Abstract
A real-time polymerase chain reaction (PCR) assay, amplifying the genes encoding lactose permease (lacY) and invasion plasmid antigen H (ipaH), was run on 121 isolates phenotypically classified as Shigella spp., enteroinvasive Escherichia coli (EIEC), or EIEC O nontypable (ONT). The results were compared with data from a generic E. coli multiple-locus variable-number of tandem repeat analysis (MLVA) and a Shigella MLVA.
The real-time PCR verified all Shigella spp. (n = 53) as Shigella (lacY negative) and all EIEC O121 (n = 15) and EIEC O124 (n = 2) as EIEC (lacY positive). However, the real-time PCR typed EIEC O164 as either EIEC (n = 2) or Shigella (n = 2) and, thus, was not suited for classifying this group of isolates. Interestingly, the majority (42/47, 89.4%) of the EIEC ONT were classified as Shigella (lacY negative) by the real-time PCR, and in nearly all cases, (92.9%, 39/42) data from both MLVA assays supported these findings. Overall, in 94.7% (114/121) of the isolates, the results from the real-time PCR were substantiated by the results from the MLVA assays.
In conclusion, the real-time PCR assay was fast and accurate in differentiating Shigella spp. from EIEC, with the exception of the EIEC O164 group. This molecular assay was particularly pragmatic for the challenging EIEC ONT group.
Keywords: EIEC, Shigella, real-time PCR, molecular differentiation, MLVA
Introduction
Shigella is a gram-negative, lactose-negative, facultative intracellular pathogen, closely related to Escherichia coli (E. coli). It was recognized as the etiologic agent of bacillary dysentery or shigellosis in the 1890s, and in the 1950s, Shigella was adopted as a genus and subgrouped into four species (spp.): Shigella dysenteriae, Shigella flexnerii, Shigella boydii, and Shigella sonnei [1]. Shigellosis remains a major cause of morbidity and mortality among children in developing countries, in which S. flexneri is the dominating species. These bacteria are also important causes of morbidity in the industrialized part of the world where S. sonnei is the most common [2]. Shiga toxins (Stx) carrying S. dysenteriae serotype 1 and, to a lesser extent, S. flexneri, are the Shigella spp. responsible for most severe diseases. Recently, Stx2, the Stx subtype associated with hemolytic uremic syndrome in patients infected with Stx-producing E. coli (STEC), was described in an S. sonnei isolate [3]. Shigella infection spreads by the fecal–oral route, and the infectious dose is low [4]. Rapid identification of Shigella spp. is thus important for outbreak control purposes. In Norway, shigellosis is a rare disease, with 100–200 cases annually. S. sonnei is the dominating species, and the majority of the cases are infected abroad (http://www.msis.no/). However, some domestic outbreaks of shigellosis have been detected in Norway, mainly associated with imported vegetables, meat, or herbs [5–8].
In the 1970s, the first invasive strains of E. coli causing Shigella-like dysentery were described [9]. Thereafter, several studies have shown that Shigella spp. and enteroinvasive E. coli (EIEC) form a single pathovar of E. coli [10–13]. In spite of this, discrimination between Shigella spp. and EIEC is essential due to clinical differences and also for epidemiological purposes [14]. However, the close relatedness between Shigella spp. and EIEC makes the distinction difficult if based on biochemical, serological, or molecular characteristics [11]. Most Shigella spp. are lactose negative, whereas EIEC isolates display variable ability to utilize lactose. It has been suggested that Shigella spp. lack the lactose permease gene (lacY), one of three genes constituting the lac operon important for lactose fermentation, or carry a lacY pseudogene. On the other hand, EIEC, as do all E. coli, harbor this particular gene [10, 14, 15]. Even though various molecular methods developed in the past few years presumably allow differentiation between Shigella spp. and EIEC, the discrimination between the two still represents a challenge [4, 16–19]. Therefore, in the present study, we aimed at establishing a rapid and reliable duplex real-time polymerase chain reaction (PCR) able to differentiate Shigella spp. from EIEC based on the presence or absence of lacY. Second, we wanted to substantiate these results by comparing them with genotyping data from two multiple-locus variable-number of tandem repeat analysis (MLVA) assays: one designed for E. coli and one for Shigella spp.
Materials and methods
Phenotypical characterization and E. coli pathotype PCR
Clinical microbiology laboratories throughout Norway mandatory forwarded presumptive Shigella and enteropathogenic E. coli isolated from stool specimens to the National Reference Laboratory for Enteropathogenic Bacteria at the Norwegian Institute of Public Health (NIPH). At NIPH, the received isolates were routinely subjected to a broad panel of single tube biochemical tests, and the results were evaluated according to established criteria [20]. Based on the biochemical findings, the isolates were tested for agglutination with either polyvalent anti-S. flexneri, anti-Shigella II and III (Sifin Diagnostics, Berlin, Germany), and anti-S. boydii 14–18 (Difco by Becton and Dickinson, Franklin Lakes, New Jersey), or polyvalent E. coli antisera, Anti-Coli I, II, and III (Sifin Diagnostics, Berlin, Germany). Positive agglutination in a polyvalent antiserum was followed by agglutination in the relevant monovalent antiserum (either Sifin or from noncommercial production at NIPH). Isolates not clearly defined as either Shigella spp. or EIEC by phenotypic typing were denoted EIEC O nontypable (ONT). Presumptive E. coli isolates were classified into well-known pathotypes by running a multiplex PCR including, among other genes, ipaH [21].
