Abstract
Upon differentiation, T cells acquire tissue-specific homing properties allowing efficient targeting of effector T cells into distinct inflamed organs. Priming of T cells within skin-draining, peripheral lymph nodes (pLNs) leads to the expression of E- and P-selectin ligands, which facilitate migration into inflamed skin, whereas activation within gut-draining, mesenteric LNs (mLNs) results in induction of chemokine receptor CCR9 and integrin α4β7, both required for migration of effector T cells into mucosal tissues. In addition to the local tissue microenvironment, both organ-specific dendritic cells and LN-resident stromal cells are critical factors to shape T cell migration properties. Here, we identify two additional homing-related molecules, CCR6 and Neuropilin-1 (Nrp1), upregulated in T cells early during differentiation solely in pLNs, but not mLNs. Surprisingly, intestinal inflammation resulted in an ameliorated induction of CCR6 and Nrp1 in pLNs, suggesting that a local inflammation within the gut can systemically alter T cell differentiation. Finally, transplantation of mLNs to a skin-draining environment revealed that LN stromal cells also contribute to efficient CCR6 induction in pLNs. Collectively, these findings identify further aspects of early T cell differentiation within skin-draining pLNs, which could be utilized to further develop tailored and highly specialized vaccination strategies.
Keywords: skin-draining lymph node, gut-draining lymph node, T cell differentiation, CCR6, Neuropilin-1, inflammation, lymph node stromal cells
Introduction
T cells continuously recirculate through the body, passing through different lymph nodes (LNs) to scan dendritic cells (DCs) for the presence of cognate antigens. Upon recognition of their respective antigen, naive T cells get activated, differentiate and acquire not only effector functions, but also the ability to migrate to the tissue drained by the priming LNs [1–3].
The induction of tissue-specific homing properties on T cells depends on molecular and cellular mediators of the tissue-specific LNs, including DCs and soluble lymph-derived factors [4–11]. In this context, skin-draining, peripheral LNs (pLNs) were shown to be a preferential induction site for E- and P-selectin ligands [12, 13], which favor the migration of effector T cells into inflamed skin. On the contrary, gut-draining mesenteric LNs (mLNs) were found to support the induction of chemokine receptor CCR9 and integrin α4β7 [12, 14, 15], thereby facilitating migration of primed T cells into mucosal tissues. More recently, novel insights into the segregation of lymphocyte homing to different segments of the intestine are emerging, as migration to the small intestine more dominantly requires expression of CCR9 and α4β7, whereas Gpr15 promotes homing to colonic lamina propria [16, 17].
The chemokine receptor CCR6 is also induced upon T cell differentiation and can be found on both Foxp3+ regulatory T cells (Tregs) as well as inflammatory Th17 cells [18, 19]. The CCR6 ligand chemokine C-C motif ligand (Ccl)20 contributes to the recruitment of CCR6-expressing cells and is highly inducible under inflammatory conditions [20]. Expression of CCR6 on regulatory and effector T cells is key for various pathological settings, including psoriasis, graft-versus-host disease (GvHD), inflammatory bowl diseases, experimental autoimmune encephalomyelitis (EAE) and rheumatoid arthritis [18, 21–26]. Although it is known that stable expression of CCR6 on effector/memory T cells is under epigenetic control [27], no preferential induction site for CCR6 expression has been reported so far. Similarly, the requirements for an efficient induction of the cell surface molecule Neuropillin-1 (Nrp1), which is known to foster the interaction of T cells with DCs [28], remain enigmatic. Several studies suggest that Nrp1 can be used to distinguish between thymus-derived Tregs (tTregs) and peripherally-induced Tregs (pTregs) under certain circumstances [29, 30], however, whether Nrp1 is induced on differentiated T cells in a tissue-specific manner is completely unknown.
Through their unique structural makeup LNs serve as a hub for immune cell migration and interaction [31]. DCs, which are constantly sampling antigens within tissues, can migrate to local draining LNs, where they only have a short functional life span of several days, being continuously replaced by newly arriving DCs [32, 33]. Importantly, incoming DCs can be influenced by the micro environment of the LN and thereby can even change their tissue-derived, T cell priming properties [7]. To a significant degree, the microenvironment of LNs is determined by LN stromal cells, which in contrast to the highly mobile DCs, are sessile and have a low turn-over under steady-state conditions [34]. Among the LN stromal cells are fibroblastic reticular cells (FRCs), which are dominating the T cell zone [35, 36] and which have been linked to several immune modulatory functions, ranging from limiting T cell expansion to regulating peripheral tolerance [37–39]. Accordingly, stromal cells of gut-draining mLNs were shown to significantly contribute to both the efficient induction of gut-homing molecules on differentiated CD4+ T cells [40, 41] as well as the efficient de novo induction of Foxp3+ Tregs [42].
