida: University of Florida
data extracted from graph
8-year progression
Patients with WHO Grade I meningioma have lengthy survival expectations (Figure 1), and hence long-term studies are required to fully understand the risks of progression and death. In studies that have included prolonged evaluation with MRI, higher than expected rates of local progression have been identified136 (Table 3). Moreover, recurrent meningioma exhibits a several-fold increased risk of progression and a shorter interval to progression than newly diagnosed tumors.19,86,87,148 Miralbell reported an 8-year PFS of 11% in recurrent tumor with surgery alone, compared to a rate of 78% following a combination of surgery and adjuvant EBRT.86 Taylor found a 5-year PFS of 30% with surgery alone for recurrent meningioma, 88% with surgery and EBRT. They also reported 5-year overall survival of 45% and 90%, respectively.148 These data support the need for prospective clinical investigation of methods to prevent recurrence, and provide impetus for research into clinical, imaging, histopathologic, and molecular predictors of response to treatment and to tumor progression.
WHO Grade I - Radiation Therapy
Multiple retrospective studies have demonstrated that various forms of radiation therapy (RT), including SRS and EBRT can provide improved and durable local control in selected patients with meningioma. RT has most commonly been utilized as an adjunct to surgery following STR, as treatment for recurrence, or for tumors of high-grade histology. Additionally, as shown in Table 4 and Table 5, many studies document excellent local control with SRS or EBRT as a primary modality. In these studies, RT was used predominantly for tumors in difficult to surgically access locations such as the optic nerve sheath or cavernous sinus, for patients regarded as inoperable for medical reasons, or for those who chose primary RT over surgery.32,66,67,71,76,80,104,116,129 These studies show that RT achieved long-term local control in 68% to 100% of WHO grade I or presumed grade I meningioma at 5 to 10 years, including patients treated post-operatively, primarily, or following recurrence. Results varied somewhat by treatment era, tumor size and location, and clinical setting.
Table 4.
Thirty-five studies of stereotactic radiosurgery, largely for WHO grade I or presumed grade I meningiomas
| Stereotactic Radiosurgery | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| First Author |
Year | n | Technique | Location | Mean FU (months) |
Mean/ Median Dose (Gy) |
5yr/10yr PFS (%) |
Clinical Improvement (%) |
Tumor Regression (%) |
Complication (%) |
Mean/Median Tumor Vol (cc) |
| Roche | 2000 | 80 | GKRS | Cav Sinus | 30.5 | 14 | 92.8 / -- | 27 | 31 | 4 | 5.8 |
| Shin | 2001 | 40 | GKRS | Cav Sinus | 42 | 18 | 91.3 / 91.3 | -- | 37.5 | 2.5 | 4.3 |
| Stafford | 2001 | 190 | GKRS | All | 47 | 16 | 93 / -- | 8 | 56 | 13 | 8.2 |
| Eustacchio | 2002 | 121 | GKRS | Skull Base | 72 | 13 | 99 / -- | 44.6 | 60 | 5 | 6.8 |
| Lee | 2002 | 159 | GKRS | Cav Sinus | 35 | 13 | 93 / 93 | 29 | 34 | 5 | 6.5 |
| Nicolato | 2002 | 111 | GKRS | Cav Sinus | 48.2 | 14.8 | 96 / -- | 66 | 61 | 4.5 | 8.1 |
| Spiegelmann | 2002 | 42 | LINAC | Cav Sinus | 36 | 14 | 97.5 / -- | 22 | 60 | 16.7 | 8.2 |
| Flickinger | 2003 | 219 | GKRS | All | 29 | 14 | 93.2 / -- | -- | -- | 8.8 | 5 |
| Iwai | 2003 | 42 | GKRS | Cav Sinus | 49 | 11 | 92 / -- | 29 | 59.5 | 4.7 | 12.4 |
| Pollock | 2003 | 62 | GKRS | All | 64 | 17.7 | 95 / -- | 13 | -- | 10 | 7.4 |
| Roche | 2003 | 32 | GKRS | Petroclival | 56 | 13 | 100 / -- | 58 | 12.5 | 9.3 | -- |
| Chuang | 2004 | 43 | LINAC | Skull Base | 74.5 | 17 | 89.7 / -- | 16 | 37 | 11 | 4.5 |
| DiBiase | 2004 | 137 | GKRS | All | 54 | 14 | 86.2 / -- | -- | 28 | 8.3 | 4.5 |
| Kreil | 2005 | 200 | GKRS | Skull Base | 94 | 12 | 98.5 / 97 | 41.5 | 56.5 | 2.5 | 6.5 |
| Pollock | 2005 | 49 | GKRS | Cav Sinus | 58 | 15.9 | 100 / -- | 26 | 59 | 14 | 10.2 |
| Zachenhofer | 2006 | 33 | GKRS | Skull Base | 103 | 17 | 94 / -- | 44 | 36 | 12 | -- |
| Davidson | 2007 | 36 | GKRS | Skull Base | 81 | 16 | 100 / 94.7 | 44 | 14 | 2.8 | 4.1 |
| Hasegawa | 2007 | 115 | GKRS | Cav Sinus | 62 | 13 | 94 / 92 | 46 | -- | 12 | 13.8 |
| Kollova | 2007 | 331 | GKRS | All | 68 | 12.5 | 98 / -- | 62 | 70 | 10 | 6.3 |
| Han | 2008 | 63 | GKRS | Skull Base | 77 | 12.7 | 90.2 / -- | 45 | 44 | 17 | 6.3 |
| Iwai | 2008 | 125 | GKRS | Skull Base | 86 | 12 | 93 / 83 | 13 | 46 | 7.2 | 8.1 |
| Kondziolka | 2008 | 972 | GKRS | All | 48 | 14 | 97 / 87 | 11 | 42 | 8 | 7.4 |
| Kondziolka | 2009 | 125 | GKRS | Convexity | 31 | 14 | 86 / -- | -- | 26 | 9.6 | 7.6 |
| Bledsoe | 2010 | 116 | GKRS | All | 70 | 15.1 | 99 / 92 | -- | -- | 23 | 17.5 |
| Flannery | 2010 | 168 | GKRS | Petroclival | 72 | 13 | 95 / -- | 26 | 49 | 14 | 7.7 |
| Korah | 2010 | 41 | LINAC | All | 60 | 14 | 94 / 94 | -- | -- | 2.4 | 4.5 |
| Skeie | 2010 | 100 | GKRS | Cav Sinus | 82 | 12.4 | 94 / 91.6 | 21 | 22 | 6 | 7.4 |
| Zada | 2010 | 116 | GKRS | All | 75 | 16 | 99 / 84 | -- | 26 | 8 | 3.4 |
| Williams | 2011 | 138 | GKRS | Parasellar | 84 | 13.7 | 95.4 / 69 | -- | 78 | 10 | 7.5 |
| Hayashi | 2011 | 66 | GKRS | Skull Base | 46 | 12 | 99 | -- | 82 | 1 | 6.6 |
| dos Santos | 2011 | 88 | LINAC | Cav Sinus | 87 | 14 | 92.5/82.5 | 51.1 | 73.8 | 19.3 | -- |
| Santacroce | 2012 | 4565 | GKRS | All | 63 | 14 | 95.2/88.6 | 53.5 | 58 | 6.6 | 4.8 |
| Unger | 2012 | 173 | LINAC | All | 21 | 15¶ | 89.3/-- | -- | -- | 8.5§ | 4.7 |
| Pollock | 2012 | 251 | GKRS | All | 62.9 | 15.8 | 99.4/99.4 | -- | 72.1 | 11.5 | 7.7 |
| Starke | 2012 | 255 | GKRS | Skull Base | 78 | 14 | 96/79 | -- | 49 | 5.1 | 5.0 |
15 Gy was the median dose with single fraction radiosurgery. With multifraction radiosurgery it was 25 Gy in 5 fractions.
symptomatic edema risk for all patients combined (12.5% with single fraction and 3.6% with multifraction radiosurgery).
Table 5.
Thirty-five studies of fractionated external beam radiation therapy for patients largely with WHO grade I or presumed grade I meningiomas.
| Fractionated External Beam Radiation Therapy | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Progression-Free Survival |
|||||||||||
| First Author |
Year |
n |
Technique |
GTR |
STR |
STR+RT |
RT Alone |
Clinical Improvement |
Tumor Shrinkage |
Late Toxicity |
Time Point |
| Adegbite | 1983 | 114 | EBRT | 74% | 34% | 82% | 10 yrs | ||||
| Mirimanoff | 1985 | 225 | EBRT | 80% | 45% | 10 yrs | |||||
| Barbaro | 1987 | 135 | EBRT | 96% | 40% | 68% | 0% | crude | |||
| Taylor | 1988 | 132 | EBRT | 77% | 18% | 82% | 10 yrs | ||||
| Glaholm | 1990 | 117 | EBRT | 96% | 43% | 77% | 46% | 38% | 10 yrs | ||
| Miralbell | 1992 | 115 | EBRT | 48% | 88% | 16% | 8 yrs | ||||
| Mahmood | 1994 | 254 | EBRT | 98% | 62% | 5 yrs | |||||
| Goldsmith | 1994 | 117 | EBRT | 77% 98%* |
3.6% | 10 yrs | |||||
| Peele | 1996 | 86 | EBRT | 52% | 100% | 5% | crude | ||||
| Condra | 1997 | 246 | EBRT | 80% | 40% | 87% | 24% | 10 yrs | |||
| Stafford | 1998 | 581 | EBRT | 75% | 39% | 10 yrs | |||||
| Nutting | 1999 | 82 | EBRT | 83% | 14% | 10 yrs | |||||
| Vendrely | 1999 | 156 | EBRT | 79% | 59% | 29% | 8% | ||||
| Pourel | 2001 | 26 | EBRT | 76% | 2.2% | 8 yrs | |||||
| Dufour | 2001 | 31 | EBRT | 93% | 71% | 29% | 3.2% | 10 yrs | |||
| Jalali | 2002 | 41 | FSRT | 100% | 26.8% | 22% | 12.1% | 3 yrs | |||
| Uy | 2002 | 40 | IMRT | 93% | 23% | 5% | 5 yrs | ||||
| Pirzkall | 2003 | 20 | IMRT | 100% | 60% | 25% | 0% | 3 yrs | |||
| Soyuer | 2004 | 92 | EBRT | 77% | 38% | 91% | 2.5% | 10 yrs | |||
| Selch | 2004 | 45 | FSRT | 97% | 20% | 18% | 0% | 3 yrs | |||
| Milker-Zabel | 2005 | 317 | IMRT | 89% | 42.9% | 23% | 0% | 10 yrs | |||
| Henzel | 2006 | 224 | FSRT | 97% | 43.4% | 46% | 0% | 3 yrs | |||
| Milker-Zabel | 2007 | 94 | IMRT | 94% | 39.8% | 20% | 4% | 4.4 yrs | |||
| Hamm | 2008 | 183 | FSRT | 93% | 23.2% | 2.7% | 3 yrs | ||||
| Litre | 2009 | 100 | FSRT | 94% | 94% | 50–81% | 9% | 0% | 5 yrs | ||
| Korah | 2010 | 41 | FSRT | 94% | 3% | 5 yrs | |||||
| Metellus | 2010 | 53 | FSRT | 94% | 94% | 58.5% | 30% | 1.9% | 10 yrs | ||
| Bria | 2011 | 60 | FSRT | 95% | 60% | 1 yr. | |||||
| Minniti | 2011 | 52 | FSRT | 96% 93% |
20% | 23% | 5.5% | 3 yrs 5 yrs |
|||
| Mahadevan | 2011 | 16 | FSRT | 100% | 19% | 4% | 2 yrs | ||||
| Morimoto | 2011 | 31 | FSRT | 87% | 1% | 5 yrs | |||||
| Onodera | 2011 | 27 | FSRT | 96.2% | 5.3 yrs | ||||||
| Ohba | 2011 | 281 | FSRT/SRS | 88.3% | 63.7% | 92.3% | 5 yrs | ||||
| Tanzler | 2011 | 146 | EBRT/FSRT/SRS | 96% 93% |
99% 99% |
6.8% | 5 yrs 10 yrs |
||||
| Paulsen | 2012 | 109 | FSRT | 98% | 21% | 5% | 5 yrs | ||||
Goldsmith et al, 10-year PFS 98% with treatment after 1980 when CT and MRI began to be used for treatment planning, versus 77% before 1980.
Stereotactic Radiosurgery (SRS)
SRS was developed more recently than fractionated EBRT, and over the past 2 to 3 decades has been used with increasing frequency. It has been used after STR or for recurrence ,61,65,139 and as a definitive primary treatment .32,116,117,118 Table 4 includes 35 studies of SRS, and demonstrates that local control was achieved in the majority of patients at 5 to 10 years.