Bacterial isolates
A total of 121 isolates from 121 patients infected within the period 2006 to 2014 were obtained from the national strain collection at NIPH. The selection was based on phenotypical findings and comprised 53 Shigella spp. (13 S. sonnei, 15 S. flexneri, 12 S. boydii, and 13 S. dysenteriae), 21 EIEC of known serotype (15 O121, four O164, and two O124), and 47 EIEC ONT. All isolates, except two S. sonnei and two S. dysenteriae serotype 2, were sporadic cases. To ensure the specificity of the real-time PCR method, the following strains were added: STEC (n = 2), enteropathogenic E. coli (EPEC) (n = 2), enteroaggregative E. coli (EAEC) (n = 2), enterotoxigenic E. coli (ETEC) (n = 3), non-diarrhea/commensal E. coli (n = 1), Salmonella Typhimurium (n = 1), Salmonella Kedougou (n = 1), and Yersinia enterocolitica (serogroups 3 and 9, respectively) (n = 2) (Table 1).
Table 1.
Bacterial isolates examined and results achieved using the duplex real-time PCR
| Pathogen | Pathotype* | Serotype | No. analyzed | Duplex real-time PCR no. | |||
|---|---|---|---|---|---|---|---|
| lacY + | ipaH + | EIEC (%) | Shigella (%) | ||||
| E. coli | EIEC | ONT† | 47 | 5 | 47 | 5 (10.6%) | 42 (89.4%) |
| O121 | 15 | 15 | 15 | 15 (100%) | 0 (0%) | ||
| O124 | 2 | 2 | 2 | 2 (100%) | 0 (0%) | ||
| O164 | 4 | 2 | 4 | 2 (50%) | 2 (50%) | ||
| STEC | O103:H2, O26:H11 | 2 | 2 | 0 | 0 (0%) | 0 (0%) | |
| aEPEC | ONT:H11, O145:H8 | 2 | 2 | 0 | 0 (0%) | 0 (0%) | |
| EAEC | O104:H4, ONT | 2 | 2 | 0 | 0 (0%) | 0 (0%) | |
| ETEC | O6, ONT (2) | 3 | 3 | 0 | 0 (0%) | 0 (0%) | |
| Non-enteropathogenic | – | 1 | 1 | 0 | 0 (0%) | 0 (0%) | |
| Shigella spp. | S. sonnei | – | 13 | 0 | 13 | 0 (0%) | 13 (100%) |
| S. flexneri | 1, 2, 3, 4, 6, and x variant | 15 | 0 | 15 | 0 (0%) | 15 (100%) | |
| S. dysenteriae | 1, 2, 3, 4, 7, and 9 | 13 | 0 | 13 | 0 (0%) | 13 (100%) | |
| S. boydii | 2, 4, 8, 10, 14, and 18 | 12 | 0 | 12 | 0 (0%) | 12 (100%) | |
| Salmonella enterica spp. | S. Typhimurium | 4, 5, 12:i:1, 2 | 1 | 0 | 0 | 0 (0%) | 0 (0%) |
| S. Kedougou | – | 1 | 0 | 0 | 0 (0%) | 0 (0%) | |
| Yersinia spp. | Y. enterocolitica | O:3 | 1 | 0 | 0 | 0 (0%) | 0 (0%) |
| O:9 | 1 | 0 | 0 | 0 (0%) | 0 (0%) | ||
*The pathotype was phenotypically determined for Shigella spp., Salmonella spp., and Yersinia spp.; however, for E. coli, the pathotype was determined running an 11-plex PCR [21]
†ONT: O nontypable
Growth conditions and extraction of DNA
All isolates were recultivated from stabbing agar on nutrient broth agar at 37 °C overnight. Suspensions of bacterial cells were boiled for 15 min and used directly as template in the real-time PCR after a brief 3 min centrifugation at 13,000 rpm.
Primer and probe design
Two primer-probe sets were used in the duplex real-time PCR (Table 2). The primer set for lacY was modified from Pavlovic et al., 2011 [19], whereas the primer set for the internal amplification control, ipaH, was adapted from Barletta et al., 2013 [22], with minor modifications. The probes for lacY [19] were modified to minor groove binder (MGB) format, and an MGB probe for ipaH was designed using PrimerExpress 3.0 (LifeTechnologies). To check the specificity of both primer pairs and the probes, a BLAST search on NCBI was performed.