Here we report that both CCR6 and Nrp1 are induced on recently activated CD4+ T cells selectively in skin-draining pLN when compared to gut-draining mLN. Interestingly, a chronic inflammation of the colon negatively influenced this preferential induction of CCR6 and Nrp1 in pLN. Finally, LN transplantation experiments revealed a significant contribution of LN stromal cells to the efficient CCR6 induction in pLNs. In conclusion, these findings contribute to a better understanding of the early stages of T cell differentiation within skin-draining pLNs, findings that could be of significance for the development of novel vaccination strategies.
Materials and Methods
Mouse strains
Foxp3hCD2xRag2–/–xDO11.10 (BALB/c), Foxp3hCD2xThy1.1 (BALB/c), Thy1.1 (BALB/c) and BALB/c mice (Janvier) were bred or kept at the Helmholtz Centre for Infection Research (Braunschweig, Germany). Water and Ova-free diet were supplied ad libitum. In all experiments, genderand age-matched mice were used. All mice were housed and handled under specific pathogen-free conditions.
Chronic DSS colitis
To induce a chronic inflammation of the colon, mice were treated for four cycles with 5% dextran sodium sulfate (DSS, 36-50 kDa, MP Biomedicals) in drinking water ad libitum for four days followed by ten days of normal drinking water.
Antibodies and flow cytometry
Fluorochrome-conjugated anti-human CD2 (clone RPA-2.10), anti-CD3 (clone 145-2C11), anti-CD4 (clone RM4-5), anti-CD196 (CCR6, clone 129816), anti-CD199 (CCR9, clone CW-1.2), anti-CD304 (Nrp1, clone 3E12), and anti-Ova-TCR (clone KJ1.26) were purchased from eBioscience, BD and Biolegend. Polyclonal anti-CD304 (Nrp1) antibodies were purchased from R&D. Live/Dead discrimination was carried out utilizing LIVE/DEAD Fixable Dead Cell Stain (Invitrogen). Flow cytometry was performed using LSRII or LSR Fogrtessa flow cytometer with Diva software (BD), and data were analyzed with FlowJo software (TreeStar).
T cell isolation, adoptive transfer and in vivo T cell differentiation
Single cell suspensions were generated from spleens and LNs of Foxp3hCD2xRag2–/–xDO11.10 mice, labeled with cell proliferation dye CPDviolet (Invitrogen) and adoptively transferred without cell sorting. Approximately 3–10·106 cells were injected in 100 μl PBS (ThermoFisher Scientific) i.v. per recipient mouse. Subsequently, in vivo T cell differentiation was initiated by injecting 20 μg Ova323–339 peptide i.v. on two consecutive days, starting one day after adoptive T cell transfer. Three days after the first immunization, cells were isolated from transplanted LNs and endogenous control LNs and analyzed by flow cytometry.
LN transplantations
For transplantations of LNs into the popliteal fossa, BALB/c recipient mice were anesthetized with ketamine (WDT) and xylazine (CP Pharma), the skin of the popliteal fossa of the right hind leg was opened, and the endogenous popliteal LN (popLN) and surrounding fat tissue was removed. popLNs or mLNs dissected from SPF-housed donor mice (BALB/c) were placed into the popliteal fossa, and the cut was sewn with absorbable suture (Catgut). Before subjecting to further experimental procedure, recipients were housed for at least ten weeks to ensure restoration of lymphatic and blood vessel connections to the LN. Successful engraftment of transplanted LNs was verified by footpad injection of 20 μl Patent V (25 mg/ml, SigmaAldrich) into CO2-euthanized mice.
Statistical analysis
Group sizes were estimated according to a presumed standard deviation (SD) and an expected type I error of <0.05. The sample size was adjusted, if required, based on initial results. For all figures, each data point represents a single mouse if not stated otherwise. Prism software (GraphPad) was utilized for statistical analysis and graphs. For comparison of unmatched groups, two-tailed Mann–Whitney test was applied. All data are presented as mean or mean ± SD and p < 0.05 are considered as significant. *p < 0.05; **p < 0.01, ***p < 0.001.
Ethics
Animals were handled with appropriate care and welfare in accordance with good animal practice as defined by FELASA and the national animal welfare body GVSOLAS under supervision of the institutional animal welfare officer, and all efforts were made to minimize suffering. Animal experiments were performed in accordance with institutional, state, and federal guidelines, and all animal experiments were approved by the Lower Saxony Committee on the Ethics of Animal Experiments as well as the responsible state office (Lower Saxony State Office of Consumer Protection and Food Safety) under the permit number 33.9-42502-04-12/1012.