SRS is considered most effective for patients with small meningiomas, typically those that are less than 3 cm in diameter or 10cc in volume, those with distinct margins, and those at sufficient distance from functionally important brain, nerves and other critical structures to permit safe delivery of an adequate target dose. For WHO grade I meningioma, excellent local control has consistently been achieved with 12 to 16 Gy (Table 4). Ganz noted that a minimum peripheral tumor dose of 10 Gy or less was associated with higher failure risk, compared with a dose of at least 12 Gy.33 Stafford reported no reduction in local control at 5 years with tumor margin doses of less than 16 Gy as compared to greater than or equal to 16 Gy.139 Similarly, Kondziolka reported no improvement with marginal doses greater than 15 Gy versus less than 15 Gy.63
With respect to tumor size, DiBiase reported a 91.9% 5-year disease-free survival for patients with meningioma less than 10 cc (equivalent diameter 2.7 cm), as opposed to 68% for larger tumors.25 Kondziolka reported excellent outcomes with SRS for meningioma up to a diameter of 3.0 cm or a volume of 7.5 cc.63 Likewise, other authors have found excellent local control (10-year 99.4%), and fewer radiation-related complications with smaller meningiomas, with complications in 4.8% of patients with tumors in the smallest quartile (<3.2cc) but in 22.6% in the largest quartile (>9.6cc).116,117
Pollock reported 188 benign or presumed benign meningioma patients treated with either surgery or SRS alone. With median follow-up of 64 months, 7-year PFS with SRS and Simpson grade I surgery were equivalent 95% and 96%, respectively. However, SRS resulted in superior tumor control when compared to less extensive surgery. The authors concluded that SRS should be a primary option when Simpson grade I resection is unlikely.118 In an updated analysis of primary SRS, Pollock found 10-year local control was 99.4%. They used a mean tumor margin dose of 15.8 Gy. No patient developed marginal recurrence. These results suggest that grade I meningioma can often be accurately defined and well controlled with SRS as primary therapy. However, emphasizing the requirement for prolonged evaluation, 2 patients developed local progression more than 12 years after SRS.116,117
SRS for meningioma has traditionally been single session. However, reports of multi- session SRS are emerging.18,34,72,76,89,150 These studies appear to demonstrate comparable local control to single fraction treatment, with perhaps fewer side effects and a lower incidence of symptomatic edema, particularly for non-basal/parasagittal or large meningiomas. In one of these reports, Unger reported on 173 patients and found that symptomatic edema was significantly less common following multifraction (typically 25 Gy in 5 fractions) SRS than single session (median 15 Gy) SRS. The respective 2-year actuarial risks were 3.2% and 12.5%. Single session SRS and tumor volume >4.9cc were significant predictors of symptomatic edema.150
Girvigian published on 30 convexity or parasagittal meningioma patients, 14 treated with single fraction and 16 with multifraction SRS. Multifraction treatment was typically 25 Gy in 5 fractions, and was used for larger tumors. Symptomatic edema occurred in 43% following single fraction, as opposed to 6.3% (1 patient) after multi-fraction SRS, and this patient had pre-treatment edema. Single doses of more than 14 Gy and larger tumor volume were predictors of edema.34
Columbo reported on 49 patients who received single fraction SRS (11–13 Gy), and 150 patients with tumors close to critical structures and/or greater than 8cc in volume who were treated with multi-fraction SRS (14–25 Gy in 2–5 fractions). For the entire cohort, 5-year PFS was 93%. They observed very few treatment related complications, even in patients with large tumors, and maintained that, with the use of multifraction SRS they were able to treat 63 patients who could not have been treated by single-fraction techniques.18
Fractionated External Beam Radiation Therapy (EBRT)
Historically meningioma has been considered resistant to irradiation, probably due to infrequent documentation of tumor regression following the use of EBRT. EBRT was also felt to produce considerable side effects, to potentiate malignant degeneration, and indeed to cause meningiomas.60,87,126,143 These concerns likely remain an issue today, and as a consequence many patients with inoperable or subtotally resected are managed by observation.3,138 A recent publication by Sughrue reported the outcomes of 373 patients with a newly diagnosed WHO grade I meningioma -- the preponderance located at the skull base -- treated with surgery alone. Simpson resection grades were I in 88 patients (23.6%), II in 114 (30.6%), III in 57 (15.3%), and IV in 114 (30.6%),144,146 indicating that many patients with a subtotally resected meningioma continue to be managed without adjuvant therapy.
Regarding the risk of radiation-associated tumor dedifferentiation (i.e. transformation to higher tumor grade), reliable estimates are difficult to ascertain. Dedifferentiation has not been definitively linked to RT, and as well is the natural history of a subgroup of recurrent or progressive meningioma.55,96 To establish radiation-induced malignant transformation, detailed histology prior to irradiation would be indispensable. Moreover, irradiation is often employed only after imaging-confirmed regrowth, without additional histology. Thus whether dedifferentiation results from irradiation or as a result of natural cellular evolution cannot be readily determined.96 This raises the question of whether some advanced imaging surrogate of histology could be developed and used to help guide therapy and predict outcomes.
The risk of developing a meningioma after cranial irradiation has been reviewed by Strojan, who reported as actuarial risk of 0.53% at 5 years, and 8.18% at 25 years.143 This risk appears to be considerably smaller with modern, highly conformal therapy. Minniti reported 426 pituitary adenoma patients treated with surgery and small field EBRT, and followed for 5,749 person-years. The risk of second brain tumor at 20 years was 2.4%. Of the 11 second tumors 5 were meningioma.85 With even smaller field treatment using SRS, and with over 9000 patients, Niranjan estimated a second tumor risk of less than 1 per 1000.96 This is smaller than the published series using larger field non-conformal EBRT, but with modern highly conformal approaches to fractionated EBRT, improved outcomes relative to older series may be expected.
Outcomes data from 35 studies of EBRT for meningioma are found in Table 5. These studies, while retrospective in nature, provide evidence that EBRT can improve PFS when used as an adjunct to STR, as salvage treatment of meningioma at recurrence, or as primary therapy. Excellent long-term outcomes from primary EBRT are reported for optic nerve sheath meningioma (ONSM). For these tumors, surgery carries a high risk of visual complications and a high rate of local recurrence, whereas EBRT alone results in more favorable outcomes than observation, surgery, or surgery plus EBRT.93,104,149 Moreover, patients with ONSM commonly experience improved visual acuity following use of EBRT.93,104,149
Primary EBRT for intracranial meningioma not involving the optic nerve sheath has also resulted in excellent local control, clinical improvement, and low rates of toxicity (Table 5). Tanzler studied 88 patients treated with definitive EBRT (mean total dose 52.7Gy). The majority of patients were diagnosed on the basis of imaging findings alone. Median follow-up for living patients was 8 years, and 10-year local control was 99%.147
Technical improvements in the delivery of EBRT have favorably impacted the outcome and side effects of this treatment modality. Treatment is now delivered with more precision and conformality, and improvements in local control have been documented. Goldsmith and Milosevic each substantiated improvements in local control with modern imaging.36,37,83 Goldsmith found that, with immobilization techniques and with CT or MRI based planning, 10-year PFS improved from 77% to 98% (p=0.002).36,37
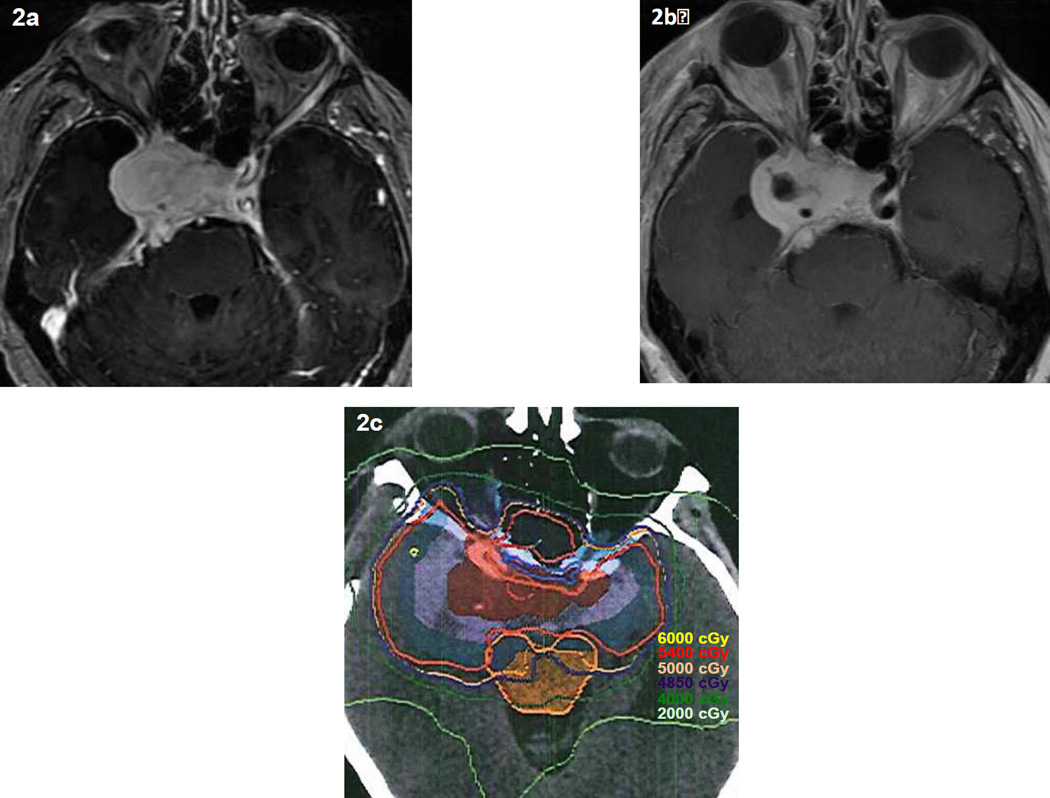
Recommended EBRT doses for benign meningiomas are generally 50 to 55 Gy with fraction sizes of 1.8 to 2.0 Gy,19,36 but a dose-response relationship has not been unequivocally established. Goldsmith reported that doses above 52 Gy resulted in improved 10-year local control, but this effect was not substantiated on multivariate analysis.37 Winkler found no clear dose response from 36–79.5 Gy (1.5 to 2.0 Gy per day).163 A common dosing schedule for WHO grade I meningioma is 54 Gy in 27 to 30 fractions, although for meningioma of the optic nerve sheath or near the anterior visual pathway lower total doses in the range of 50 Gy and even modestly lower doses per fraction have achieved good results.93,132 Figure 2 displays pre-operative and post-operative MRIs and the dosimetry plan CT for EBRT on a patient with a subtotally resected WHO grade I meningioma. The prescription dose was 5400 cGy in 30 fractions.
Figure 2.
Pre-operative (2a) and post-operative (2b) MRIs as well as the dosimetry plan CT (2c) for EBRT on a patient with a subtotally resected WHO grade I meningioma. The prescription dose is 5400 cGy in 30 fractions (180 cGy per fraction). Courtesy of Heyoung McBride, MD and Terry Thomas, MS, Barrow Neurological Institute, Phoenix, AZ.
Radiation treatment-related edema has rarely been reported with EBRT. Table 5 summarizes data from 35 studies with 4389 patients. Less than 0.5% of patients are reported to have developed treatment-related edema. It should be noted, however, that many studies did not specifically assess edema, and some patients with treatment-related edema, especially if asymptomatic, may have escaped detection. However, Selch specifically examined the rate of treatment-related edema in 45 patients and found no cases of post-EBRT edema with a median follow-up of 3-years.130 Tanzler studied 146 patients treated with EBRT and two (1.4%) developed edema.147 It appears that edema is a less likely a consequence of EBRT than of single-fraction SRS. Delayed neurotoxicity is also an important consideration, but little is known with specific reference to patients with meningioma, and represents an avenue for further research.
WHO Grade II (Atypical) Meningioma
Although grade II meningiomas were for decades identified in only about 5% of cases, with the adoption of the 2000 and 2007 WHO criteria they now constitute 20–35% of newly diagnosed meningioma.15,105,107,162 Given this magnitude of change in their identification, investigation is needed to redefine the natural history expectations for these tumors, and to better define the results of treatment. Furthermore, assessment is needed to determine how uniformly the new WHO diagnostic criteria are being implemented, and to define the rates of inter-observer and inter-institutional concordance in diagnosis. These investigations are crucial since, as shown in Figure 1, atypical meningioma carries a 7 to 8 fold increased risk of recurrence at 5 years, and an increased rate of mortality compared to WHO grade 1 meningioma.107
Surgery
When evaluating the impact of treatment on atypical meningioma, it is critical to keep in mind that the literature consists of retrospective reports, and that most include patients diagnosed using pre-WHO pathologic criteria, which underreported the incidence of atypical meningioma. Both the recently completed RTOG and EORTC prospective trials included central review of pathology, and analysis of their pathologic material is eagerly awaited. There is general agreement, but not consensus, that subtotal resection alone is insufficient treatment for WHO grade II meningioma. Surveys among neurosurgeons in Germany and the United Kingdom indicated that 26% and 41%, respectively, do not recommended adjuvant therapy after STR of an atypical meningioma.77,133 Another single institution series reported a 10-year local control rate of 17% following STR of atypical meningioma, but could not document a significant benefit associated with the use of post-operative radiation therapy.39 In general, neurosurgeons have used the strategy of serial re-resection to manage grade II meningioma recurrence.
There is considerably less agreement regarding adjuvant treatment after GTR. In Germany 84% of centers (47 of 56) recommended surgery alone for initially diagnosed, gross totally resected WHO grade II meningioma,133 similar to the United Kingdom where 80% made the same recommendation.77 A number of other reports have suggested that GTR alone is sufficient for these patients.39,73,75,102,105 Jaaskelainen reported a 38% 5-year local recurrence after GTR, and did not find that adjuvant RT was of utility.55 However, no randomized trials have been completed; many of the studies in the literature had small cohorts, used pre-WHO 2000 grading criteria, included patients with newly diagnosed and recurrent tumors, or used RT doses that were, as will be discussed subsequently, likely too low to be effective.