Table 2.
ipaH and lacY primers and probes used in the present study
| Gene | Primer or probe* | Sequence (5′–3′) | Melting point (°C) | PCR product (bp) | Fluorochrome (5′ end) | Reference |
|---|---|---|---|---|---|---|
| lacY† | lacY-F | ACCAGACCCAGCACCAGATAAG | 59 | 104 | [19] | |
| lacY-R | TTCTGCTTCTTTAAGCAACTGGC | 58.9 | Modified from [19] | |||
| lacY-MGB-p1 | CATACATATTGCCCGCCAGTA | 70 | FAM | Modified from [19] | ||
| lacY-MGB-p2 | CATACATATGCCCGCCAGA | 70 | FAM | Modified from [19] | ||
| ipaH | ipaH-F | GACGGACAACAGAATACACTCCATC | 59.8 | 108 | Modified from [22] | |
| ipaH-R | ATGTTCAAAAGCATGCCATATCTGT | 59.8 | [22] | |||
| ipaH-MGB-p | CGGAAAACAAACAATCTGATGT | 69 | VIC | Modified from [22] |
*All probes were conjugated with minor groove binder (MGB) and had a “Black Hole Quencher” at the 3′ end
†Due to sequence variation in the lacY gene of certain EIEC strains, two different lacY probes were used to detect all EIEC strains [19]
Conventional PCR and sequencing
Two conventional PCRs, including either the lacY or the IpaH primer set, were conducted to verify the expected PCR product size and to check the specificity of each primer set. EIEC O121 (lacY and ipaH positive) and S. dysenteriae (lacY negative, but ipaH positive) were used as positive controls in each run. PCR was performed using the Qiagen Multiplex PCR kit (Qiagen, Hilden, Germany), as described by the manufacturer. The PCRs were run in a GeneAmp 9700 machine (Life Technologies, Carlsbad, California, USA) with a temperature profile as indicated for the Qiagen Multiplex PCR kit and an annealing temperature of 58 °C. PCR products were diluted 1:10 prior to capillary electrophoresis on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California, USA). DNA 1000 LabChip kit series II was prepared and loaded with samples as recommended by the manufacturer (Agilent Technologies, Santa Clara, California, USA). The specificity of each primer pair was verified by direct sequencing of the PCR product of the positive control.
Real-time PCR; efficiencies and detection limits
For each primer-probe set, a 20× primer-probe mix was prepared with a final concentration of 2.5 μM of the respective primers and probes. Each reaction mix consisted of 10 μl 2× QuantiTect Multiplex RT-PCR Rox Master-mix (Qiagen, Hilden, Germany), 1 μl of 20× primer-probe mix for ipaH and/or lacY, 4 μl template DNA diluted 1:10, and sterile PCR grade water (Qiagen, Hilden, Germany) to bring the final volume to 20 μl. Real-time PCR was run in a StepOnePlus machine (Life Technologies, Carlsbad, California, USA) with the following PCR program: initial activation step of 15 min at 95 °C followed by 30 cycles of denaturation for 60 s at 94 °C and annealing/extension for 60 s at 58 °C. DNA from EIEC O121 was used as template, and a dilution series ranging from 50 ng/μl to 0.5 pg/μl was measured. Triplicates of the dilution series were run, and PCR efficiencies were calculated as described previously [23].
MLVA typing
All 121 isolates were examined by a 10-loci E. coli generic MLVA assay (GECM10) as described by Løbersli et al. [24] and an MLVA specific for Shigella spp. as described by Rawal et al. [8].
Ethical considerations
At the NIPH, all Shigella spp. and EIEC strains are routinely collected for disease surveillance and outbreak detection. The current study is descriptive of a bacterial collection and microbiological characteristics are not combined with clinical data. Ethical approval was therefore not required. Also, the Norwegian Act relating to control of communicable diseases (https://lovdata.no/dokument/NL/lov/1994-08-05-55?q=Smittevernloven) obliges the NIPH to monitor the Shigella spp. and EIEC populations within the country on a regular basis. For these reasons, consent was not obtained from the patients to analyze the bacterial samples for this research project.
Results
Duplex real-time PCR; efficiencies, detection limits, sensitivity, and specificity
The NCBI BLAST search confirmed that the lacY primers were absent in published sequences of Shigella spp. but present in E. coli. The ipaH primers were exclusively seen in Shigella spp. and EIEC. By conventional PCR, both PCR products showed expected base pair sizes and no scatter bands were observed. Sequencing of the PCR products confirmed the correct sequences (data not shown). The PCR efficiencies for lacY primer-probes were 106.3% in singleplex PCR and 93.1% in duplex PCR, whereas the values for ipaH primer-probe were 109.4% and 90.4%, respectively. The detection limit for both genes was 5 pg/ μl. All E. coli isolates, except the majority of the EIEC ONT group and two EIEC O164 isolates, were positive for lacY. On the other hand, the Shigella spp., Salmonella spp., and Yersinia spp. were all negative for this specific gene (Table 1). As expected, ipaH was detected in all EIEC and Shigella spp. isolates, but in no other pathogens. Thus, the duplex real-time PCR had a high sensitivity and specificity.