Results
CCR6 and Nrp1 are induced during early T cell differentiation selectively in skin-draining pLN
Numerous studies have addressed tissue-specific properties of the LN environment shaping T cell differentiation. Particular progress was made with regard to integrins and chemokine receptors required for gut homing, namely α4β7 and CCR9, as well as E- and P-selectin ligands, supporting homing of effector T cells towards the inflamed skin [2, 12–15].
To determine additional differences during early T cell differentiation taking place in skin-draining pLNs and gut-draining mLNs, an adoptive transfer model utilizing TCR-transgenic, Ova-specific naive Foxp3–CD4+ T cells from Foxp3hCD2xRag2–/–xDO11.10 mice was applied in the present study. Subsequent to adoptive transfer of T cells, recipient mice were immunized systemically with Ova-peptide (Ova323–339) via the i.v. route. This systemic antigen application circumvents the uptake of antigen within the tissue and thus allows studying intrinsic properties of all LNs simultaneously, independent of the route of antigen delivery. At day 3 after antigen application, flow cytometric analysis revealed a comparable proliferation of Ova-specific T cells within all pLN and mLN (Fig. 1a). In line with previously published data [41, 42], a significantly higher induction of CCR9 and Foxp3 was observed in mLN when compared to pLN (Fig. 1a, b). In contrast, pLN showed a significantly higher induction of CCR6 and Nrp1 on Ova-specific T cells when compared to mLN, and strikingly hardly any CCR6+ cells could be detected among Ova-specific T cells within mLN at all (Fig. 1a, b).
Fig. 1.
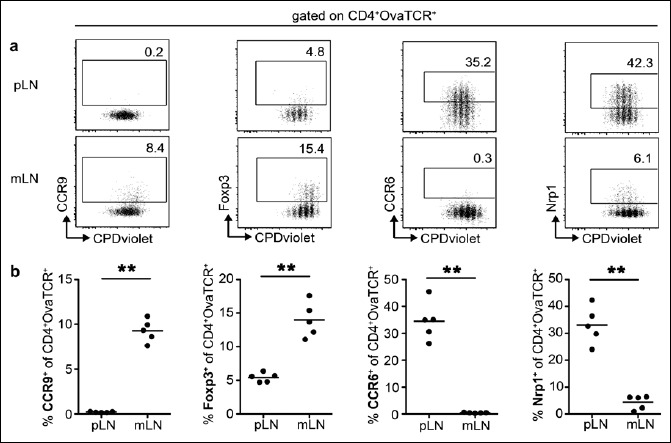
Induction of CCR9, Foxp3, CCR6 and Nrp1 in LNs is taking place in a tissue-specific manner. BALB/c mice received CPDvioletlabeled cells from Foxp3hCD2xRag2–/–xDO11.10 mice. Repetitive i.v. injection of Ova323–339 peptide was performed on the two consecutive days post adoptive transfer, and cells were analyzed on day 3 after the first immunization. (a) Exemplary dotplots show expression of CCR9, Foxp3, CCR6 and Nrp1 on adoptively transferred T cells (gated as CD4+OvaTCR+ cells) over cell division for pLNs and mLNs. Numbers indicate frequencies in gates. (b) Scatterplots summarize frequencies of CCR9+, Foxp3+, CCR6+ and Nrp1+ cells among adoptively transferred CD4+OvaTCR+ T cells for pLNs and mLNs. One representative of two independent experiments is shown (n = 5)
To determine whether the endogenous T cell populations mirror the higher induction of CCR6 and Nrp1 among adoptively transferred Ova-specific T cells within pLN, flow cytometric analysis was performed. No difference of the frequency of CCR6+ among total CD4+ T cells was identified, when comparing mLNs and pLNs (Fig. 2a, b). Importantly, when dissecting the T cell compartment into Foxp3+ Tregs and Foxp3– conventional T cells, we observed slightly, but significantly elevated frequencies of CCR6+ cells among Foxp3+ Tregs within pLNs as compared to mLNs (Fig. 2a, b). However, the frequency of Nrp1+ cells among total CD4+ T cells, Foxp3+ Tregs and Foxp3– conventional T cells was similar for the analyzed LNs (Fig. 2c). Together, these results suggest that the tissue-specific microenvironment of the skin fosters the induction of CCR6 and Nrp1 during early T cell differentiation, but does not result in an accumulation of CCR6+ or Nrp1+ effector T cells within skin-draining pLNs.
Fig. 2.