Employing WHO 2000/2007 criteria and higher EBRT doses, Aghi analyzed 108 patients with atypical meningioma. Following Simpson grade I surgery alone, the 5-year local recurrence was 50%.2 A more recent report by Komotar reviewed outcomes among 45 patients, each with a gross totally resected atypical meningioma. GTR was defined as Simpson grade I or II, confirmed by post-operative MRI. Thirty-two of their 45 patients (71%) were treated initially with surgery alone, and experienced a 5-year actuarial risk of recurrence of 55%.62
The clinical impact of tumor recurrence in patients with atypical meningioma appears to be more significant than in patients with WHO grade I tumors. Mair found that neither the extent of salvage resection nor the use of RT was predictive of outcome for patients with recurrent grade II meningioma.75 Aghi reported a 10-year disease-specific survival after first recurrence of 69%.2 With a median follow-up of 44.1 months, Komotar noted crude overall survival of 69.2% following first recurrence, very similar to Aghi,2 and concluded that recurrences resulted in shortened overall survival, as well as additional treatment burden.62
Radiation Therapy
Various forms of RT have been used for grade II meningioma following STR, including SRS5,58,135,139 and EBRT.2,8,17,19,52,83 Even following GTR, many have advocated RT for these patients,2,19,44,51,52,103,163 but others recommend observation.39,75,105 Irradiation is also commonly employed as a primary modality for some meningioma, but as there was no pathological confirmation it is unclear how many were, in reality, WHO grade II tumors. The determination of grade requires tissue confirmation, and there is very limited data on primary RT after biopsy alone.
Achieving local control for patients with atypical meningioma is an important endpoint with RT, and appears to be paramount. As aforementioned, Aghi reported a 69% 10-year disease-specific survival after first recurrence.2 Skeie found that 6 of 7 recurrent patients died of disease at a mean 25 months after regrowth.135 Stafford noted that patients with prior surgery or EBRT fared worse, and that patients with recurrent atypical tumors continued to exhibit worse cause-specific survival despite aggressive salvage therapy.139
Stereotactic Radiosurgery (SRS)
Reports of SRS for grade II meningioma are, with near exclusivity, in the STR or recurrent settings, mostly the latter. Table 6 summarizes 8 series. Reported local control at 2 years and beyond spans a wide range from 0% up to 90%, with most in the 50% to 80% range. These studies suggest that dose, target volume and treatment timing are key elements in improving outcomes. Kano reported that 5-year PFS for lesions treated below 20 Gy was 29.4%, as compared to 68% for those receiving 20 Gy (p=.0139).58 However, Stafford identified a 5-year local control rate of 68% using a moderately lower dose, median 16 Gy (range 12–36 Gy), and found no clear correlation between SRS dose and local control.139
Table 6.
Eight studies of stereotactic radiosurgery for atypical meningioma.
| Stereotactic Radiosurgery for Atypical Meningioma | ||||||
|---|---|---|---|---|---|---|
| Author (year) |
n |
Follow-upa |
Dosea |
Local Control |
Time Frame |
Comments |
| Stafford (2001) | 13 | 47 mo | 16 Gy | 68 % | 5yr actuarial | Majority were recurrent before SRS. 5y LC for Gr I 93%. 5y OS: 76% for Gr II vs 92% Gr I, worse CSS for Gr II. Predictors: Prior Surg or EBRT, larger tumor vol, location (non-basal) |
| Harris (2003) | 18 | 46 mo | 14.9 Gy | 83% | 5yr actuarial | Mean of 2 resections prior to SRS. 5y OS 59%. Better with smaller tumor vol and early SRS. Median neurologic progression: 15 mo early SRS vs 61 mo late SRS |
| Huffmann (2005) | 15 | 35 mo | 16 Gy | 60 % | crude 18–36 mo | 67% recurrent before SRS. 6 progressed after SRS, only 1 in field after 15 Gy, but all within the surgical bed. “Recurrence was essentially outside the SRS field” |
| Kano (2007) | 12b | 43 mo | 18 Gy | 48.3 % |
2yr actuarial | All recurrent before SRS. 5y PFS 29.4% < 20 Gy vs 63.1% 20 Gy. 19 lesions progressed, 13 in field, and 6 out of field. Predictors: Gr III (vs II), dose < 20 Gy (vs 20 Gy) |
| Attia (2009) | 24 | 26 mo | 14 Gy | 76% 52% 58% |
1yr actuarial 2yr ” 5yr ” |
Tumor vol and dose not predictive of LC, but CI was predictive. Mean CI 1.7 if recurrent vs 4.6 if no recurrence |
| Skeie (2010) | 7 | 82 mo | 12.4 Gy | 0 % | mean 43 mo | 100 cavernous sinus meningiomas, 7 Gr II. 5/7 progressed w/in 15 mo. Predictors: Gr II, tumor vol, dose, and suboptimal coveragec |
| Choi (2010) | 25 | 28 mo | 22 Gyd | 90%e 90% e 62% e |
1yr 2yr 3yr |
15 treated immed after STR, 10 after progression. 9 failures: 3 local, 6 regional. Predictors: # recurrences, delayed SRS, age >60 |
| Hardesty (2013) | 32 | 52 mo | 14 Gy | 94%f 73% f 62% f |
1yr 3yr 8yr |
No significantly different from GTR or STR alone. No recurrence in 10 patients with GTR and SRS |
n: number of patients, vol: volume, yr: year, mo: month, SRS: stereotactic radiosurgery, LC: local control, CSS: cause-specific survival, PFS: progression-free survival, Surg: surgery, EBRT: external beam radiation therapy, OS: overall survival, Gr: grade, vs: versus, w/in: within, Gy: Gray, CI: conformality index (treatment volume ÷ tumor volume), immed: imediately, LRC: locoregional control, STR: sub-total resection, #: number, signif: significant
Mean or median.
10 of the 12 had atypical primaries, 2 anaplastic.
suboptimal coverage defined as <88%
Choi et al, median marginal dose 22Gy in 1–4 fractions (median 1).
Percentages refer to loco-regional control (i.e. SRS target & resection bed).
Data derived from graph.
Attia, studying dose and conformality index (CI = treatment volume ÷ tumor volume) in residual or recurrent grade II tumors, shed further light on this issue. Their median dose was 14 Gy (range 12–18 Gy). Local recurrence, defined as within 2 cm of the original tumor margin, developed in 48% at 5 years, median time to recurrence 25 months. When CI was considered, margin dose was not predictive of local control.5 The mean CI was 1.7 in the patients who recurred, and 4.6 in those who did not (p=.038). This raises the possibility that higher doses in some studies58 might in part be a proxy for a larger CI.
This finding is supported by other studies showing that atypical meningioma may recur outside of the SRS target, yet inside the resection bed. Huffmann treated 15 patients to a median 16 Gy. At 18 to 36 months, 9 were progression-free, for a crude local control of 60%. Six (40%) progressed, 1 (17%) in field, but all within the surgical approach or resection bed.51 Choi reviewed 25 grade II patients, median marginal dose 22 Gy (range 16–30 Gy) in 1–4 fractions (median 1). Recurrence was identified in nine, 3 (33%) within the targeted region (local failure), 5 (56%) elsewhere in the resection bed (regional failure), and 1 (11%) locoregionally.13 These findings suggest that, for atypical meningioma, a volume beyond the residual or recurrent enhancement is at risk, and that this includes the entire tumor and resection bed. Further patterns of failure analyses will help define the best approaches of target definition.
Timing of treatment may also influence outcome. Choi showed improved local control with immediate (within 6 months of surgery) post-operative SRS as opposed to SRS at recurrence or progression.13 Harris, defining “late” as after radiographic progression and “early” as after craniotomy without imaging evidence of progression, found a median time to neurologic progression of 15 months after “late” SRS, versus 61 months with “early” treatment.44
Multi-session SRS has also been employed for grade II meningioma, often for larger or critically located tumors, involving for instance the anterior optic apparatus, or the sagittal sinus where edema may occur after single fraction SRS.18,34,150 Local control results have been essentially equivalent to single fraction therapy,18 possibly with a lower risk of side effects.18,34,150 Vernimmen reported multi-fraction SRS using protons. With a mean follow-up of 40 months, 88% remained under control. With the multifraction approach, they were able to treat larger tumors, up to 63cc.156 Presently, multi-fraction SRS data specific to atypical meningioma is limited. Its role and proper dose-volume constraints remain important research questions.
Fractionated External Beam Radiation Therapy (EBRT)
Several investigators have reviewed EBRT for atypical meningioma, some recommending EBRT irrespective of resection extent,19,52,163 but others have questioned its benefit. Goyal reported local control of 87% at 5 and 10 years among 22 patients. EBRT was used in 8, with a median dose 54 Gy, but did not significantly impact outcome.39 Hoffmann identified 10 grade II patients. The post-operative recurrence rate was 50%. They suggested a benefit to EBRT, especially when radical surgery could not be achieved, and recommended a higher total dose of 60 Gy.50
Aghi published an analysis of 108 patients with atypical meningioma and Simpson grade 1 resection. One hundred (93%) had surgery alone, and 8 (7%) surgery + EBRT, mean 60.2 Gy. The target volume was described as 1 cm beyond the resection bed. Five-year recurrence after GTR alone was 45%, but 0% following surgery + EBRT. This difference did not reach statistical significance (p=0.1), perhaps owing to the relatively small number of events. They assessed the clinical consequences of recurrence, and found that all 30 patients with recurrence ultimately received either EBRT or SRS, and 73% underwent repeat surgery, with a mean number of craniotomies of 2.7. Only 1 meningioma had transformed to WHO grade III, but at 7 years 33% had died as a result of recurrence.2
Similarly, Komotar reported on 45 patients with atypical meningioma and with a Simpson grade I or II resection. Thirty-two had GTR alone and 13 GTR + EBRT, median 59.4 Gy, to a target described as the tumor cavity plus a 0.5 to 1.0 cm margin. After surgery alone, 13 patients (41%) recurred at a median 19 months. After GTR + EBRT 1 patient (8%) recurred at 52.5 months. Following GTR alone versus GTR + EBRT, the respective 6-year actuarial recurrence risks were 65% versus 20% (p=0.085).62 Other recent analyses have supported EBRT in this setting. Park reported 5-year PFS rates of 46.4% with GTR alone, 77.9% with GTR + EBRT, 0% with STR alone, and 55.6% for STR + EBRT. PFS was improved by EBRT, regardless of resection extent.103
Others have reached different conclusions. Mair suggested EBRT was not appropriate following GTR, and advised SRS rather than EBRT following STR.75 In spite of this contention, their report did confirm that EBRT improved PFS when comparing surgery alone to surgery + EBRT. Four-year PFS rates were respectively, 13% following surgery alone versus 72% with surgery and EBRT (p=.043). These results were not stratified by extent of resection, and they used a relatively low mean EBRT dose of 51.8 Gy in 28 fractions.75 Hardesty reported improved outcomes with GTR, but no significant improvement in recurrence rate with radiation therapy (either EBRT or SRS) following “aggressive microsurgical resection” of an atypical meningioma. Gross total resection, defined as Simpson grade I or II, was achieved in 58% of patients. Appreciating the lack of statistical significance it is notable that no patient is this study treated with a GTR and post-operative radiation therapy experienced recurrence, with actuarial data extending 7 to 9 years.43 In this series, the number and length of follow-up of patients managed with GTR and radiation therapy was limited. Their median RT dose, 54 Gy with 1.8 to 2.0 Gy fractions, as discussed below, may be lower than optimal, but in spite of these, there were no recurrences in patients treated with GTR and radiation therapy.
A SEER-based analysis by Stessin et al reviewed 657 patients treated for a non-benign meningioma from 1988–2007.141 Two hundred and forty-four (37%) received adjuvant EBRT. After controlling for WHO grade (II vs. III), tumor size, extent of resection, and date of diagnosis (i.e. considering the 2000 WHO reclassification), EBRT was found not to impart a survival or disease-specific survival benefit. Paradoxically, they found significantly lower survival for patients receiving adjuvant EBRT than for those receiving no irradiation, possibly reflecting a treatment selection bias for patients with poor overall prognosis. Stessin did not analyze local control, and did not factor in EBRT doses or target definition parameters.141
This may be of critical importance since higher EBRT doses appear to improve outcome for grade II meningioma. Park found an improved PFS using a mean dose of 61.2 Gy.103 Aghi observed no local recurrences with 59.4 to 61.2 Gy,2 and Komotar had numerically better outcomes with a median EBRT dose of 59.4 Gy. The RTOG trial (0539), which recently completed accrual, used 54 Gy in 30 fractions for newly diagnosed atypical meningioma following GTR, and 60 Gy in 30 fractions following STR or for recurrent grade II tumors of any resection extent. The current EORTC trial (22042–26042) employs 60 Gy following a GTR, and adds a 10 Gy boost after STR. This trial will ultimately provide important guidance regarding dose-escalation for atypical meningioma.