Evaluating the duplex real-time PCR with other typing methods
A 100% (53/53) concordance between phenotypic typing and the duplex real-time PCR was seen for all Shigella spp. isolates (Table 1). Similar results were observed for EIEC O121 and O124 (100%, 17/17), whereas only 10.6% (5/47) of the isolates phenotypically determined as EIEC ONT were confirmed as EIEC by duplex real-time PCR. Furthermore, of the four EIEC O164 isolates, two were verified as EIEC (lacY positive) and two were identified as Shigella (lacY negative) (Fig. 1). In total, disagreement between the real-time PCR and the phenotypic typing was observed in 36.4% (44/121) of the isolates examined, and the majority of the discrepant cases was seen within the EIEC ONT group (42/44, 95.5%).
Fig. 1.
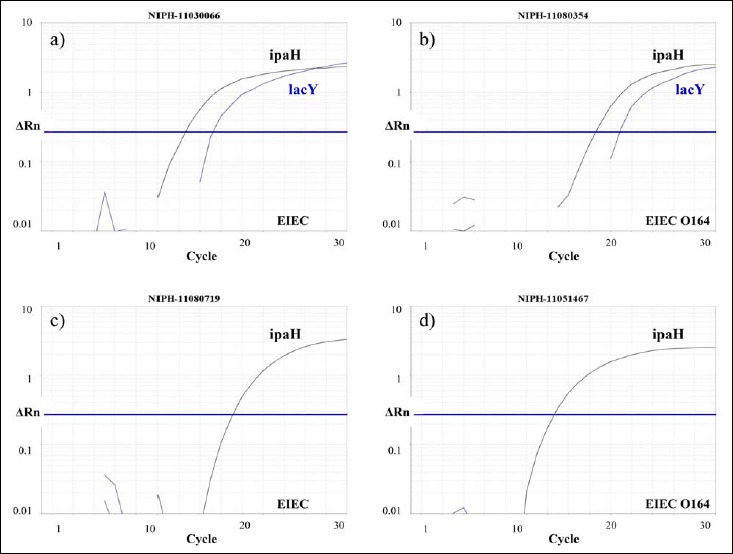
Four strains phenotypically determined as EIEC O164 were either classified as EIEC (lacY positive) or Shigella (lacY negative) by the real-time PCR. a) NIPH-11030066 and b) NIPH-11080354 carried lacY and ipaH and were classified as EIEC. However, c) NIPH-11080719 and d) NIPH-11051467 harbored ipaH only and, thus, designated Shigella by the real-time PCR. Phenotypically, except for the lactose fermentation in NIPH-11080354, they could not be distinguished
Results from generic E. coli MLVA and Shigella MLVA showed six main groups of E. coli MLVA profiles (I–VI) and seven groups of Shigella MLVA profiles (A–G) (Table 3, Fig. 2). E. coli MLVA group I included seven Shigella spp. and seven EIEC ONT isolates. All these 14 isolates were classified as Shigella (lacY negative) by the duplex real-time PCR, and they belonged to one of two Shigella MLVA groups (A and B). The second E. coli group (II) constituted 16 Shigella spp. and 27 EIEC ONT. The real-time PCR assay identified all 43 isolates as Shigella (lacY negative), and they all fell into Shigella MLVA group B (Table 3). E. coli group III included 17 EIEC with known O groups (15 EIEC O121 and two EIEC O124) and four isolates phenotypically defined as EIEC ONT. Of these, 18/21 (85.7%) were verified as EIEC (lacY positive) by real-time PCR and they belonged to Shigella MLVA group C. The three last isolates, all EIEC ONT, were classified as Shigella (lacY negative) and were assigned to one of two Shigella MLVA groups (C or D) (Table 3). Interestingly, these three latter isolates, although not unambiguous, were phenotypically typed as EIEC ONT, but agglutinated with S. boydii serotype 9 (2/3) or S. dysenteriae serotype 3. The fourth E. coli MLVA group (IV) harbored 24 Shigella spp., three EIEC O164, and two EIEC ONT isolates. All Shigella spp. were verified as Shigella (lacY negative) by real-time PCR, and they were placed in Shigella MLVA groups A, E, or G. However, only two EIEC O164 were confirmed as EIEC (lacY positive), whereas the last EIEC O164 was classified as Shigella (lacY negative). All three EIEC O164 belonged to Shigella MLVA group C. Both EIEC ONT were lacY negative and clustered within Shigella MLVA group A, supporting the real-time PCR results (Table 3). Within E. coli MLVA group V, one EIEC O164 and four EIEC ONT were defined. The four EIEC ONT were determined as EIEC (lacY positive), and all but one belonged to Shigella MLVA group C. Although clustering within Shigella MLVA group C, the EIEC O164 isolate was defined as Shigella (lacY negative) by real-time PCR. The last E. coli MLVA group (VI) included eight isolates, five Shigella, and three EIEC ONT, all found as Shigella (lacY negative) by real-time PCR and all belonging to Shigella MLVA group F (Table 3). In conclusion, in 94.7% (114/121) of the cases, MLVA profiles both from the generic E. coli and Shigella assays supported the findings achieved by duplex real-time PCR. E. coli MLVA groups I, II, and VI, and Shigella MLVA groups A, B, E, and F were exclusively seen in isolates defined as Shigella (lacY negative) by the real-time PCR. On the other hand, E. coli MLVA groups III and V, and Shigella MLVA profile C, were associated with isolates defined as EIEC (lacY positive). Overall, a discrepancy between the real-time PCR and the MLVA assays was seen for the O164 EIEC group (n = 4) and in three EIEC ONT isolates (Table 3). Repeated biochemical analyses of the four EIEC O164 isolates showed that one of two was verified as EIEC (lacY positive) by real-time PCR fermented lactose, whereas no other biochemical differences among the isolates were revealed. All four EIEC O164 agglutinated weakly in monovalent antiserum against S. dysenteriae serotype 3. Of the 47 EIEC ONT examined, only five were defined as EIEC (lacY positive) by real-time PCR. All five showed E. coli MLVA profiles belonging to group III or V, and all but one clustered within Shigella MLVA group C, supporting the finding of these isolates as EIEC (Fig. 2). Moreover, 39/42 (92.9%) EIEC ONT defined as Shigella (lacY negative) showed MLVA profiles associated with Shigella spp., indicating that the real-time PCR classification was correct (Table 3 and Fig. 2).