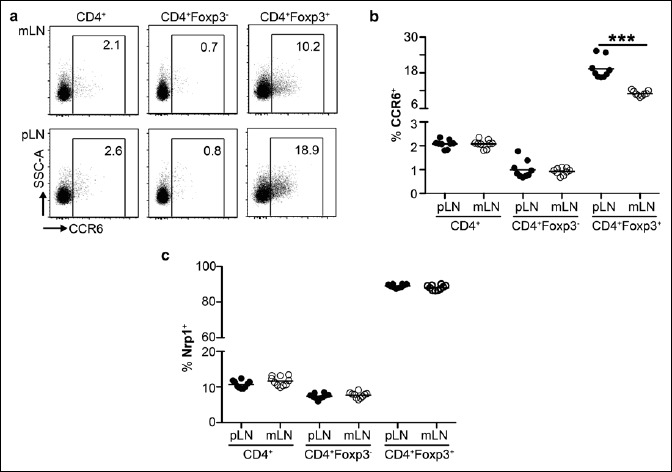
No enrichment of CCR6+ or Nrp1+ effector T cells within skin-draining pLNs. (a) Exemplary dotplots show expression of CCR6 in indicated T cell populations of mLNs (upper row) or pLNs (lower row). Numbers indicate frequencies in gates. (b) Scatterplot summarizes frequencies of CCR6+ cells within indicated T cell populations for pLNs (filled circles) and mLNs (open circles). (c) Scatterplot summarizes frequencies of Nrp1+ cells within indicated T cell populations for pLNs (filled circles) and mLNs (open circles). Pooled data from two independent experiments are shown (n = 10–18)
Chronic intestinal inflammation reduces CCR6 and Nrp1 induction in skin-draining pLNs
The in vivo T cell differentiation studies had revealed that hardly any CCR6+ and only very low frequencies of Nrp1+ cells could be detected among Ova-specific T cells within mLN. To determine whether intestinal inflammation would alter the induction of CCR6 and Nrp1 within mLNs, the chronic DSS colitis model was utilized to achieve long-lasting inflammation [43]. Thereto, mice were treated with four cycles of DSS in drinking water for four days followed by ten days of normal drinking water (Fig. 3a). Each DSS treatment cycle caused a transient body weight loss, while repetitive DSS treatment resulted in chronic inflammation as indicated by shortened colon length and increased spleen size (data not shown). Next, we analyzed the CCR6- and Nrp1-inducing properties of mLNs from DSS-treated mice by adoptive transfer of naive Ova-specific T cells and subsequent systemic immunization as described above. We could not observe any impact of chronic intestinal inflammation on the proliferation of adoptively transferred T cells in both pLNs and mLNs (Fig. 3b). Furthermore, the frequency of CCR6+ and Nrp1+ Ova-specific T cells remained low within mLNs of DSS-treated mice (Fig. 3c, d). However, we observed a significant reduction in the induction of both CCR6 as well as Nrp1 in pLNs of DSS-treated compared to untreated control mice (Fig. 3c, d), suggesting that a chronic inflammation within the intestine can impact functional properties of skin-draining pLNs and alter their T cell differentiation capacities.
Fig. 3.
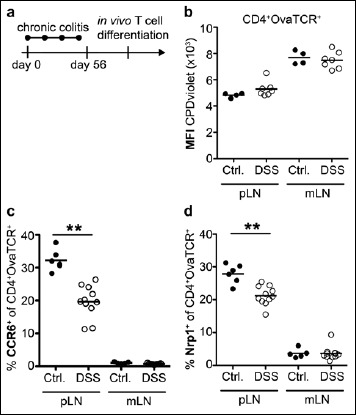
Chronic intestinal inflammation ameliorates CCR6 and Nrp1 induction in skin-draining pLNs. (a) BALB/c mice (Janvier) aged eight weeks were treated with 5% DSS in drinking water ad libitum for four days followed by ten days of normal drinking water. This treatment cycle was repeated four times. Subsequently, BALB/c mice received CPDviolet-labeled cells from Foxp3hCD2xRag2–/–xDO11.10 mice. Repetitive i.v. injection of Ova323–339 peptide was performed on the two consecutive days post adoptive transfer, and cells were analyzed on day 3 after the first immunization. (b) Scatterplot depicts geometric mean fluorescence intensity (MFI) of CPDviolet from adoptively transferred OvaTCR+CD4+ T cells for pLNs and mLNs in DSS-treated (open circles) and untreated mice (filled circles). One representative of two independent experiments is shown (n = 4–7). (c, d) Scatterplots summarize frequencies of CCR6+ (c) and Nrp1+ (d) cells among adoptively transferred CD4+OvaTCR+ T cells for pLNs and mLNs in DSS-treated (open circles) and untreated mice (filled circles). Pooled data from two independent experiments are shown (n = 6–11)
LN stromal cells critically contribute to efficient CCR6 induction within pLNs
We had previously shown that LN stromal cells significantly contribute to the unique functional properties of gut-draining mLNs, as they had a direct impact on both the efficient induction of gut-homing molecules on differentiated CD4+ T cells [41] as well as on the efficient de novo induction of Foxp3+ Tregs [42]. To investigate whether LN stromal cells also contribute to the efficient induction of CCR6 among activated T cells within pLN, we here performed LN transplantation experiments. Upon transplantation of LNs, all hematopoietic cells of donor origin are completely replaced by hematopoietic cells of the recipient mice as early as five weeks after LN transplantation, whereas non-hematopoietic stromal cells are largely retained in transplanted LNs [41]. Here, mLNs of healthy donor mice were transplanted into the popliteal fossa of recipient mice after excision of the