Studies of proton radiotherapy further illuminate questions of dose. Hug published results of 15 patients with atypical meningioma. Approximately half of all patients received EBRT with photons and half combined photons and protons, with total doses from 40 to 72 CGE (Cobalt-Gray-Equivalent). Local control was significantly improved with doses > 60 CGE, with 5-year local control 90% with > 60 CGE, and 0% < 60 CGE. They noted improved results with combined photon and proton therapy, but this was not an independent factor, rather a reflection of higher doses with the use of protons.52 Boskos published outcomes with 24 high-grade meningioma patients, typically treated following STR. Nineteen (79%) were WHO grade II. Cause-specific survival at 5 years was 80% with > 60 Gy versus 24% with < 60 Gy (p=0.01). There was a trend toward further improvement with doses above 65 Gy (p=0.06).8
Optimal dosing regimens, and choices among varying radiation modalities, are important matters for further study. Dose escalation may have a role for high-grade meningioma, but caution with dose escalation is warranted. Using accelerated hyperfractionated EBRT with or without an SRS boost, Katz found a high rate of complications with no improvement in tumor control.59 Future research of RT dosing and other critical issues will be strengthened by uniform adoption of WHO grading standards, and by studies that stratify patients into de novo and recurrent categories.
WHO Grade III (Anaplastic / Malignant) Meningioma
Less than 3% of newly diagnosed meningiomas are WHO grade III (also termed anaplastic or malignant). Consequently, there are only about 300 newly-diagnosed anaplastic meningiomas per year in the USA.50 With such rarity, firm conclusions regarding optimal treatment are problematic.
These are aggressive tumors with considerably poorer local control and overall survival than lower grade meningioma. In studies used to determine WHO grading, median overall survival has been less than 2 to 3 years (Figure 1).111,112 There is little discrepancy in recommendations for aggressive treatment, typically including surgery and radiation therapy (RT), but regarding the required extent of surgery, the preferred type of RT, and its dosing and target volume constraints, treatment remains controversial. Even with aggressive management, local control remains difficult to attain, and metastasis, although uncommon, can occur. Improved treatment paradigms are needed.
Surgery
In most cases of aggressive meningioma, surgery serves as the first-line therapy, as well as establishing a diagnosis. As is the case with lower grade meningioma, recurrence corresponds to the extent of tumor removal.28,39,102,111 However, the success of surgery alone has not been satisfactory. Jaaskelainen reported a 5-year recurrence rate of 78% following GTR for patients with anaplastic meningioma, less than half of whom received any adjuvant therapy.55 Among patients with malignant histology treated with surgery alone, Dzuik encountered a 5-year PFS of 28% after GTR, and 0% after STR.28 Most investigators now recommend adjuvant therapy.29,116,144
When a clear plane between the tumor and surrounding normal structures can be identified, GTR remains the goal of surgery for anaplastic meningioma.144 Sughrue recently analyzed resection extent for WHO grade III patients. All patients were also referred for post-operative EBRT. They found that heroic surgical efforts did not improve survival, and even compromised neurologic outcome. Specifically, they found improved overall survival with near total resection (NTR) as opposed to GTR. NTR implied >90% tumor removal.144
Surgery appears of benefit at recurrence as well. Correcting for other prognostic factors, Sughrue found a survival benefit from repeat operation, with median survivals of 53 months with salvage surgery versus 25 months without (p=.02). All patients received EBRT, and some also received radiosurgery or brachytherapy. As with their patients in the de novo setting, NTR resulted in superior median survival to GTR, 77 versus 42 months (p=.005).144 In contrast, other investigators have found that the mode of salvage therapy for WHO grade III patients did not significantly affect time to subsequent progression.127
Radiation Therapy
There are no randomized trials to document the efficacy of multimodality therapy for patients with malignant meningioma, but retrospective studies, using varying definitions of anaplasia, have reported measurable benefits.19,28,83,127,136 As documented in Table 7, both EBRT and SRS have been used. Outcomes vary, perhaps in part by treatment technique, but also in relation to the extent of surgery, the histologic grading standards employed, the extent and type of follow-up, and the timing of irradiation.
Table 7.
Eleven selected series reporting treatment outcomes for patients with anaplastic meningioma.
| Anaplastic (WHO Grade III) Meningioma | |||||||
|---|---|---|---|---|---|---|---|
| Author (year) |
n |
F/Ua (study period) |
Grading Scheme |
Treatment Regimen |
RT Dosea |
Outcome |
Comments |
| Jaaskelainen (1986) | 11 | not reported (1953–1980) |
Modified WHO 1979 |
Post-op & salvage surgery alone or surgery + EBRT |
not reported |
PFS 5 yr 22% |
Atypia and anaplasia developed in previously benign tumors without radiotherapy. 4 of 5 anaplastic tumors treated w/ surg + EBRT recurred. EBRT doses not reported. |
| Milsoevic (1996) | 42 | Not reported (1966–1990) |
Modified WHO 1979 |
Post-op & salvage surg + EBRT or salvage EBRT alone. |
EBRT 50 Gy |
CSS 2 yr 63% 5 yr 34% |
Malignancy often diagnosed (60%) by brain invasion (60%), some hemangiopericytomas. Negative predictive fxs (CSS): age ≥58, EBRT before 1975, dose <50Gy. Morbidity 3.4%. Recommend immediate post-op EBRT. |
| Dziuk (1998) | 38 | 29–39 mob (1984–1992) |
Russell & Rubinstein 1977 [R&R 1977] |
Initial & salvage surg alone or with EBRT |
EBRT 54Gy |
PFS 2 yr 24% 5 yr 25% |
5yr PFS: 39% after GTR, 0% STR. 28% GTR alone, 57% GTR+EBRT. Intital post-op EBRT improved 5yr PFS 15% to 80%, and salvage EBRT 2yr PFS 50% to 89%, but no benefit at 60 mo. 11 had hemangiopericytoma. No distant failures. |
| Hug (2000) | 16 | 59 mo (1973–1995) |
WHO 1993 | Post-op & salvage photon + proton EBRT |
EBRT photon + proton 58 CGE |
LC 5 yr 52% 8 ys 17% |
LC & OS improved w/ EBRT ≥60 Gy. 5 & 8yr LC 100% & 33% w/ ≥60 CGE versus 0% w/ <60 CGE. Late morbidity 9%. |
| Mattozo (2007) | 5 | 42 mo (1992–2004) |
WHO 2000 | Post-op & salvage SRS or stereotactic EBRT |
SRS 15.5 Gy EBRT 49.3Gy |
PFS 3 yr 0% |
All patients had recurrent tumors. Median SRS treatment vol 2.2cc, median EBRT vol 21.3cc. 77% of recurrences were within the original resection cavity. |
| Kondziolka (2008) | 29 | 48 mo (not reported) |
Based upon “previous histo-path” |
Post-op & salvage single fraction SRS |
SRS 14 Gy |
LC 15mo 17% 5yr 9%¶ |
Median tumor vol 7.4cc. 5y CSS 22%¶ Morbidity 7%, including symptomatic edema in 4% |
| Boskos (2009) | 5 | 32 mo (1999–2006) |
WHO 1993 | Post-op & salvage photon + proton EBRT. 1 patient hypofractionated protons alone |
EBRT photon + proton 65 CGE |
Mean RFI 23 mo |
Median CTV 151cc. Mean RFI for grade II tumors 28.3mo. OS & CSS improved w/ EBRT >60 Gy, and possibly further w/ >65 Gy. Late morbidity in 1 patient, necrosis. |
| Rosenberg (2009) | 13 | not reported (1984–2006) |
WHO 2007 | Post-op & salvage EBRT or SRS. 2 received systemic therapy (1 temozolomide, 1 immunotherapy) |
EBRT 50–60 Gy SRS 14–24 Gy 5 Gy x 5 |
PFS 1 yr 52% 2 yr 17% 3 yr 8.7% |
Median time to recurrence 9.6 mo. Med OS 2.5 yr w/o vs 5.4 yr w/ initial EBRT (p=.13). 5 & 8 yr OS 47.2%, 12.2%. RT morbidity 2 patients, both necrosis. Recommend upfront RT. |
| Sughrue (2010) | 63 | 60 mo (not reported) |
WHO 2007 | Post-op fractionated EBRT & some salvage brachy or SRS, newly diagnosed & recurrent. 25% pre- operative embolization |
EBRT doses not reported |
PFS 2yr 80% 5yr 57% 10yr 40% |
Mean tumor vol 78cc. 2,5&10 yr OS 82%, 61%, 40%. Better survival with NTR than with GTR. Signif neuro morbidity from attempted GTR. |
| El-Khatib (2011) | 7 | 60 mo (1990–2003) |
WHO 2007 | Post-op & salvage single fraction SRS, newly diagnosed & recurrent |
SRS 14 Gy |
PFS 3yr 57% 5yr 57% 10yr 43% |
Median tumor vol 4.8cc. PFS 57% 5yr, 43% 10yr. Negative predictive fx (tumor control) age ≥50. Morbidity 3.5%. |
| Pollock (2012) | 13 | 38 mo (1990–2008) |
WHO 2000 & 2007 |
Post-op & salvage single fraction SRS, newly diagnosed & recurrent |
SRS 15 Gy |
CSS 1 yr 69% 5 yr 27% |
Median tumor vol 14.6cc. Negative predictive fxs (CSS): prior EBRT & tumor vol >14.6cc. Morbidity 26%. Emphasize early SRS. |
n: number of patients, F/U: follow-up, RT: radiation therapy, WHO: World Health Organization, Post-op: post-operative, EBRT: external beam radiation therapy, PFS: progression-free survival, w/: with, surg: surgery, fx(s): factor(s), CSS: cause-specific survival, mo: month, GTR: gross total resection, STR: subtotal resection, yr: year,LC: local control, CGE: cobalt Gray equivalent), vol: volume, histo-path: histo-pathology, RFI: relapse-free interval, CTV: clinical target volume, OS: overall survival,SRS: stereotactic radiosurgery, brachy: brachytherapy, NTR: near total resection (>90% removal), neuro: neurological, vs: versus.
Actuarial percentage measured from graph.
Mean or median
follow-up listed by study groups, and varied accordingly.
Stereotactic Radiosurgery (SRS)
Some authors have argued that SRS is not indicated for malignant meningioma,88 however, several studies have reported outcomes with SRS (Table 7). Kondziolka treated 29 WHO grade III patients with post-operative SRS, mean margin dose 14 Gy, and found PFS rates of 17% at 15 months, and 9% (extrapolated from graph) at 5 years.65 In a separate publication of convexity meningioma, the same group treated 5 WHO grade III patients. With follow-up extending to 47 months, none maintained local control, and 4 of 6 died of tumor progression.64
El-Khatib reported 7 patients with WHO grade III meningioma, using a 14 Gy margin dose. They found considerably higher rates of PFS, 57% at 3-years and 43% at 10 years. This study employed similar tumor margin doses to Kondziolka. The mean target volumes were modestly smaller in the El-Khatib study (4.8 versus 7.4 cc). Both studies included newly diagnosed and recurrent tumors. The Kondziolka study graded tumors based upon “previous histopathology” (often diagnosed before the advent of the WHO criteria) whereas El-Khatib used the WHO 2007 criteria. These differences in diagnostic criteria may play a role in accounting for the differences in results.
Pollock recently published an experience with 50 WHO grade II or III patients, treated in both the de novo and salvage settings. Thirteen had anaplastic meningioma. Their median treatment volume was larger at 14.6 cc, and median dose modestly higher at 15 Gy. Disease-specific survival at 1 and 5 years for the WHO grade III patients was 69% and 27%. They did not specify PFS for malignant meningioma alone, but for their entire group of 50 high-grade tumors PFS at 1 year was 76%, and at 5 years 40%. For patients who had failed prior EBRT, PFS was lower, 19% at 3 years.117
Fractionated External Beam Radiation Therapy (EBRT)
The early experiences of Milosevic83 and Dziuk28 provide evidence of benefit from surgery followed by EBRT, and indeed for the use of EBRT initially rather than at progression, now accepted as a standard approach for anaplastic meningiomas. Melosevic found that patients who received < 50 Gy experienced inferior cause-specific survival, as did those treated before 1975 (i.e. before CT based planning).83 Dziuk found that EBRT improved 5-year PFS from 50% to 80% compared to surgery alone. When EBRT was added following initial resection, 5-year PFS significantly improved from 15% to 80%. They recommended a total EBRT dose of 6000 cGy “be administered coincident with an initial complete resection, with a 4 cm margin for the initial 5000 cGy.”28
The use and extent of a margin in radiation therapy treatment planning is a topic of particular interest when comparing EBRT and SRS for malignant meningiomas. With SRS, Pollock described tumor progression, “away from the original irradiated tumor,” in 30% of patients with atypical or anaplastic meningioma, occurring at a median of 15 months after SRS. Most (80%) were marginal, meaning “adjacent to the irradiated tumor.116 Analyzing SRS and stereotactic EBRT for recurrent high-grade meningioma, Mattozo found that 77% of recurrences were within the original resection cavity, and recommended that “the whole cavity receive radiation therapy,” with an SRS boost to the recurrent nodule if desired. They suggested that EBRT to treat the entire tumor cavity after initial surgery may be appropriate to reduce the risk of any relapse.78
Indeed the timing of RT appears to be an important factor. Some studies have shown modest benefit from irradiation in the recurrent setting,28 but others have suggested little or no improvement from salvage RT.78,127,144 Dziuk reported that EBRT improved local control with malignant meningioma over surgery alone. Even in the recurrent group, 2-year PFS improved from 50% to 89% (p=.002) with EBRT, although it had no impact at 5-years.28 Following initial resection, several investigators have found outcome improvement with RT (Table 7).28,44,83,127
Other RT factors may play important roles. As with atypical meningioma, higher RT doses appear to improve local tumor control for patients with malignant histology. Reviewing WHO grade II and III patients, Milosevic found a 5-year cause-specific survival of 42% with ≥50 Gy versus 0% with <50 Gy.83 With malignant lesions, Goldsmith reported a 5-year PFS of 63% using >53 Gy versus 17% with ≤ 53 Gy,37 and Dziuk recommend a total EBRT dose of 60 Gy, even after GTR.28 More recent studies have specifically evaluated doses of this magnitude.