Table 3.
E. coli MLVA and Shigella MLVA profiles in concordance with the duplex real-time PCR results
| E. coli MLVA group* | Pathotype† | Serotype | No. analyzed | Duplex real-time PCR | Shigella MLVA group‡ |
|---|---|---|---|---|---|
| I | S. boydii | 18 | 1 | Shigella | A |
| S. dysenteriae | 3, 4, and 9 | 6 | Shigella | A/B | |
| EIEC | ONT# | 7 | Shigella | A | |
| II | S. boydii | 2, 4, 8, 10, 14, and 16 | 12 | Shigella | B |
| S. dysenteriae | 7 | 1 | Shigella | B | |
| S. flexneri | 6 | 3 | Shigella | B | |
| EIEC | ONT | 27 | Shigella | B | |
| III | EIEC§ | ONT | 3 | Shigella | C (n = 1)/D |
| 1 | EIEC | C | |||
| EIEC | O121 and O124 | 17 | EIEC | C | |
| IV | S. flexneri | 1, 2, 3, 4, and x variant | 11 | Shigella | A/E |
| S. sonnei | – | 13 | Shigella | G | |
| EIEC | O164 | 1 | Shigella | C | |
| O164 | 2 | EIEC | C | ||
| ONT | 2 | Shigella | E | ||
| V | EIEC | O164 | 1 | Shigella | C |
| ONT | 4 | EIEC | C/G (n = 1) | ||
| VI | S. dysenteriae | 2 | 5 | Shigella | F |
| EIEC | ONT | 3 | Shigella | F | |
| Other MLVA profiles not seen in EIEC | S. dysenteriae | 1 | 1 | Shigella | G |
| S. flexneri | 4 | 1 | Shigella | B |
*Six main groups of E. coli MLVA profiles are defined; each group was given a Roman numeral (I–VI). Within each group, different copy number profiles are seen: I, 4-NA-NA-X-NA-X-X-2-NA-NA; II, 4-2-NA-X-X-X-X-2-NA-NA; III, 5-2-NA-X-X-X-X-X-X-NA; IV, 6-NA-NA-X-X-X-X-X-X-NA; V, 6-2-NA-X-3-X-X-X-X-NA; and VI, 11-2-NA-9-X-X-5-2-NA-NA. The repeat number of each allele is designated as suggested by ref. [24]; however, absence of PCR product is designated with NA instead of a negative number (-2). X assign the presence of a PCR product; however, different copy numbers of the specific locus exist
†The pathotype was phenotypically determined for Shigella spp.; however, for E. coli, the pathotype was determined running an 11-plex PCR [21]
‡The MLVA group for Shigella spp. is designated by letters (A–F). Seven different MLVA groups were defined: A, X-X-0-5-4-0-0; B, X-5-0-X-X-0-0; C, X-5-5-5-4-0-0; D, 5-X-5-5-X-0-0; E, X-X-0-5-5-0-X; F, X-X-5-5-3-0-0; and G, X-5-X-5-4-X-0. The allele number of each locus is designated as suggested by ref. [8]. Within each letter variation of MLVA, profiles exist, but each letter has from four to five identical loci. X assigns the presence of a PCR product; however, different allele numbers of the specific locus exist. Absence of PCR product is designated zero (0)
#ONT: O nontypable
§Bold indicate isolates (7/121, 5.8%) showing disagreement between the real-time PCR method and one or both MLVA assays. In total, 94.2% (114/121) of the strains showed concordance when comparing these molecular methods
Fig. 2.