endogenous, skin-draining popliteal LN (popLN). popLNs were transplanted as controls. After engraftment of the transplanted LNs, the in vivo T cell differentiation potential of transplanted LNs was analyzed as described above (Fig. 4a). Proliferation of adoptively transferred T cells was similar within transplanted popLNs and mLNs (Fig. 4b). Importantly, CCR6 induction among Ova-specific T cells was similar in transplanted popLN when compared to endogenous popLN (Fig. 4b, c). However, transplanted mLNs contained significantly lower frequencies of induced CCR6+ cells among Ova-specific T cells when compared to transplanted popLN (Fig. 4c), suggesting that pLN stromal cells significantly contribute to the efficient induction of CCR6 among activated T cells within skin-draining LNs.
Fig. 4.
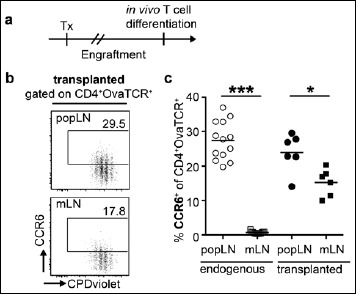
LN stromal cells support efficient CCR6 induction within pLNs. (a) The endogenous popLN was removed and replaced by mLNs or popLNs from 10–12-week-old BALB/c donor mice. After engraftment of the transplanted LN, recipient mice received CPDviolet-labeled cells from Foxp3hCD2xRag2–/–xDO11.10 mice. Repetitive i.v. injection of Ova323–339 peptide was performed on the two consecutive days post adoptive transfer, and cells were analyzed on day 3 after the first immunization. (b) Exemplary dotplots show expression of CCR6 on adoptively transferred T cells (gated as CD4+OvaTCR+ cells) over cell division for transplanted popLNs (upper row) and mLNs (lower row). Numbers indicate frequencies in gates. (c) Scatterplot summarizes frequencies of CCR6+ Tregs among adoptively transferred CD4+OvaTCR+ T cells recovered from endogenous (open symbols) and transplanted indicated LNs (filled symbols). Pooled data from two independent experiments are shown (n = 6–13). Tx, transplantation
Discussion
Differential induction of E- and P-selectin ligands, integrin α4β7 and chemokine receptor CCR9 during early T cell differentiation had been reported for skin- and gut-draining LNs [2, 12, 13]. In the present study, we identified both CCR6 and Nrp1 to be predominantly induced in skin-draining pLN, but not in gut-draining mLN, and could observe a significant contribution of LN stromal cells to the efficient CCR6 induction in pLNs.
The cell surface receptor Nrp1 has been suggested as a marker for tTregs [29, 30]. However, Nrp1 expression is also associated with T cell activation, differentiation and inflammation, and under certain conditions can be even induced in pTregs [29, 30]. Accordingly, numerous Foxp3–Nrp1+ T cells were previously found within human secondary lymphoid organs, and Nrp1 could be induced on peripheral blood-derived T cells upon in vitro activation [44]. However, the molecular factors supporting Nrp1 induction remain largely unknown. Results from the present study suggest that the skin-specific microenvironment promotes Nrp1 induction. Further insights into the induction and function of Nrp1 have been derived from studies on different cancer types, where Nrp1 expression promotes proliferation and survival of tumor cells and limits apoptosis [45]. Induction of Nrp1 can be mediated by epidermal growth factor (EGF) and vascular endothelial growth factor (VEGF) [46]. Importantly, FRCs are the major source of VEGF in pLNs [47], and display a higher VEGF expression than FRCs derived from mLNs [48], suggesting VEGF as a potential contributor to promote the induction of Nrp1. Although it has also been reported that Nrp1 expressed on Foxp3+ Tregs can regulate the anti-tumor immune response by guiding Tregs into tumor sites in response to tumor-derived VEGF [49], it is highly unlikely that the higher frequency of Nrp1+ Ova-specific T cells in pLNs observed in the present study is a consequence of a selective recruitment of Nrp1+ cells to pLNs since the induction of Nrp1 was monitored at an early time point after T cell stimulation, excluding a significant contribution of T cell recruitment. Furthermore, Nrp1 has been described to enhance the interaction of Nrp1+ T cells with DCs [28] via a semaphorin-4a (Sema4a) mechanism [50], thereby promoting sensitivity to antigenic material presented by DCs. As DCs from skin-draining LNs express Sema4a at higher levels than their analogues from mLNs (www.im-mgen.org), the enhanced expression of Nrp1 by recently primed T cells might provide survival and proliferation signals.
