Abstract
Objective. Opioids are frequently prescribed for chronic low back pain (CLBP), but there are broad individual differences in the benefits and risks of opioid therapy, including the development opioid-induced hyperalgesia. This study examined quantitative sensory testing (QST) data among a group of CLBP patients undergoing sustained oral opioid treatment. We investigated whether individual differences in psychological characteristics were related to opioid-induced changes in pain perception and pain modulation.
Design. The six-month, open-label trial evaluated patients with low to high levels of negative affect (e.g., symptoms of distress, depression and anxiety); participants underwent QST at baseline (prior to initiating treatment) and during oral opioid treatment.
Setting. A chronic pain management center.
Patients. The 31 study participants had chronic discogenic back pain, with a pain intensity rating >3/10. Participants were divided into groups with high vs. low levels of Negative Affect (NA).
Results. In the previously-published manuscript describing the clinical outcomes of the trial, high NA patients achieved only about half of the analgesic effect observed in the low NA group (Wasan AD, Michna E, Edwards RR, et al. Psychiatric comorbidity is associated prospectively with diminished opioid analgesia and increased opioid misuse in patients with chronic low back pain. Anesthesiology 2015;123:861–72). The QST findings reported here suggested that tolerance to experimental (cold pressor) pain and conditioned pain modulation tended to decrease in the high NA group over the course of opioid treatment, while temporal summation of mechanical pain declined in the low NA group.
Conclusions. These results reveal that while the low NA group seemed to exhibit a generally adaptive, analgesic pattern of changes during opioid management, the high NA group showed a pattern more consistent with opioid-induced hyperalgesic processes. A greater susceptibility to hyperalgesia-promoting changes in pain modulation among patients with high levels of distress may contribute to a lower degree of benefit from opioid treatment in high NA patients.
Keywords: Chronic Low Back Pain, Negative Affect Opioids, Catastrophizing, Quantitative Sensory Testing, Conditioned Pain Modulation, Temporal Summation
Introduction
Persistent pain is a serious therapeutic challenge and a public health epidemic; it affects over 100 million Americans per year, is among the leading causes of reduced quality of life, and carries direct and indirect costs of over 600 billion dollars annually in the U.S. alone [1]. In addition, long-term administration of analgesic medications (such as opioids) involves a variety of risks [2–5]. Collectively, while opioid analgesics remain a treatment of choice for the management of cancer pain, and are widely used to treat chronic non-cancer pain [6–8], moderate- to long-term opioid use is potentially associated with a variety of adverse impacts [9,10]. One such outcome is a paradoxical amplification of pain sensitivity, a phenomenon termed opioid-induced hyperalgesia (OIH). An increasing body of literature from both basic and clinical science studies has documented this phenomenon, and OIH appears to pose a significant clinical challenge in acute, chronic, and cancer pain settings [11–14]. While the early OIH clinical literature was largely anecdotal, several recent cross-sectional quantitative sensory testing (QST) studies have revealed that individuals taking either morphine or methadone are hyperalgesic relative to controls [15], and that opioid-maintained chronic pain patients showed reduced heat pain thresholds and enhanced temporal summation of pain relative to both controls and matched non-opioid chronic pain patients [16]. Some prospective work has been done as well, with some trials observing OIH [17,18] and some not reporting significant changes in pain responses [19].
As is true for human studies of opioid analgesia [20], individual differences in the development of OIH appear to be substantial [14,21]. To date, it is not understood why some individuals experience OIH while others on even larger doses of opioids do not. A variety of individual difference variables are likely to play a role; for example, a recent clinical study of healthy volunteers found that polymorphisms of the catechol O-methyl transferase gene affected acute changes in pain sensitivity after administration of a potent parenteral opioid [22]. Such findings suggest that opioid-induced maladaptive sensory changes might be restricted to subgroups of patients, which could conceal OIH effects when analyzing group-level effects. For example, important biopsychosocial factors such as pain-related catastrophizing and negative affect (NA) are known to shape individual differences in a variety of pain responses, and some recent data from our lab suggests that they may systematically place patients at elevated risk for the development of OIH [23]. Interestingly, patients scoring very high on measures of psychological distress tend to be systematically excluded from RCTs, even though this subgroup of patients is highly prevalent within the chronic pain population [24,31,43].
There is reason to believe that opioids might impact pain modulation as well as more traditional measures of pain sensitivity and tolerance. To date, several cross-sectional studies have shown disrupted endogenous pain modulation systems in patients maintained on opioid therapy. Long-term opioid users showed significantly greater temporal summation of pain (TSP) than non-users [16], while another study reported that conditioned pain modulation (CPM) was significantly lower in long-term opioid users [24]. In humans, TSP and CPM are well-established psychophysical tools commonly used for assessing endogenous pain-facilitation and pain-inhibition, respectively, and these pain-modulatory systems play an important role in shaping pain transmission and perception at spinal and supraspinal levels [25–27]. Prior work has shown repeatedly that enhanced TSP and diminished CPM are associated with heightened clinical pain across various types of chronic pain conditions [28–30].