Using either photons or combined photons and protons, DeVries24 and Hug52 showed dramatic increases in local control and survival with a total dose exceeding 60 Gy. Hug, studying a mixed group of WHO grade II and III meningiomas, identified 5-year local control of 100% for patients receiving ≥60 CGE versus 0% with lower doses (p=.0006). The respective 8-year figures were 33% and 0%. For the subgroup with malignant meningioma, improved local control corresponded with improved 5 and 8 year overall survival: 87% with ≥60 CGE and 15% with <60 CGE.52 As mentioned with WHO grade II tumors, some caution is prudent with dose escalation. Katz found no benefit from accelerated hyperfractionated RT, on occasion with an SRS boost, but did encounter unacceptable toxicity.59
Summary
Meningiomas are the most common primary intracranial tumor.15 The majority are histologically benign (WHO grade I), but even if benign can be clinically formidable. Owing to a lack of prospective, randomized trials, standardized treatment guidelines are difficult to formulate. Furthermore, uniformly applied guidelines have been difficult to achieve given the typical pattern of slow growth and given the availability of several management options. Granting these limitations, a growing body of largely retrospective evidence does permit inferences.
Small, incidental meningiomas can often be carefully observed, as recommended in the NCCN guidelines. For most other patients, gross total resection (GTR) remains the benchmark. However, complete removal within the constraints of acceptable morbidity is not always achievable. Many meningiomas arise at or near critical neural or vascular structures or in sites with limited surgical access, and can be very challenging for surgeons.142 Based upon these concerns and upon other key features such as WHO grade, clinically significant subgroups of patients cannot be managed successfully by resection alone. When a GTR is not accomplished, postoperative RT, including SRS or EBRT, are important considerations. In this setting, numerous studies indicated improvements in local control. Some have shown significant cause-specific survival advantages as well. In spite of this, there remains controversy regarding most appropriate therapy after subtotal resection (STR), particularly as to whether patients should be observed and treated at progression, or treated preemptively. Some patients do well for many years after STR alone, while others progress and develop larger, symptomatic tumors more promptly.
Adding further controversy, there is increasing retrospective evidence in support of SRS or EBRT not only in the adjuvant or salvage setting, but also as primary therapy. The relative efficacy of these approaches has not yet been tested in rigorously designed prospective clinical trials, but results with SRS and EBRT, at least for the majority of patients with known or presumed benign (WHO grade I) meningiomas, have been remarkably similar, whether comparing them to each other or to reported results from surgery. Either SRS or EBRT can be recommended for many patients but not for all. EBRT is suitable for a broader range of patients, whereas excellent outcome with SRS has been realized among more distinct cohorts, taking neurovascular anatomy, location, edema risk, and tumor diameter or volume into careful account. At present, surgery retains a central role in management, acquires tissue for histologic and molecular analysis, and promptly addresses rapidly progressive tumors or tumor-related symptoms. However, with this important caveat, excellent long-term results have been attained using SRS or EBRT administered either adjuvantly or primarily.
Many significant questions remain in the more common setting of benign meningioma, and with higher grade meningioma these uncertainties are magnified. Current data support adjuvant irradiation for WHO grade III meningioma irrespective of resection extent, and for grade II meningioma at least following STR. Considerable controversy persists for patients with an newly diagnosed and gross totally resected WHO grade II meningioma. At present they may be managed with post-operative irradiation or with close observation. A randomized clinical trial has been designed to address this very question, and is expected to open in the near future. This is becoming a more clinically relevant question. There have been notable increases in the incidence of WHO grade II meningioma with broader implementation of the current WHO grading criteria. The RTOG (0539) and EORTC (22042–22062) have recently completed accrual to phase II clinical trials. From these studies there will likely be clinical outcome analyses to help integrate imaging, operative, central pathology, genotyping, immunohistochemical, microarray, and molecular (serum and urine) correlative findings.
A growing body of investigators is committed to the design and completion of prospective multicenter studies of meningioma, and is active in the above-mentioned studies and in the development of other trials. A companion article will evaluate the role of systemic therapies for patients with meningioma. Additionally, RANO is currently completing a manuscript proposing standardized endpoints and response criteria, providing investigators an opportunity to design trials and publish outcomes in a more uniform and consonant fashion.
Footnotes
- Leland Rogers: Principle Investigator for a research grant from the National Cancer Institute in support of the clinical trial RTOG 0539
- Igor Barani: BrainLab Research Grant & NAGKC Research Grant
Bibliography
- 1.Adegbite AB, Kahn MI, Paine KWE, Tan LK. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51–56. doi: 10.3171/jns.1983.58.1.0051. [DOI] [PubMed] [Google Scholar]
- 2.Aghi MK, Carter BS, Cosgrove GR, Ojemann RG, Amin-Hanjami S, Martuza RL, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64:56–60. doi: 10.1227/01.NEU.0000330399.55586.63. [DOI] [PubMed] [Google Scholar]
- 3.Akeyson E, McCutcheon I. Management of benign and aggressive intracranial meningiomas. Oncology. 1996;10:747–756. [PubMed] [Google Scholar]
- 4.Asgharian B, Chen YJ, Patronas NJ, Peghini PL, Reynolds JC, Vortmeyer A, et al. Meningiomas may be a component tumor of multiple endocrine neoplasia type 1. Clinical Cancer Research. 2004;10:869–880. doi: 10.1158/1078-0432.ccr-0938-3. [DOI] [PubMed] [Google Scholar]
- 5.Attia A, Chan M, Seif D, Russel B, Bourland JD, Deguzman A, et al. Treatment of atypical meningiomas with GammaKnife Radiiosurgery: the role of conformality index and margin dose. Int J Radiat Oncol Biol Phys. 2009;75(3):S226. (abst) [Google Scholar]
- 6.Barbaro NM, Gutin PH, Wilson CB, Sheline GE, Boldrey EB, Wara WM. Radiation therapy in the treatment of partially resected meningiomas. Neurosurgery. 1987;20:525–528. doi: 10.1227/00006123-198704000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Bledsoe JM, Link MJ, Stafford SL, Park PJ, Pollock BE. Radiosurgery for large-volume (>10cc) benign meningiomas. Journal of Neurosurgery. 2010;112:951–956. doi: 10.3171/2009.8.JNS09703. [DOI] [PubMed] [Google Scholar]
- 8.Boskos C, Feuvret L, Noel G, Habrand JL, Pommier P, Alapetite C, et al. Combined \proton and photon conformal radiotherapy for intracranial atypical and malignant meningioma. Int J Radiat Oncol Biol Phys. 2009;75(2):399–406. doi: 10.1016/j.ijrobp.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 9.Bria C, Wegner RE, Clump DA, Vargo JA, Mintz AH, Heron DE, et al. Fractionated stereotactic radiosurgery for the treatment of meningiomas. J Cancer Res Ther. 2011;7(1):52–57. doi: 10.4103/0973-1482.80462. [DOI] [PubMed] [Google Scholar]
- 10.Central Brain Tumor Registry in the United States (CBTRUS) Central Brain Tumor Registry of the United States. Chicago: CBTRUS; 2000. Statistical report: Primary brain tumors in the Unites States, 1992–1997; pp. 11–26. [Google Scholar]
- 11.Central Brain Tumor Registry of the United States (2010) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2006. 2010 Feb; http://www.cbtrus.org/2010-NPCRSEER/CBTRUSWEBREPORT-Final-3-2-10.pdf.
- 12.Chamoun R, Krisht KM, Couldwell WT. Incidental meningiomas. Neurosurg Focus. 2011;31(6):E19. doi: 10.3171/2011.9.FOCUS11220. [DOI] [PubMed] [Google Scholar]
- 13.Choi CY, Soltys SG, Gibbs IC, Harsh GR, Jackson PS, Lieberson RE, et al. Cyberknife stereotactic radiosurgery for treatment of atypical (WHO Grade II) cranial meningiomas. Neurosurgery. 2010;67:1180–1188. doi: 10.1227/NEU.0b013e3181f2f427. [DOI] [PubMed] [Google Scholar]
- 14.Chuang CC, Chang CN, Tsang NM, Wei KC, Tseng CK, Chang JT, et al. Linear accelerator-based radiosurgery in the management of skull base meningiomas. J Neurooncol. 2004;66:241–249. doi: 10.1023/b:neon.0000013500.11150.36. [DOI] [PubMed] [Google Scholar]
- 15.Claus E, Bondy M, Schildkraut J, Wiemels J, Wrensch M, Black P. Epidemiology of intracranial meningioma. Neurosurgery. 2005;57(6):1088–1095. doi: 10.1227/01.neu.0000188281.91351.b9. [DOI] [PubMed] [Google Scholar]
- 16.Claus EB, Calvocoressi L, Bondy ML, Schildkraut JM, Wiemels JL, Wrensch M. Family and personal medical history and risk of meningioma. Journal of Neurosurgery. 2011;115:1072–1077. doi: 10.3171/2011.6.JNS11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coke C, Corn B, Werner-Wasik M, Xie Y, Curran WJ. Atypical and malignant meningiomas: an outcome report of 17 cases. J Neurooncology. 1998;29:65–70. doi: 10.1023/a:1005981731739. [DOI] [PubMed] [Google Scholar]
- 18.Columbo F, Casentini Cavedon C, Scalchi P, Cora S, Francescon P. Cyberknife radiosurgery for benign meningiomas: short-term results in 199 patients. Neurosurgery. 2009;64(2):A7–A13. doi: 10.1227/01.NEU.0000338947.84636.A6. (February 2009 suppl) [DOI] [PubMed] [Google Scholar]
- 19.Condra K, Buatti J, Mendenhall W, Friedman WA, Marcus RB, Jr, Rhoton Al. Benign meningiomas: primary treatment selection affects survival. Int J Radiation Oncology Biol Phys. 1997;39:427–436. doi: 10.1016/s0360-3016(97)00317-9. [DOI] [PubMed] [Google Scholar]
- 20.Cushing H. The meningiomas (dural endotheliomas). Their source and favoured seats of origin. Brain. 1922;45:282–316. [Google Scholar]
- 21.Davidson L, Fishback D, Russin JJ, Weiss MH, Yu C, Pagnini PG, et al. Postoperative Gamma Knife surgery for benign meningiomas of the cranial base. Neurosurg Focus. 2007;23(4):E6. doi: 10.3171/FOC-07/10/E6. [DOI] [PubMed] [Google Scholar]
- 22.Davis FGKupelian V, Freels S, McCarthy B, Surawicz T. Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro-Oncology. 2001;3:152–158. doi: 10.1093/neuonc/3.3.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeMonte F, Marmor E, Al-Mefty O. In: Meningiomas, in Brain Tumors. 2nd. Andrew Kaye, Edward Laws., editors. London: Churchill Livingstone; 2001. pp. 719–750. [Google Scholar]
- 24.DeVries A, Munzenrider JE, Hedley-Whyte T, Hug EB. The role of radiotherapy in the treatment of malignant meningiomas. Strahlenther Onkol. 1999;33:239–253. doi: 10.1007/BF02753844. [DOI] [PubMed] [Google Scholar]
- 25.DiBiase SJ, Kwok Y, Yovina S, Arena C, Nagvi S, Temple R, et al. Factors predicting local tumor control after Gamma Knife stereotactic radiosurgery for benign intracranial meningiomas. Int J Radiation Oncology Biol Phys. 2004;60:1515–1519. doi: 10.1016/j.ijrobp.2004.05.073. [DOI] [PubMed] [Google Scholar]
- 26.Dos Santos MA, de Salcedo JBP, Diaz JAG, Calvo FA, Samblas J, Marsiglia H, et al. Long-term outcomes of stereotactic radiosurgery for treatment of cavernous sinus meningiomas. Int J Radiation Oncology Biol Phys. 2011;81(5):1436–1441. doi: 10.1016/j.ijrobp.2010.07.2002. [DOI] [PubMed] [Google Scholar]