Increased frequencies of CCR6+ regulatory and effector T cells were found in various inflammatory models and/or diseases [18, 21–26]. CCR6 expression has been associated with the capacity of effector T cells to produce IL-17 [19] and allows migration of both inflammatory Th17 cells and Foxp3+ Tregs to sites of inflammation mediated via Ccl20 [51]. However, although a high frequency of CCR6+ cells had been observed in the present study among Ova-specific T cells within skin-draining pLNs, no increased capacity to produce IL-17 was detectable (data not shown), suggesting that the priming conditions of the present study only result in the induction of CCR6 expression, but not acquisition of IL-17-producing properties at these early stages of T cell differentiation. A number of cytokines such as IL-2, IL-1β, IL-6 and TGF-β have been reported to promote co-expression of CCR6 and IL-17 [52, 53]. Future studies will have to dissect which molecular mediators or signaling pathways selectively induce either CCR6 or IL-17, and this knowledge could e.g. be used for the generation of inflammation-seeking CCR6+ Tregs lacking the capacity to produce pro-inflammatory IL-17.
Results from the present study suggest a contribution of LN stromal cells to the efficient induction of CCR6 among Ova-specific T cells within skin-draining pLNs. We recently had demonstrated that pLN stromal cells have a negative impact on the de novo induction of Foxp3+ Tregs as we observed a significantly reduced frequency of Ova-specific Foxp3+ Tregs within pLNs that had been transplanted to the mesenteries [42]. Accordingly, when mLNs were transplanted to the skin-draining popliteal fossa, the induction of CCR6 was reduced, indicating that mLN stromal cells alter the skin-draining environment and change early T cell differentiation. The LN transplant setting not only identifies LN stromal cells as important contributors in CCR6 induction, but also underlines the stability of the phenotype of mLN stromal cells, since mLNs not only partially retained their functional properties upon transplantation into a skin-draining environment, but also could resist inflammatory perturbations as no increase in the induction of CCR6 was observed in mLNs after chronic DSS treatment.
In summary, we could demonstrate that the cell surface molecules CCR6 and Nrp1 were differentially induced in a LN-specific manner and that LN stromal cells significantly contribute to modulate CCR6 induction. Utilizing the intrinsic properties of different LNs to generate antigen-specific responses might enable the development of novel, highly tailored vaccination approaches to confer pathogen- and tissue-specific protection.
Acknowledgements
We thank Rainer Glauben for support with the chronic DSS colitis model and Maria Ebel for technical assistance.
Funding Statement
Founding sources This work was supported by the Hannover Biomedical Research School (HBRS) and the Center for Infection Biology (ZIB).