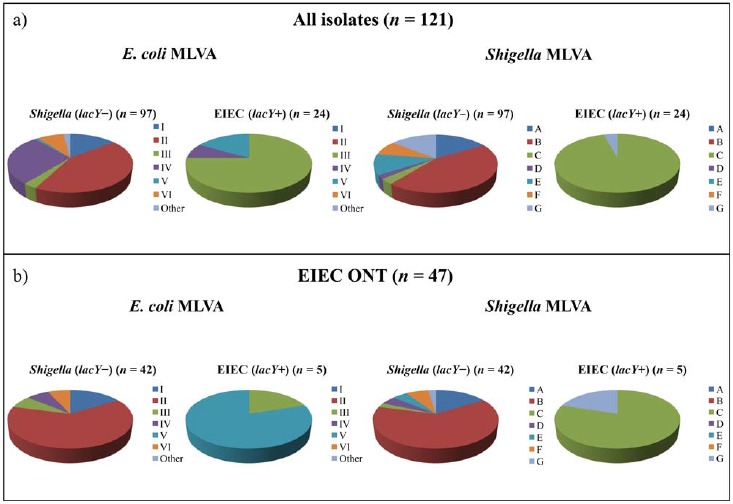
Generic E. coli MLVA and Shigella MLVA groups compared with duplex real-time PCR results. a) All Shigella spp. and EIEC isolates (n = 121) were included. E. coli MLVA groups I, II, and VI and Shigella MLVA groups A, B, E, and F were exclusively detected in isolates classified as Shigella (lacY–) by real-time PCR. On the other hand, E. coli MLVA groups III and V and Shigella MLVA group C were preferentially associated with strains classified as EIEC (lacY+) by the real-time PCR. b) Only EIEC ONT isolates (n = 47) were included. Interestingly, a) and b) showed comparable patterns, indicating that the duplex real-time PCR was suited to classify the phenotypically challenging EIEC ONT group
Discussion
Discrimination of Shigella spp. from EIEC has been challenging using phenotypical typing methods and molecular typing techniques [16–19, 25]. However, due to clinical differences between Shigella spp. and EIEC and also from an epidemiological point of view, discriminating the two is essential [13, 14, 19, 26]. The lac operon, responsible for fermentation of lactose, consists of three functional genes; lacZ, lacY, and lacA. Shigella spp. do not ferment lactose or do so slowly due to lacY deficiency or presence of a lacY pseudogene [10, 15]. Although S. sonnei and S. dysenteriae serotype 1 carry the lacY pseudogene [10, 15], this is not detected by our lacY primers since no match was observed during the NCBI BLAST search and no positive results were seen in the S. sonnei and S. dysenteriae serotype 1 isolates examined. This is in concordance with previous reports demonstrating the absence of lacY in Shigella spp. [19, 27]. Thus, it is tempting to speculate that the structural changes at the 5′ end of the lacY pseudogene described in S. sonnei and S. dysenteriae serotype 1 inhibited binding of the lacY primers [28]. Considering EIEC, previous studies have suggested the presence of lacY in this bacterium [19, 29]. A probe based real-time PCR assay detecting all known variants of lacY, using uidA (encoding the β-glucuronidase) as an internal amplification control, has previously been developed and shown to differentiate Shigella spp. from EIEC [19]. In the current study, this assay was established but with some modifications. Surprisingly, 25% (3/12) of the strains initially examined (1 EIEC O164, 1 EIEC ONT, and 1 S. boydii serotype 13) did not amplify uidA using these uidA primers (data not shown). Thus, uidA was replaced by ipaH, a gene known to be present in all Shigella spp. and EIEC isolates [13]. Additionally, to ensure the specificity of the lacY and ipaH probes, these were redesigned to MGB format [30]. In the study by Pavlovic et al. [19], only 11 EIEC and 18 Shigella spp. were examined and they did not include more than two uncharacterized Shigella spp. [19]. The latter group, defined as EIEC ONT in our study, is the most challenging and cumbersome in a phenotypical diagnostic perspective. Therefore, a molecular method rapidly classifying these isolates as either Shigella or EIEC was sought. In the present study, as many as 47 EIEC ONT strains were examined. Interestingly, most of these strains were detected as Shigella by the duplex real-time PCR, and the two MLVA assays supported our findings in the majority of the cases. This indicated that the real-time PCR was able to classify the challenging EIEC ONT group. However, for three EIEC ONT isolates typed as Shigella by real-time PCR, the MLVA assays disagreed with this classification. Interestingly, these three EIEC ONT isolates agglutinated with Shigella antisera. Nonetheless, they were phenotypically defined as EIEC due to biochemical characteristics [31]. It has been suggested that EIEC is an intermediate stage between noninvasive E. coli and Shigella [11, 14]. These EIEC ONT isolates might be precursors of “full-blown” Shigella and, thus, were either classified as Shigella or EIEC depending on the characteristics examined. Furthermore, the EIEC O164 group was not unambiguously classified molecularly, although being so by phenotypical typing. It is well known that some EIEC O antigens are identical to O antigens present in Shigella spp., and this complicates serological differentiation [11, 14, 32]. Cross-reactivity between O-antigens from EIEC O164 and S. dysenteriae serotype 3 has been described [32, 33], an observation also detected in our study. Therefore, based on the present knowledge, we cannot conclude on the molecular classification of the EIEC O164 group. Whole genome sequencing of the EIEC O164 strains, as well as the three EIEC ONT strains, is in progress and will hopefully help us understand the discrepancies observed.