- 27.Dufour H, Muracciole X, Metellus P, Regis J, Chinot O, Grisoli F. Long-term tumor control and functional outcome in patients with cavernous sinus meningiomas treated by radiotherapy with or without previous surgery: is there an alternative to aggressive tumor removal? Neurosurgery. 2001;48:285–296. doi: 10.1097/00006123-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Dziuk TW, Woo S, Butler EB, Thornby J, Grossman R, Dennis WS, et al. Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. Journal of Neuro-Oncology. 1998;37:177–188. doi: 10.1023/a:1005853720926. [DOI] [PubMed] [Google Scholar]
- 29.El-Khatib M, Majdoub FE, Hoevels M, Kocher M, Muller RP, Steiger HJ, et al. Stereotactic LINAC radiosurgery for incompletely resected or recurrent atypical and anplastic meningiomas. Acta Neurochir. 2011;153:1761–1767. doi: 10.1007/s00701-011-1073-7. [DOI] [PubMed] [Google Scholar]
- 30.Eustacchio S, Trummer M, Fuchs I, Schrottner O, Sutter B, Pendl G. Preservation of cranial nerve function following Gamma Knife radiosurgery for benign skull base meningiomas: experience in 121 patients with follow-up of 5 to 9.8 years. Acta Neurochir Suppl. 2002;84:71–76. doi: 10.1007/978-3-7091-6117-3_8. [DOI] [PubMed] [Google Scholar]
- 31.Flannery TJ, Kano H, Lunsford LD, Sirin S, Tormenti M, Miranjan A, et al. Long-term control of petroclival meningiomas through radiosurgery. Journal of Neurosurgery. 2010;112:957–964. doi: 10.3171/2009.8.JNS09695. [DOI] [PubMed] [Google Scholar]
- 32.Flickinger JC, Kondziolka D, Maitz AH, Lundsford LD. Gamma Knife radiosurgery of imaging-diagnosed intracranial meningioma. Int J Radiation Oncology Biol Phys. 2003;56(3):801–806. doi: 10.1016/s0360-3016(03)00126-3. [DOI] [PubMed] [Google Scholar]
- 33.Ganz JC, Backlund EO, Thorsen FA. The results of Gamma Knife surgery for meningiomas, related to size of tumor and dose. Stereotact Funct Neurosurg. 1993;61(suppl 1):23–29. doi: 10.1159/000100656. [DOI] [PubMed] [Google Scholar]
- 34.Girvigian MR, Chen JCT, Rahimian J, Miller MJ, Tome M. Comparison of early complications for patients with convexity and paragaggital meningiomas treated with either stereotactic radiosurgery or fractionated stereotactic radiotherapy. Neurosurgery. 2008;62:A19–A28. doi: 10.1227/01.neu.0000325933.34154.cb. [DOI] [PubMed] [Google Scholar]
- 35.Glaholm J, Bloom HJG, Crow JH. The role of radiotherapy in the management of intracranial meningiomas: the Royal Marsden Hospital experience with 186 patients. Int J Radiation Oncology Biol Phys. 1990;18(4):755–761. doi: 10.1016/0360-3016(90)90394-y. [DOI] [PubMed] [Google Scholar]
- 36.Meningioma Goldsmith B, editor; Leibel S, Phillips T, editors. Textbook of Radiation Oncology. Philadelphia: WB Saunders; 1998. pp. 324–340. [Google Scholar]
- 37.Goldsmith B, Wara W, Wilson C, Larson DA. Postoperative irradiation for subtotally resected meningiomas. J Neurosurg. 1994;80:195–201. doi: 10.3171/jns.1994.80.2.0195. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein RA, Jorden MA, Harsh GR. Meningiomas: natural history, diagnosis, and imaging. In: Black PM, Loeffler JS, editors. Cancer Nervous of the System. 2nd. Philadelphia: Peter Lippincott, Williams &Wilkins; 2005. pp. 279–313. [Google Scholar]
- 39.Goyal LK, Suh JH, Mohan DS, Prayson RA, Lee J, Barnett GH. Local control and overall survival in atypical meningioma: a retrospective study. Int J Radiat Oncol Biol Phys. 2000;46(1):57–61. doi: 10.1016/s0360-3016(99)00349-1. [DOI] [PubMed] [Google Scholar]
- 40.Hamm K, Hensel M, Gross MW, Surber G, Kleinert G, Engenhart-Cabillic R. Radiosurgery/Stereotactic Radiotherapy in the therapeutical concept for skull base meningiomas. Zentralbl Neurochir. 2008;69(1):14–21. doi: 10.1055/s-2007-992138. [DOI] [PubMed] [Google Scholar]
- 41.Han JH, Kim DG, Chung HT, Park CK, Paek SH, Kim CY, Jung HW. Gammaknife radiosurgery for skull base meningiomas: long-term radiologic and clinical outcome. Int J Radiat Oncol Biol Phys. 2008;72(5):1324–1332. doi: 10.1016/j.ijrobp.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 42.Hanft S, Canoll P, Bruce JN. A review of malignant meningiomas: diagnosis, characteristics, and treatment. J Neurooncol. 2010;99:433–443. doi: 10.1007/s11060-010-0348-9. [DOI] [PubMed] [Google Scholar]
- 43.Hardesty DA, Wolf AB, Brachman DG, McBride HL, Youssef E, Nakaji P, et al. The inpact of adjuvant stereotactic radiosurgery on atypical meningioma recurrence following aggressive microsurgical resection. Journal of Neurosurgery, published online February. 2013;8 doi: 10.3171/2012.12.JNS12414. [DOI] [PubMed] [Google Scholar]
- 44.Harris AE, Lee JYK, Omalu B, Flickinger JC, Kondziolka D, Lunsford LD. The effect of radiosurgeryduring management of aggressive meningiomas. Surgical Neurology. 2003;60(4):298–305. doi: 10.1016/s0090-3019(03)00320-3. [DOI] [PubMed] [Google Scholar]
- 45.Hasegawa T, Kida Y, Yoshimoto M, Koike J, Iizuka H, Ishii D. Long-term outcomes of Gamma Knife surgery for cavernous sinus meningioma. Journal of Neurosurgery. 2007;107(4):745–751. doi: 10.3171/JNS-07/10/0745. [DOI] [PubMed] [Google Scholar]
- 46.Hashiba T, Hashimoto N, Izumoto S, Suzuki T, Kagawa N, Maruno M, et al. Serial volumetric assessment of the natural history and growth pattern of incidentally discovered meningiomas. Journal of Neurosurgery. 2009;110:675–684. doi: 10.3171/2008.8.JNS08481. [DOI] [PubMed] [Google Scholar]
- 47.Hasseleid BF, Meling TR, Rønning P, Scheie D, Helseth E. Surgery for convexity meningioma: Simpson grade I as the goal. Journal of Neurosurgery. 2012;117:999–106. doi: 10.3171/2012.9.JNS12294. [DOI] [PubMed] [Google Scholar]
- 48.Hayashi M, Chernov M, Tamura N, Izawa M, Muragaki Y, Iseki H, et al. Gamma Knife robotic microradiosurgery for benign skull base meningiomas: tumor shrinkage may depend on the amount of radiation energy delivered per lesion volume (unit energy) Stereotactic and Functional Neurosurgery. 2011;89(1):6–16. doi: 10.1159/000321184. [DOI] [PubMed] [Google Scholar]
- 49.Henzel M, Gross MW, Hamm K, Surber G, Kleinert G, Failing T, et al. Stereotactic radiotherapy for meningiomas. Strahlenther Onkol. 2006;182:382–388. doi: 10.1007/s00066-006-1535-7. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro-Oncology. 2006;8:27–27. doi: 10.1215/S1522851705000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huffmann BC, Reinacher PC, Gilsbach JM. Gamma knife surgery for atypical meningiomas. J Neurosurg. 2005;102(suppl):283–285. doi: 10.3171/jns.2005.102.s_supplement.0283. [DOI] [PubMed] [Google Scholar]
- 52.Hug EB, Devries A, Thornton AF, Munzenrider JE, Pardo S, Hedley-Whyte ET, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D–conformal radiation therapy. J Neurooncology. 2000;48:151–160. doi: 10.1023/a:1006434124794. [DOI] [PubMed] [Google Scholar]
- 53.Iwai Y, Yamanaka K, Ikeda H. Gamma knife radiosurgery for skull base meningioma: long-term results of low-dose treatment. Journal of Neurosurgery. 2008;109:804–810. doi: 10.3171/JNS/2008/109/11/0804. [DOI] [PubMed] [Google Scholar]
- 54.Iwai Y, Yamanaka K, Ishiguro T. Gamma knife radiosurgery for the treatment of cavernous sinus meningiomas. Neurosurgery. 2003;52(3):517–524. doi: 10.1227/01.neu.0000047814.18819.9f. [DOI] [PubMed] [Google Scholar]
- 55.Jääskeläinen J. Seemingly complete removal of histologically benign intracranial meningioma: late recurrence rate and factors predicting recurrence in 657 patients; a multivariate analysis. Surg Neurol. 1986;26(5):461–469. doi: 10.1016/0090-3019(86)90259-4. [DOI] [PubMed] [Google Scholar]
- 56.Jalali R, Loughrey C, Baumert B, Perks J, Warrington AP, Traish D, et al. High precision focused irradiation in the form of fractionated stereotactic conformal radiotherapy (SCRT) for benign meningiomas predominately in the skull base location. Clinical Oncology. 2002;14(2):103–109. doi: 10.1053/clon.2001.0040. [DOI] [PubMed] [Google Scholar]
- 57.Jensen R, Lee J. Predicting outcomes of patients with meningiomas using molecular markers of hypoxia, vascularity, and proliferation. Neurosurgery. 2012;71:146–156. doi: 10.1227/NEU.0b013e3182567886. [DOI] [PubMed] [Google Scholar]
- 58.Kano H, Takahashi JA, Katsuki T, Araki N, Oya N, Hiraoka M, et al. Stereotactic radiosurgery for atypical and anaplastic meningoimas. J Neurooncology. 2007;84:41–47. doi: 10.1007/s11060-007-9338-y. [DOI] [PubMed] [Google Scholar]
- 59.Katz TS, Amdur RJ, Yachnis AT, Mendenhall WM, Morris CG. Pushing the limits of radiotherapy for atypical and malignant meningioma. American Journal of Clinical Oncology. 2005;28(1):70–74. doi: 10.1097/01.coc.0000139958.88481.5c. [DOI] [PubMed] [Google Scholar]
- 60.King D, Chang C, Pool J. Radiotherapy in the management of meningiomas. Acta Radiol Ther Phys Biol. 1966;5:26–33. doi: 10.3109/02841856609139540. [DOI] [PubMed] [Google Scholar]
- 61.Kollova A, Liscak R, Novotny J, Vilibald V, Simonova G, Janouskova L. Gamma knife surgery for benign meningioma. J Neurosurg. 2007;107:325–336. doi: 10.3171/JNS-07/08/0325. [DOI] [PubMed] [Google Scholar]
- 62.Komotar RJ, Iorgulescu JB, Raper DMS, Holland EC, Beal K, Bilsky MH, et al. The role of radiotherapy following gross-total resection of atypical meningiomas. Journal of Neurosurgery. 2012 Aug 24; doi: 10.3171/2012.7.JNS112113. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 63.Kondziolka D, Flickinger J, Perez B. Gamma Knife Study Group: Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Neurosurgery. 1998;43:405–414. doi: 10.1097/00006123-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Kondziolka D, Madhok R, Lunsford LD, Mathieu D, Miranjan A, Flickinger JC. Stereotactic radiosurgery for convexity meningiomas. Journal of Neurosurgery. 2009;111:458–463. doi: 10.3171/2008.8.JNS17650. [DOI] [PubMed] [Google Scholar]
- 65.Kondziolka D, Mathieu D, Lunsford LD, Martin JJ, Madhok R, Niranjan A, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62(1):53–60. doi: 10.1227/01.NEU.0000311061.72626.0D. [DOI] [PubMed] [Google Scholar]
- 66.Korah MP, Nowlan AW, Johnstone PA, Crocker IR. Radiation therapy alone for image defined maningiomas. Int J Radiation Oncology Biol Phys. 2010;76(1):181–186. doi: 10.1016/j.ijrobp.2009.01.066. [DOI] [PubMed] [Google Scholar]
- 67.Kriel W, Luggin J, Fuchs I, Weigl V, Eustacchio S, Papaefthymiou G. Long term experience of gamma knife radiosurgery for benign skull base meningioma. J Neurol Neurosurg Psychiatry. 2005;76:1425–1430. doi: 10.1136/jnnp.2004.049213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee JY, Niranjan A, McInerney J, Kondziolka D, Lundsford LD. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurgery. 2002;97(1):65–72. doi: 10.3171/jns.2002.97.1.0065. [DOI] [PubMed] [Google Scholar]
- 69.Lin NU, Lee EQ, Aoyama H, Barani IJ, Baumert BG, Brown PD, et al. Response Assessment in Neuro-Oncology (RANO) group. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol. 2013;14(10):e396–e406. doi: 10.1016/S1470-2045(13)70311-5. [DOI] [PubMed] [Google Scholar]
- 70.Lin NU, Wefel JS, Lee EQ, Schiff D, van den Bent MJ, Soffietti R, et al. Response Assessment in Neuro-Oncology (RANO) group. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive neurological quality-of-life outcomes. A report from the RANO group. Lancet Oncol. 2013;14(10):e407–e416. doi: 10.1016/S1470-2045(13)70308-5. [DOI] [PubMed] [Google Scholar]
- 71.Litre CF, Colin P, Noudel R, Peruzzi P, Bazin A, Sherpereel B, et al. Fractionated stereotactic radiotherapy treatment of cavernous sinus meningiomas: a study of 100 cases. Int J Radiation Oncology Biol Phys. 2009;74(4):1012–1017. doi: 10.1016/j.ijrobp.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 72.Mahadevan A, Floyd S, Wong E, Chen C, Kasper E. Clinical outcome after hypofractionated stereotactic radiotherapy (HSRT) for benign skull base tumors. Somput Aided Surg. 2011;16(3):112–120. doi: 10.3109/10929088.2011.565160. [DOI] [PubMed] [Google Scholar]