References
- 1.Campbell DJ, Debes GF, Johnston B, Wilson E, Butcher EC: Targeting T cell responses by selective chemokine receptor expression. Semin Immunol 15, 277–286 (2003) [DOI] [PubMed] [Google Scholar]
- 2.Agace WW: Tissue-tropic effector T cells: generation and targeting opportunities. Nat Rev Immunol 6, 682-692 (2006) [DOI] [PubMed] [Google Scholar]
- 3.Masopust D, Schenkel JM: The integration of T cell migration, differentiation and function. Nat Rev Immunol 13, 309–320 (2013) [DOI] [PubMed] [Google Scholar]
- 4.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH: Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature 424, 88–93 (2003) [DOI] [PubMed] [Google Scholar]
- 5.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W: Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med 198, 963–969 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY: Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004) [DOI] [PubMed] [Google Scholar]
- 7.Dudda JC, Lembo A, Bachtanian E, Huehn J, Siewert C, Hamann A, Kremmer E, Forster R, Martin SF: Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur J Immunol 35, 1056–1065 (2005) [DOI] [PubMed] [Google Scholar]
- 8.Campbell DJ. Control of Homing Receptor Expression during Lymphocyte Differentiation Activation and Function. In Hamann A., Engelhardt B. (Eds.) Leukocyte Trafficking. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim: 2005, pp 131–153. [Google Scholar]
- 9.Johansson-Lindbom B, Svensson M, Pabst O, Palmqvist C, Marquez G, Forster R, Agace WW: Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med 202, 1063–1073 (2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milling SW, Jenkins CD, Yrlid U, Cerovic V, Edmond H, McDonald V, Nassar M, Macpherson G: Steady-state migrating intestinal dendritic cells induce potent inflammatory responses in naive CD4+ T cells. Mucosal Immunol 2, 156–165 (2009) [DOI] [PubMed] [Google Scholar]
- 11.McCully ML, Ladell K, Hakobyan S, Mansel RE, Price DA, Moser B: Epidermis instructs skin homing receptor expression in human T cells. Blood 120, 4591–4598 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell DJ, Butcher EC: Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med 195, 135–141 (2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siewert C, Menning A, Dudda J, Siegmund K, Lauer U, Floess S, Campbell DJ, Hamann A, Huehn J: Induction of organ-selective CD4+ regulatory T cell homing. Eur J Immunol 37, 978–989 (2007) [DOI] [PubMed] [Google Scholar]
- 14.Stenstad H, Ericsson A, Johansson-Lindbom B, Svensson M, Marsal J, Mack M, Picarella D, Soler D, Marquez G, Briskin M, Agace WW: Gut associated lymphoid tissue primed CD4+ T cells display CCR9 dependent and independent homing to the small intestine. Blood 107, 3447–3454 (2006) [DOI] [PubMed] [Google Scholar]
- 15.Gorfu G, Rivera-Nieves J, Ley K: Role of beta7 integrins in intestinal lymphocyte homing and retention. Curr Mol Med 9, 836–850 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SV, Xiang WV, Kwak C, Yang Y, Lin XW, Ota M, Sarpel U, Rifkin DB, Xu R, Littman DR: GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science 340, 1456–1459 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Houston SA, Cerovic V, Thomson C, Brewer J, Mowat AM, Milling S: The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol 9, 468–478 (2016) [DOI] [PubMed] [Google Scholar]
- 18.Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rotzschke O, Falk K: CCR6 expression defines regulatory effector/memory-like cells within the CD25+CD4+ T-cell subset. Blood 105, 2877–2886 (2005) [DOI] [PubMed] [Google Scholar]
- 19.Romagnani S, Maggi E, Liotta F, Cosmi L, Annunziato F: Properties and origin of human Th17 cells. Mol Immunol 47, 3–7 (2009) [DOI] [PubMed] [Google Scholar]
- 20.Williams IR: CCR6 and CCL20: partners in intestinal immunity and lymphorganogenesis. Ann N Y Acad Sci 1072, 52-61 (2006) [DOI] [PubMed] [Google Scholar]
- 21.Varona R, Cadenas V, Flores J, Martinez AC, Marquez G: CCR6 has a non-redundant role in the development of inflammatory bowel disease. Eur J Immunol 33, 2937–2946 (2003) [DOI] [PubMed] [Google Scholar]
- 22.Varona R, Cadenas V, Gomez L, Martinez AC, Marquez G: CCR6 regulates CD4+ T-cell-mediated acute graft-versushost disease responses. Blood 106, 18–26 (2005) [DOI] [PubMed] [Google Scholar]
- 23.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S: Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med 204, 2803–2812 (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedrick MN, Lonsdorf AS, Shirakawa AK, Richard Lee CC, Liao F, Singh SP, Zhang HH, Grinberg A, Love PE, Hwang ST, Farber JM: CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J Clin Invest 119, 2317–2329 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villares R, Cadenas V, Lozano M, Almonacid L, Zaballos A, Martinez AC, Varona R: CCR6 regulates EAE pathogenesis by controlling regulatory CD4+ T-cell recruitment to target tissues. Eur J Immunol 39, 1671–1681 (2009) [DOI] [PubMed] [Google Scholar]
- 26.Kitamura K, Farber JM, Kelsall BL: CCR6 marks regulatory T cells as a colon-tropic, IL-10-producing phenotype. J Immunol 185, 3295–3304 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinfelder S, Floess S, Engelbert D, Haeringer B, Baron U, Rivino L, Steckel B, Gruetzkau A, Olek S, Geginat J, Huehn J, Hamann A: Epigenetic modification of the human CCR6 gene is associated with stable CCR6 expression in T cells. Blood 117, 2839–2846 (2011) [DOI] [PubMed] [Google Scholar]