Culture-independent assays for detecting gastrointestinal pathogens at clinical microbiological laboratories are increasingly used. These multiplex PCR assays particularly focus on ipaH and, therefore, do not distinguish Shigella spp. from EIEC. Hence, after isolation of ipaH positive bacteria, the herein described real-time PCR will be an important supplement for fast and reliable molecular differentiation of these two entities.
Conclusion
A high correlation between the real-time PCR method, the two MLVA assays (generic E. coli MLVA and Shigella MLVA), and phenotypical typing was achieved. This indicated that the real-time PCR was well suited for discriminating Shigella spp. from EIEC and especially fruitful for the challenging EIEC ONT group. Phenotypical typing methods distinguishing Shigella spp. from EIEC are labor intensive and sometimes nonconclusive. Thus, implementing the herein described real-time PCR method is advantageous for a fast and reliable discrimination between Shigella spp. and EIEC.
Acknowledgements
We would like to thank all medical microbiological laboratories in Norway for isolating Shigella spp. and EIEC from patient samples and forwarding the isolates to the NIPH for further characterization. Additionally, Anne Marie Sørgaard and Marit Hindrum at NIPH are gratefully acknowledged for skillful technical assistance.
Footnotes
Abbreviations: EIEC, enteroinvasive E. coli; ipaH, invasive plasmid antigen H; lacY, lactose permease; MLVA, multiple-locus variable-number of tandem repeat analysis; ONT, O nontypable; spp., species; Stx, shiga toxin
References
- 1.Hale TL: Genetic basis of virulence in Shigella species. Microbiol Rev 55, 206–224 (1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lima IF, Havt A, Lima AA: Update on molecular epidemiology of Shigella infection. Curr Opin Gastroenterol 31, 30–37 (2015) [DOI] [PubMed] [Google Scholar]
- 3.Nyholm O, Lienemann T, Halkilahti J, Mero S, Rimhanen-Finne R, Lehtinen V, Salmenlinna S, Siitonen A: Characterization of Shigella sonnei isolate carrying Shiga toxin 2-producing gene. Emerg Infect Dis 21, 891–892 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ojha SC, Yean Yean C, Ismail A, Singh KK: A pentaplex PCR assay for the detection and differentiation of Shigella species. Biomed Res Int 2013, 412370 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapperud G Rorvik LM Hasseltvedt V Hoiby EA Iversen BG Staveland K Johnsen G Leitao J Herikstad H Andersson Y et al.: Outbreak of Shigella sonnei infection traced to imported iceberg lettuce. J Clin Microbiol 33, 609–614 (1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman-Herrador B, Vold L, Comgelli H, MacDonald E, Heier BT, Wester AL, Stavnes TL, Jensvoll L, Lindegard Aanstad A, Severinsen G, Aasgaard Grini J, Werner Johansen O, Cudjoe K, Nygard K: Outbreak of Shigella sonnei infection in Norway linked to consumption of fresh basil, October 2011. Euro Surveill 16 (2011) [PubMed] [Google Scholar]
- 7.Heier BT, Nygard K, Kapperud G, Lindstedt BA, Johannessen GS, Blekkan H: Shigella sonnei infections in Norway associated with sugar peas, May–June 2009. Euro Surveill 14, (2009) [DOI] [PubMed] [Google Scholar]
- 8.Rawal M, Hoff E, Aas-Pedersen L, Haugum K, Lindstedt BA: Rapid multiple-locus variable-number tandem-repeats analysis of Shigella spp. using multicolour capillary electrophoresis. J Microbiol Methods 83, 279–285 (2010) [DOI] [PubMed] [Google Scholar]
- 9.Levine MM: Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis 155, 377–389 (1987) [DOI] [PubMed] [Google Scholar]
- 10.Yang F, Yang J, Zhang X, Chen L, Jiang Y, Yan Y, Tang X, Wang J, Xiong Z, Dong J, Xue Y, Zhu Y, Xu X, Sun L, Chen S, Nie H, Peng J, Xu J, Wang Y, Yuan Z, Wen Y, Yao Z, Shen Y, Qiang B, Hou Y, Yu J, Jin Q: Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic Acids Res 33, 6445–6458 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lan R, Alles MC, Donohoe K, Martinez MB, Reeves PR: Molecular evolutionary relationships of enteroinvasive Escherichia coli and Shigella spp. Infect Immun 72, 5080–5088 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukiya S, Mizoguchi H, Tobe T, Mori H: Extensive genomic diversity in pathogenic Escherichia coli and Shigella Strains revealed by comparative genomic hybridization microarray. J Bacteriol 186, 3911–3921 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Beld MJ, Reubsaet FA: Differentiation between Shigella, enteroinvasive Escherichia coli (EIEC) and noninvasive Escherichia coli. Eur J Clin Microbiol Infect Dis 31, 899–904 (2012) [DOI] [PubMed] [Google Scholar]