- 73.Mahmood A, Caccamo DV, Tomacek FJ, Malik GM. Atypical and malignant meningiomas: a clinicopathological review. Neurosurgery. 1993;33(6):955–963. doi: 10.1227/00006123-199312000-00001. [DOI] [PubMed] [Google Scholar]
- 74.Mahmood A, Qureshi NH, Malik GM. Intracranial meningiomas: analysis of recurrence after surgical treatment. Acta Neurochir (Wien) 1994;126:53–58. doi: 10.1007/BF01476410. [DOI] [PubMed] [Google Scholar]
- 75.Mair R, Morris K, Scott I, Carroll TA. Radiotherapy for atypical meningiomas. Journal of Neurosurgery. 2011;115(4):811–819. doi: 10.3171/2011.5.JNS11112. [DOI] [PubMed] [Google Scholar]
- 76.Marchetti M, Bianchi S, Milanesi I, Bergantin A, Bianchi L, Broggi G, et al. Multisession radiosurgery for optic nerve sheath meningiomas - an effective option: Preliminary results of a single0center experience. Neurosurgery. 2011;69:1116–1123. doi: 10.1227/NEU.0b013e31822932fe. [DOI] [PubMed] [Google Scholar]
- 77.Marcus HJ, Price SJ, Wilby M, Santarius T, Kirollos RW. Radiotherapy as an adjunct in the management of intracranial meningiomas: are we practicing evidence-based medicine. British Journal of Neurosurgery. 2008;22:520–528. doi: 10.1080/02688690802308687. [DOI] [PubMed] [Google Scholar]
- 78.Mattozo CA, De Salles AAF, Klement I, Gorgulho A, McArthur D, Ford JM, et al. Stereotactic radiation treatment for recurrent nonbenign meningiomas. Journal of Neurosurgery. 2007;106(5):846–854. doi: 10.3171/jns.2007.106.5.846. [DOI] [PubMed] [Google Scholar]
- 79.McDermott MW, Wilson CB. Meningiomas. In: Youmans JR, editor. Neurologic Surgery. 4. Philadelphia: WB Suanders; 1996. pp. 2282–2825. [Google Scholar]
- 80.Metellus P, Batra S, Karkar S, Kapoor S, Weiss S, Kleinberg L, et al. Fractionated conformal radiotherapy in the management of cavernous sinus meningiomas: Long-term functional outcome and tumor control at a single institution. Int J Radiation Oncology Biol Phys. 2010;78(3):836–843. doi: 10.1016/j.ijrobp.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 81.Milker-Zabel S, Zabel A, Schulz-Ertner D, Schlegel W, Wannenmacher M, Debus J. Fractionated stereotactic radiotherapy in patients with benign or atypical intracranial meningioma: long-term experience and prognostic factors. Int J Radiat Oncol Biol Phys. 2005;61(3):809–816. doi: 10.1016/j.ijrobp.2004.07.669. [DOI] [PubMed] [Google Scholar]
- 82.Milker-Zabel S, Zabel-Du Bois A, Huber P, Schlegel W, Debus J. Intensity-modulated radiotherapy for complex shaped meningioma of the skull base: long-term experience of a single institution. Int J Radiation Oncol Biol Phys. 2007;68(3):858–863. doi: 10.1016/j.ijrobp.2006.12.073. [DOI] [PubMed] [Google Scholar]
- 83.Milosevic MF, Frost PJ, Laperriere NJ, Wong CS, Simpson WJ. Radiotherapy for atypical or malignant intracranial meningioma. Int J Radiat Oncol Biol Phys. 1996;34(4):817–822. doi: 10.1016/0360-3016(95)02166-3. [DOI] [PubMed] [Google Scholar]
- 84.Minniti G, Clarke E, Cavallo L, Osti MF, Esposito V, Contore G, et al. Fractionated stereotactic conformal radiotherapy for large benign skull base meningiomas. Radiation Oncology. 2011;6:36–42. doi: 10.1186/1748-717X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Minniti G, Traish D, Ashley A, Gonsalves A, Brada M. Risk of second brain tumor after conservative surgery and radiatherapy for pituitary adenoma: update after an additional 10 years. J Clin Endocrinol Metab. 2005;90:800–804. doi: 10.1210/jc.2004-1152. [DOI] [PubMed] [Google Scholar]
- 86.Miralbell R, Linggood RM, De la Monte S, Convery K, Munzenrider JE, Mirimanoff RO. The role of radiotherapy in the treatment of subtotally resected benign meningiomas. J Neurosurg. 1992;13:157–164. doi: 10.1007/BF00172765. [DOI] [PubMed] [Google Scholar]
- 87.Mirimanoff R, Dosoretz D, Linggood R, Ojemann RG, Martuza RL. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18–24. doi: 10.3171/jns.1985.62.1.0018. [DOI] [PubMed] [Google Scholar]
- 88.Modha A, Gutin PH. Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery. 2005;57:538–550. doi: 10.1227/01.neu.0000170980.47582.a5. [DOI] [PubMed] [Google Scholar]
- 89.Morimoto M, Yoshioka Y, Shiomi H, Isohashi F, Konishi K, Kotsuma T, et al. Singnificance of tumor volume related to peritumoral edema in intracranial meningioma treated with extreme hypofractionated stereotactic radiation therpay in three to five fractions. Jpn J Clin Oncol. 2011;41(5):609–616. doi: 10.1093/jjco/hyr022. [DOI] [PubMed] [Google Scholar]
- 90.Morokoff AP, Zauberman J, Black PM. Surgery for convexity meningiomas. Neurosurgery. 2008;63:427–434. doi: 10.1227/01.NEU.0000310692.80289.28. [DOI] [PubMed] [Google Scholar]
- 91.Nakamura M, Roser F, Michel J, Jacobs C, Samii M. The natural history of incidental meningiomas. Neurosurgery. 2003;53:62–71. doi: 10.1227/01.neu.0000068730.76856.58. [DOI] [PubMed] [Google Scholar]
- 92.Nakasu S, Nakasu Y, Fukami T, Jito J, Nozaki K. Growth curve analysis of asymptomatic meningiomas. J Neurooncol. 2011;102:303–310. doi: 10.1007/s11060-010-0319-1. [DOI] [PubMed] [Google Scholar]
- 93.Narayan S, Cornblath WT, Sandler HM, Elner V, Hayman JA. Preliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2003;56(2):537–543. doi: 10.1016/s0360-3016(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 94. [Accessed Oct10, 2013];National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines, Central Nervous System Cancers, Version 2.2013 Meningiomas.
- 95.Nicolato A, Foroni R, Alessandrini F, Maluta S, Bricolo A, Gerosa M. The role of gamma knife radiosurgery in the management of cavernous sinus sinus meningiomas. Int J Radiation Oncology Biol Phys. 2002;53(4):992–1000. doi: 10.1016/s0360-3016(02)02802-x. [DOI] [PubMed] [Google Scholar]
- 96.Niranjan A, Kondziolka D, Lunsford LD. Neoplastic transformation after radiosurgery or radiotherapy: risk and realities. Otolaryngol Clin N Am. 2009;42:717–729. doi: 10.1016/j.otc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 97.Nutting C, Brada M, Brazil L, Sibtain A, Saran F, Westbury C, et al. Radiotherapy in the treatment of benign meningioma of the skull base. Journal of Neurosurgery. 1999;90:823–827. doi: 10.3171/jns.1999.90.5.0823. [DOI] [PubMed] [Google Scholar]
- 98.Ohba S, Kobayashi M, Horiguchi T, Onozuka S, Yoshida K, Ohira T, et al. Long-term surgical outcome and biological prognostic factors in patients with skull base meningiomas. Journal of Neurosurgeruy. 2011;114:1278–1287. doi: 10.3171/2010.11.JNS10701. [DOI] [PubMed] [Google Scholar]
- 99.Onodera S, Aoyama H, Katoh N, Taguchi H, Yasuda K, Yoshida D, et al. Long-term outcomes of fractionated stereotactic radiotherapy for intracranial skull base benign meningiomas in single institution. Jpn J Clin Oncol. 2011;41(4):462–468. doi: 10.1093/jjco/hyq231. [DOI] [PubMed] [Google Scholar]
- 100.Oya S, Kawai K, Nakatomi H, Saito N. Significance of Simpson grading system in modern meningioma surgery: integration of the grade with MIB-1 labeling index as a key to predict the recurrence of WHO Grade I meningiomas. Journal of Neurosurgery. 2012;117:121–128. doi: 10.3171/2012.3.JNS111945. [DOI] [PubMed] [Google Scholar]
- 101.Oya S, Kim SH, Sade B, Lee JH. The natural history of intracranial meningioma. Journal of Neurosurgery. 2011;114:1250–1256. doi: 10.3171/2010.12.JNS101623. [DOI] [PubMed] [Google Scholar]
- 102.Palma L, Celli P, Franco C, Cervoni L, Cantore G. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. Journal of Neurosurgery. 1997;86:793–800. doi: 10.3171/jns.1997.86.5.0793. [DOI] [PubMed] [Google Scholar]
- 103.Park H, Kim I, Jung H. Atypical meningioma: outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 2009;75(3):S238. (abst) [Google Scholar]
- 104.Paulsen F, Doerr S, Wilhelm H, Becker G, Bamberg M, Classen J. Fractionated stereotactic radiotherapy in patients with optic nerve sheath meningioma. Int J Radiation Oncology Biol Phys. 2012;82(2):773–778. doi: 10.1016/j.ijrobp.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 105.Pearson BE, Markert JM, Fisher WS, Guthrie BL, Fiveash JB, Palmer CA, et al. Hitting a Moving Target: Evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24(5):E3. doi: 10.3171/FOC/2008/24/5/E3. [DOI] [PubMed] [Google Scholar]
- 106.Peele KA, Kennerdall JS, Maroon JC, Kalnicki S, Kazim M, Gardner T, et al. The role of postoperative irradiation in the management of sphenoid wing meningiomas. A preliminary report. Ophthalmology. 1996;103(11):1761–1766. doi: 10.1016/s0161-6420(96)30430-2. [DOI] [PubMed] [Google Scholar]
- 107.Perry AMeningiomas. In: Russell & Rubinstein’s Pathology of Tumors of the Nervous System. 7th. McLendon R, Rosenblum M, Bigner DD, editors. London, England: Hodder Arnold (Publisher); 2006. pp. 427–474. [Google Scholar]
- 108.Perry A, Dehner LP. Meningeal tumors of childhood and infancy. An update and literature review. Brain Pathol. 2003;13(3):386–408. doi: 10.1111/j.1750-3639.2003.tb00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Perry A, Giannini C, Raghavan R, Scheithauer BW, Banerjee R, Margraf L, et al. Aggressive phenotypic and genotypic features in pediatric and NF2-associated meningio- mas: a clinicopathologic study of 53 cases. J Neuropathol Exp Neurol. 2001;60(10):994–1003. doi: 10.1093/jnen/60.10.994. [DOI] [PubMed] [Google Scholar]
- 110.Perry A, Louis DN, Scheithauer BW, Budka H, von Deimling A. Meningeal tumours. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC; 2007. pp. 164–172. [Google Scholar]
- 111.Perry A, Scheithauer BW, Stafford SL, Lohse CM, Wollan PC. “Malignancy” in meningiomas: a clinicopathologic study of 116 patients. Cancer. 1999;85:2046–2056. doi: 10.1002/(sici)1097-0142(19990501)85:9<2046::aid-cncr23>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 112.Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM. Meningioma grading: an analysis of histologic parameters. Amer J Surg Pathol. 1999;21:1455–1465. doi: 10.1097/00000478-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 113.Pirzkall A, Debus J, Haering P, Wannenmacher M. Intensity modulated radiotherapy (IMRT) for recurrent, residual, or untreated skull-base meningiomas: preliminary clinical experience. Int J Radiation Oncology Biol Phys. 2003;55(2):362–372. doi: 10.1016/s0360-3016(02)03809-9. [DOI] [PubMed] [Google Scholar]
- 114.Pollock BE, Stafford SL. Results of stereotactic radiosurgery for patients with imaging defined cavernous sinus meningiomas. Int J Radiation Oncology Biol Phys. 2005;62(5):1427–1431. doi: 10.1016/j.ijrobp.2004.12.067. [DOI] [PubMed] [Google Scholar]
- 115.Pollock BE, Stafford SL, Link MJ. Gamma Knife radiosurgery for skull base meningiomas. Neurosurgery Clinics of North America. 2000;11:659–666. [PubMed] [Google Scholar]
- 116.Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Single-fraction radiosurgery for presumed intracranial meningiomas: efficacy and complications from a 22-year experience. Int J Radiation Oncology Biol Phys. 2012;83(5):1414–1418. doi: 10.1016/j.ijrobp.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 117.Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Stereotactic radiosurgery of World Health Organization grade II and III intracranial meningiomas. Cancer. 2012;118:1048–1054. doi: 10.1002/cncr.26362. [DOI] [PubMed] [Google Scholar]
- 118.Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA. Stereotactic radiosurgery provides equivalent tumor contgrol to a Simpson Grade I resection for patients with small-to medium-sized meningiomas. Int J Radiation Oncology Biol Phys. 2003;55:1000–1005. doi: 10.1016/s0360-3016(02)04356-0. [DOI] [PubMed] [Google Scholar]