- 28.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG: Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity 28, 402–413 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhust CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, Yang Y, Floess S, Huehn J, Oh S, Li MO, Niec RE, Rudensky AY, Dustin ML, Littman DR, Lafaille JJ: Neuropilin-1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ Treg cells. J Exp Med 209, 1723–1742 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, von Schack D, Bluestone JA: Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med 209, 1713–1722 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forster R, Braun A, Worbs T: Lymph node homing of T cells and dendritic cells via afferent lymphatics. Trends Immunol 33, 271–280 (2012) [DOI] [PubMed] [Google Scholar]
- 32.Kamath AT, Henri S, Battye F, Tough DF, Shortman K: Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood 100, 1734–1741 (2002) [PubMed] [Google Scholar]
- 33.Cerovic V, Houston SA, Scott CL, Aumeunier A, Yrlid U, Mowat AM, Milling SW: Intestinal CD103- dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol 6, 104–113 (2013) [DOI] [PubMed] [Google Scholar]
- 34.Roozendaal R, Mebius RE: Stromal cell-immune cell interactions. Annu Rev Immunol 29, 23–43 (2011) [DOI] [PubMed] [Google Scholar]
- 35.Malhotra D, Fletcher AL, Turley SJ: Stromal and hematopoietic cells in secondary lymphoid organs: partners in immunity. Immunol Rev 251, 160–176 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fletcher AL, Acton SE, Knoblich K: Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol 15, 350–361 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier AR, Turley SJ: Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol 12, 1096–1104 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegert S, Huang HY, Yang CY, Scarpellino L, Carrie L, Essex S, Nelson PJ, Heikenwalder M, Acha-Orbea H, Buckley CD, Marsland BJ, Zehn D, Luther SA: Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS One 6, e27618 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baptista AP, Roozendaal R, Reijmers RM, Koning JJ, Unger WW, Greuter M, Keuning ED, Molenaar R, Goverse G, Sneeboer MM, den Haan JM, Boes M, Mebius RE: Lymph node stromal cells constrain immunity via MHC class II self-antigen presentation. Elife 3, e04433 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Forster R, Pabst O: Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med 205, 2483–2490 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Molenaar R, Greuter M, van der Marel AP, Roozendaal R, Martin SF, Edele F, Huehn J, Forster R, O’Toole T, Jansen W, Eestermans IL, Kraal G, Mebius RE: Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol 183, 6395–6402 (2009) [DOI] [PubMed] [Google Scholar]
- 42.Cording S, Wahl B, Kulkarni D, Chopra H, Pezoldt J, Buettner M, Dummer A, Hadis U, Heimesaat M, Bereswill S, Falk C, Bode U, Hamann A, Fleissner D, Huehn J, Pabst O: The intestinal micro-environment imprints stromal cells to promote efficient Treg induction in gut-draining lymph nodes. Mucosal Immunol 7, 359–368 (2014) [DOI] [PubMed] [Google Scholar]
- 43.Manicassamy S, Manoharan I: Mouse models of acute and chronic colitis. Methods Mol Biol 1194, 437–448 (2014) [DOI] [PubMed] [Google Scholar]
- 44.Milpied P, Renand A, Bruneau J, Mendes-da-Cruz DA, Jacquelin S, AsnafiV Rubio MT, MacIntyre E, Lepelletier Y, Hermine O: Neuropilin-1 is not a marker of human Foxp3+ Treg. Eur J Immunol 39, 1466–1471 (2009) [DOI] [PubMed] [Google Scholar]
- 45.Ellis LM: The role of neuropilins in cancer. Mol Cancer Ther 5, 1099-1107 (2006) [DOI] [PubMed] [Google Scholar]
- 46.Parikh AA, Fan F, Liu WB, Ahmad SA, Stoeltzing O, Reinmuth N, Bielenberg D, Bucana CD, Klagsbrun M, Ellis LM: Neuropilin-1 in human colon cancer: expression, regulation, and role in induction of angiogenesis. Am J Pathol 164, 2139–2151 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chyou S, Ekland EH, Carpenter AC, Tzeng TC, Tian S, Michaud M, Madri JA, Lu TT: Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J Immunol 181, 3887–3896 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fletcher AL, Malhotra D, Acton SE, Lukacs-Kornek V, Bellemare-Pelletier A, Curry M, Armant M, Turley SJ: Reproducible isolation of lymph node stromal cells reveals site-dependent differences in fibroblastic reticular cells. Front Immun 2, 35 (2011), doi: 10.3389/fimmu.2011.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, Albert J, Sparwasser T, Sakaguchi S, Westendorf AM, Schadendorf D, Buer J, Helfrich I: Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med 209, 2001–2016 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA: Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501, 252–256 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, Craig S, Watowich SS, Jetten AM, Tian Q, Dong C: CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol 181, 8391–8401 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manel N, Unutmaz D, Littman DR: The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol 9, 641–649 (2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Kang SG, Lee J, Sun Z, Kim CH: The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol 2, 173–183 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]