- 14.Ud-Din A, Wahid S: Relationship among Shigella spp. and enteroinvasive Escherichia coli (EIEC) and their differentiation. Braz J Microbiol 45, 1131–1138 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito H, Kido N, Arakawa Y, Ohta M, Sugiyama T, Kato N: Possible mechanisms underlying the slow lactose fermentation phenotype in Shigella spp. Appl Environ Microbiol 57, 2912–2917 (1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu BM, Wu SF, Huang SW, Tseng YJ, Ji DD, Chen JS, Shih FC: Differentiation and identification of Shigella spp. and enteroinvasive Escherichia coli in environmental waters by a molecular method and biochemical test. Water Res 44, 949–955 (2010) [DOI] [PubMed] [Google Scholar]
- 17.Kingombe CI, Cerqueira-Campos ML, Farber JM: Molecular strategies for the detection, identification, and differentiation between enteroinvasive Escherichia coli and Shigella spp. J Food Prot 68, 239–245 (2005) [DOI] [PubMed] [Google Scholar]
- 18.Zhao J, Kang L, Hu R, Gao S, Xin W, Chen W, Wang J: Rapid oligonucleotide suspension array-based multiplex detection of bacterial pathogens. Foodborne Pathog Dis 10, 896–903 (2013) [DOI] [PubMed] [Google Scholar]
- 19.Pavlovic M, Luze A, Konrad R, Berger A, Sing A, Busch U, Huber I: Development of a duplex real-time PCR for differentiation between E. coli and Shigella spp. J Appl Microbiol 110, 1245–1251 (2011) [DOI] [PubMed] [Google Scholar]
- 20.Jorgensen HJ, Pfaller MA, Carroll KC, Funke G, Landry ML, Richter S, Warnock DW. (2015): Manual of Clinical Microbiology, vol. 1, 11th Edition, ASM Press, Washington. [Google Scholar]
- 21.Brandal LT, Wester AL, Lange H, Lobersli I, Lindstedt BA, Vold L, Kapperud G: Shiga toxin-producing Escherichia coli infections in Norway, 1992–2012: characterization of isolates and identification of risk factors for haemolytic uremic syndrome. BMC Infect Dis 15, 324 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barletta F, Mercado EH, Lluque A, Ruiz J, Cleary TG, Ochoa TJ: Multiplex real-time PCR for detection of Campylobacter, Salmonella, and Shigella. J Clin Microbiol 51, 2822–2829 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29, e45 (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobersli I, Haugum K, Lindstedt BA: Rapid and high resolution genotyping of all Escherichia coli serotypes using 10 genomic repeat-containing loci. J Microbiol Methods 88, 134–139 (2012) [DOI] [PubMed] [Google Scholar]
- 25.Sahl JW, Morris CR, Emberger J, Fraser CM, Ochieng JB, Juma J, Fields B, Breiman RF, Gilmour M, Nataro JP, Rasko DA: Defining the phylogenomics of Shigella species: a pathway to diagnostics. J Clin Microbiol 53, 951–960 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson JR: Shigella and Escherichia coli at the crossroads: machiavellian masqueraders or taxonomic treachery? J Med Microbiol 49, 583–585 (2000) [DOI] [PubMed] [Google Scholar]
- 27.Horakova K, Mlejnkova H, Mlejnek P: Specific detection of Escherichia coli isolated from water samples using polymerase chain reaction targeting four genes: cytochrome bd complex, lactose permease, beta-D-glucuronidase, and beta-D-galactosidase. J Appl Microbiol 105, 970–976 (2008) [DOI] [PubMed] [Google Scholar]
- 28.Denamur E, Picard B, Tenaillon O. (2010). In: Bacterial Population Genetics in Infectious Disease, eds. Robinson DA, Feil EJ, Falush D, Wiley–Blackwell, Hoboken, pp. 269–286 [Google Scholar]
- 29.Leonard SR, Lacher DW, Lampel KA: Draft Genome sequences of the enteroinvasive Escherichia coli strains M4163 and 4608-58. Genome Announc 3, (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kutyavin IV, Afonina IA, Mills A, Gorn VV, Lukhtanov EA, Belousov ES, Singer MJ, Walburger DK, Lokhov SG, Gall AA, Dempcy R, Reed MW, Meyer RB, Hedgpeth J: 3′-Minor groove binder-DNA probes increase sequence specificity at PCR extension temperatures. Nucleic Acids Res 28, 655–661 (2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rezwan F, Lan R, Reeves PR: Molecular basis of the in-dole-negative reaction in Shigella strains: extensive damages to the tna operon by insertion sequences. J Bacteriol 186, 7460–7465 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheasty T, Rowe B: Antigenic relationships between the enteroinvasive Escherichia coli O antigens O28ac, O112ac, O124, O136, O143, O144, O152, and O164 and Shigella O antigens. J Clin Microbiol 17, 681–684 (1983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linnerborg M, Weintraub A, Widmalm G: Structural studies of the O-antigen polysaccharide from the enteroinvasive Escherichia coli O164 cross-reacting with Shigella dysenteriae type 3. Eur J Biochem 266, 460–466 (1999) [DOI] [PubMed] [Google Scholar]