- 119.Pourel N, Auque J, Bracard S, Hoffstetter S, Luporsi Vignaud JM, et al. Efficacy of external fractionated radiation therapy in the treatment of meningiomas: a 20-year experience. Radiotherapy and Oncology. 2001;61:65–70. doi: 10.1016/s0167-8140(01)00391-7. [DOI] [PubMed] [Google Scholar]
- 120.Reardon DA, Galanis E, DeGroot JF, Cloughesy TF, Wefel JS, Lamborn KR, et al. Clinical trial end points for high-grade glioma: the evolving landscape. Neuro Oncol. 2011;13(3):353–361. doi: 10.1093/neuonc/noq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5(12):1045–1054. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- 122.Roche PH, Pellet W, Fuentes S, Thomassin JM, Regis J. Gamma knife radiosurgical management of petroclival meningiomas: results and indications. Acta Neurochir (Wien) 2003;145(10):883–888. doi: 10.1007/s00701-003-0123-1. [DOI] [PubMed] [Google Scholar]
- 123.Roche PH, Regis J, Dufour H, Fournier HD, Delsanti C, Pellet W, et al. Gamma knife radiosurgery in the management of cavernous sinus meningiomas. Journal of Neurosurgery. 2000;93(suppl 3):68–73. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 124.Rockhill J, Mrugala M, Chamberlain MC. Intracranial meningiomas: an overview of diagnosis and treatment. Neurosurg Focus. 2007;23(4):E1. doi: 10.3171/FOC-07/10/E1. [DOI] [PubMed] [Google Scholar]
- 125.Rohringer M, Sutherland GR, Louw DF, Sima AA. Incidence and clinicopathological features of meningioma. J Neurosurg. 1989;71(5 pt 1):665–672. doi: 10.3171/jns.1989.71.5.0665. [DOI] [PubMed] [Google Scholar]
- 126.Ron E, Modan B, Boice JD, Alfandary E, Stovall M, Chetrit A, et al. Tumors of the brain and nervous system after radiotherapy in childhood. N Engl J Med. 1988;319:1033–1039. doi: 10.1056/NEJM198810203191601. [DOI] [PubMed] [Google Scholar]
- 127.Rosenberg LA, Prayson RA, Lee J, Reddy C, Chao ST, Barnett GH, et al. Long-term experience with World Health Organization grade III (malignant) meningiomas at a single institution. Int J Radiation Oncology Biol Phys. 2009;74(2):427–432. doi: 10.1016/j.ijrobp.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 128.Russell DS, Rubinstein LJ. Pathology of tumors of the nercous system. 4th. London: Edward Arnold; 1977. pp. 66–91. [Google Scholar]
- 129.Santacroce A, Walier M, Regis J, Liscak R, Motti E, Lindquist C, et al. Long-term tumor control of benign intracranial meningiomas after radiosurgery in a series of 4565 patients. Neurosurgery. 2012;70:32–39. doi: 10.1227/NEU.0b013e31822d408a. [DOI] [PubMed] [Google Scholar]
- 130.Selch MT, Ahn E, Laskari A, Lee SP, Agazaryan N, Solberg TD, et al. Stereotactic radiotherapy for treatment of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2004;59(1):101–111. doi: 10.1016/j.ijrobp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 131.Shin M, Kurita H, Sasaki T, Kawamoto S, Tago M, Kawahara N, et al. Analysis of treatment outcome after stereotactic radiosurgery for cavernous sinus meningiomas. J Neurosurgery. 2001;95:435–439. doi: 10.3171/jns.2001.95.3.0435. [DOI] [PubMed] [Google Scholar]
- 132.Shrieve DC, Hazard L, Boucher K, Jensen RL. Dose fractionation in stereotactic radiotherapy for parasellar meningiomas: radiobiological considerations of efficacy and optic nerve tolerence. J Neurosurg. 2004;101(Suppl 3):390–395. [PubMed] [Google Scholar]
- 133.Simon M, Bostrom J, Kock P, Schromm J. Interinstitutional variance of post-operative radiotherapy and follow-up for meningiomas in Germany: Impact of changes of the WHO classification. J Neurol Neurosurg Psychiatry. 2006;77:767–773. doi: 10.1136/jnnp.2005.077974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Skeie BS, Enger PO, Skeie GO, Thorsen F, Pedersen PH. Gamma Knife Surgery of meningiomas involving the cavernous sinus: long-term follow-up of 100 patients. Neurosurgery. 2010;66:661–669. doi: 10.1227/01.NEU.0000366112.04015.E2. [DOI] [PubMed] [Google Scholar]
- 136.Soyuer S, Chang EL, Selek U, Shi W, Maor MH, DeMonte F. Radiotherapy after surgery for benign cerebral meningioma. Radiotherapy and Oncology. 2004;71:85–90. doi: 10.1016/j.radonc.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 137.Spiegelmann R, Nissim O, Menhel J, Alezra D, Pfeffer MR. Linear Accelerator radiosurgery for meningiomas in and around the cavernous sinus. Neurosurgery. 2002;51(6):1373–1379. [PubMed] [Google Scholar]
- 138.Stafford S, Perry A, Suman V, Meyer FB, Scheithauer BW, Lohse CM, et al. Primarily resected meningiomas: Outcome and prognostic factors in 581 Mayo Clinic patients, 1978 through 1988. Mayo Clin Proc. 1998;73:936–942. doi: 10.4065/73.10.936. [DOI] [PubMed] [Google Scholar]
- 139.Stafford SL, Pollock BE, Foote RL, Link MJ, Gorman DA, Schomberg PJ, et al. Meningioma radiosurgery: Tumor control, outcomes, and complications in 190 consecutive patients. Neurosurgery. 2001;49(5):1029–1038. doi: 10.1097/00006123-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 140.Starke RM, Williams BJ, Hiles C, Nguyen JH, Elsharkawy MY, Sheehan JP. Gamma Knife surgery for skull base meningiomas. Journal of Neurosurgery. 2012;116(3):588–597. doi: 10.3171/2011.11.JNS11530. [DOI] [PubMed] [Google Scholar]
- 141.Stessin AM, Schwartz A, Judanin G, Pannullo SC, Boockvar JA, Schwartz TH, et al. Does adjuvant external-beam radiotherapy improve outcomes for nonbenign meningiomas? A Surveillence, Epidemiology, and End Results (SEER)-based analysis. Journal of Neurosurgery. 117(4):669–675. doi: 10.3171/2012.7.JNS111439. [DOI] [PubMed] [Google Scholar]
- 142.Strang RD, Al-Mefty O. Comment on Stereotactic Radiosurgery for Meningioma. In: Pollock BE, editor. Contemporary Stereotactic Radiosurgery: Technique and Evaluation. Armonk, NY: Futuraq Publishing Company; 2002. pp. 172–180. [Google Scholar]
- 143.Strojan P, Popovic M, Jereb B. Secondary intracranial meningiomas after high-dose cranial irradiation: report of five cases and review of the literature. Int J Radiat OncolBiol Phys. 2000;48:65–73. doi: 10.1016/s0360-3016(00)00609-x. [DOI] [PubMed] [Google Scholar]
- 144.Sughrue ME, Kane AJ, Shangari G. The relevance of Simpson grade I and II resection in modern neurosurgical treatment of World Health Organization grade I meningioma. J Neurosurgery. 2010;113:1029–1035. doi: 10.3171/2010.3.JNS091971. [DOI] [PubMed] [Google Scholar]
- 145.Sughrue ME, Rutkowski MJ, Aranda D, Barani IJ, McDermott MW, Parsa AT. Treatment decision making based on the published natural history and growth rate of small meningiomas. Journal of Neurosurgery. 2010;113:1036–1042. doi: 10.3171/2010.3.JNS091966. [DOI] [PubMed] [Google Scholar]
- 146.Sughrue ME, Sanai N, Shangari G, Parsa AT, Berger MS, McDermott MW. Outcome and survival following primary and repeat surgery for World Health Organization Geade III meningiomas. Journal of Neurosurgery. 2010;113(2):202–209. doi: 10.3171/2010.1.JNS091114. [DOI] [PubMed] [Google Scholar]
- 147.Tanzler E, Morris CG, Kirwan JM, Amdur RJ, Mendenhall WM. Outcomes of WHO grade I meningiomas receiving definitive or post-operative radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(2):508–513. doi: 10.1016/j.ijrobp.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 148.Taylor BW, Marcus RB, Friedman WA, Ballinger WE, Jr, Million RR. The meningioma controversy: Post-operative radiation therapy. Int J Radiat Oncol Biol Phys. 1988;15:299–304. doi: 10.1016/s0360-3016(98)90008-6. [DOI] [PubMed] [Google Scholar]
- 149.Turbin RE, Thompson CS, Kennerdell JS, Cockerham KP, Kupersmith MJ. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology. 2002;109:890–899. doi: 10.1016/s0161-6420(02)01017-5. [DOI] [PubMed] [Google Scholar]
- 150.Unger KR, Lominska CE, Chanyasulkit J, Randolf-Jackson P, White RL, Ausili E, et al. Risk factors for posttreatment edema in patients treated with stereotactic radiosurgery for meningiomas. Neurosurgery. 2012;70:639–645. doi: 10.1227/NEU.0b013e3182351ae7. [DOI] [PubMed] [Google Scholar]
- 151.Uy NW, Woo SY, Teh BS, Mai WY, Carpenter LS, Chiu JK, et al. Intensity modulated radiation therapy (IMRT) for meningioma. Int J Radiat Oncol Biol Phys. 2002;53(5):1265–1270. doi: 10.1016/s0360-3016(02)02823-7. [DOI] [PubMed] [Google Scholar]
- 152.Vagefi MR, Larson DA, Horton JC. Optic nerve sheath meningioma: visual improvement during radiation treatment. Am J Ophthalmol. 2006;142:343–344. doi: 10.1016/j.ajo.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 153.van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM. End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald’s Criteria. J Clin Oncol. 2009;27(18):2905–2908. doi: 10.1200/JCO.2009.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.van den Bent MJ, Wefel JS, Schiff D, Taphoorn MJ, Jaeckle K, Junck L, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 155.Vendrely V, Maire JP, Darrouzet V, Bonichon N, San Galli F, Celerier D, et al. Radiotherapie fractionnee des meningiomes intracraniens: 15 ans d’experience au centre hospitalier universitaire de Bordeaux. Cancer Radiother. 1999;3(4):311–317. doi: 10.1016/s1278-3218(99)80073-0. [DOI] [PubMed] [Google Scholar]
- 156.Vernimmen FJ, Harris JK, Wilson JA, Melvill R, Smit BJ, Slabbert JP. Stereotactic proton beam therapy of skull base meningiomas. Int J Radiat Oncol Biol Phys. 2001;49(1):99–105. doi: 10.1016/s0360-3016(00)01457-7. [DOI] [PubMed] [Google Scholar]
- 157.Vogelbaum MA, Jost S, Aghi MK, Heimberger AB, Sampson JH, Wen PY, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery. 2012;70(1):234–243. doi: 10.1227/NEU.0b013e318223f5a7. [DOI] [PubMed] [Google Scholar]
- 158.Wara WM, Sheline GE, Newman H, Townsend JJ, Boldrey EB. Radiation therapy of meningiomas. American Journal of Roentgenology, Radium Therapy and Nuclear Medicine. 1975;123(3):453458. doi: 10.2214/ajr.123.3.453. [DOI] [PubMed] [Google Scholar]
- 159.Weber DC, Lovbad KO, Rogers L. New pathology classification, imagery techniques and prospective trials for meningiomas: the future looks bright. Current Opinion in Neurology December. 2010;23(6):563–570. doi: 10.1097/WCO.0b013e328340441e. [DOI] [PubMed] [Google Scholar]
- 160.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 161.Williams BJ, Yen CP, Starke RM, Basina B, Nguyen J, Rainey J, et al. Gamma Knife surgery for parasellar meningiomas: long-term results including complications, predictive factors, and progression-free survival. Journal of Neurosurgery. 2011;114:1571–1577. doi: 10.3171/2011.1.JNS091939. [DOI] [PubMed] [Google Scholar]
- 162.Willis J, Smith C, Ironside JW, Erridge S, Whittle IR, Everington D. The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol. 2005;31:141–149. doi: 10.1111/j.1365-2990.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 163.Winkler C, Dornfeld S, Schwarz R, Friedrich S, Baumann M. The results of radiotherapy in meningiomas with a high risk of recurrence. A retrospective analysis. Strahlenthe Onkol. 1998;174:624–628. doi: 10.1007/BF03038510. [DOI] [PubMed] [Google Scholar]
- 164.Zachenhofer I, Wolfsberger S, Aichholzer M, Bertalanffy A, Roessler K, Kitz K, et al. Gamma-Knife radiosurgery for cranial base meningiomas: experience of tumor control, clinical course, and morbidity in a follow-up of more than 8 years. Neurosurgery. 2006;58:28–36. doi: 10.1227/01.neu.0000190654.82265.a3. [DOI] [PubMed] [Google Scholar]
- 165.Zada G, Pagnini PG, Yu C, Erickson KT, Hirschbein J, Zelman V, et al. Long-term outcomes and patterns of tumor progression after Gamma Knife radiosurhery for benign meningiomas. Neurosurgery. 2010;67:322–329. doi: 10.1227/01.NEU.0000371974.88873.15. [DOI] [PubMed] [Google Scholar]