Here we present results from a secondary analysis of an open-label oral opioid trial that was designed to investigate differences in opioid analgesia between subgroups of patients with low and high levels of NA [1]. Prior work had suggested that CLBP patients with high levels of comorbid NA have diminished opioid analgesia compared with CLBP patients with low levels of NA [31,32], and the primary outcome analyses for this study replicated those findings. This manuscript presents data from quantitative sensory testing (QST; i.e., the application of standardized noxious stimuli in a controlled environment) evaluations, including TSP and CPM assessment, performed at baseline and during oral opioid treatment. We analyzed these QST data to evaluate whether the high and low NA groups differed in putative OIH-related changes in pain sensitivity and pain modulation over the course of approximately 4 months of oral opioid therapy.
Methods
Study Sample and Design
As described in the prior publication [1], this was a ∼6-month prospective cohort study of oral opioid therapy, with a 1-week baseline, a 2-week run-in of placebo (for one week) and active drug (subjects could choose oxycodone or morphine for 1 week) in randomized order, a 3-week titration period, a 4-month maintenance phase, and a 1-month taper (ClinicalTrials.gov Identifier: NCT01502644). CLBP patients with a discogenic pain syndrome of at least 6 months’ duration and an average daily pain intensity of >3/10 were recruited. Patients could not be on opioid therapy at the start of the study. Subjects taking opioids prior to enrollment underwent a 2-week weaning period and then remained off opioids for at least 1 week prior to initiating treatment. In total, 34% of participants were on opioids prior to enrolling in this study, with no difference between the low and high NA groups [1]. The study was approved by the Institutional Review Board at Brigham and Women’s Hospital, and all participants provided written and verbal informed consent.
Patients were phenotyped for negative affect using the combined depression and anxiety subscale scores of the Hospital Anxiety Depression Scale (HADS total score), as in previous studies [31–33]. High NA was defined as >8 on both the depression and anxiety subscales, as suggested in a review of HADS findings for non-cancer medical patients [34], and low NA was defined as <6 on each subscale. Other baseline measures included: the Neuroticism Subscale of the NEO Personality Inventory, the Pain Catastrophizing Scale [35], the Pain Anxiety Symptoms Scale [36], and the Beck Depression Inventory. In addition, subjects were evaluated for medication misuse with the Drug Misuse Index (DMI), which summarizes assessment of opioid misuse in 3 domains: patient self-report on the Current Opioid Misuse Measure [37], provider assessment of misuse on the Addiction Behaviors Checklist [38], and results of urine toxicology screening [39]. The DMI is converted to a categorical variable reflecting adherence to opioids; a positive finding of misuse on any of these three measures results in a positive DMI., If all three measures are negative for misuse, the DMI is negative [1,40]. In total, participants came to BWH for 13 in-person study visits over the course of the trial. These are described in detail in Wasan et al [1]. Overall, 81 subjects enrolled in the study, with a total of n = 24 Low NA subjects and n = 24 High NA subjects completing the trial [1].
Quantitative Sensory Testing
At baseline, prior to being treated with opioids, and at study visit 9 or 10 (which occurred during the opioid maintenance phase, when subjects had been at their individually-titrated dose for 3-4 months), participants underwent a brief battery of quantitative sensory tests to assess pain sensitivity and pain modulation. QST has been recommended as both a phenotyping and outcome measure in clinical trials of analgesics [26,41], and we have used these standardized psychophysical tests in a number of prior studies [23,42,43].
Mechanical pain thresholds were assessed using a digital pressure algometer (Somedic; Sollentuna, Sweden). Pressure pain thresholds (PPThs) were determined bilaterally at the trapezius muscle, with 2 trials performed on each side of the body. Mechanical force was applied using a 0.5 cm2 probe covered with polypropylene pressure-transducing material; pressure was increased at a steady rate of 30 kPA/s until the subject indicated that the pressure was “first perceived as painful.” Subjects then underwent an assessment of mechanical temporal summation using weighted pinprick stimulators, as in previous studies [23]. The lowest-force stimulator that produced a sensation of discomfort (128 or 256 mN for most subjects) was used to apply a train of 10 stimuli to the skin on the dorsum of the hand at the rate of 1 per second. Participants rated the painfulness of the first, fifth, and tenth stimulus; temporal summation was quantified as the difference in pain intensity ratings between the tenth and the first stimulus. Reaction to prolonged pressure pain was ascertained via cuff pressure algometry (CPA) [44,45]. We used a Hokanson rapid cuff inflator; a standard blood pressure cuff was wrapped comfortably around the lower leg, over the gastrocnemius muscle, and a computer-controlled air compressor determined the pressure level that was individually tailored, for each subject, to produce a pain intensity rating of 40/100. Finally, responses to noxious cold were evaluated using a repeated cold pressor task, involving immersion of the right hand in a circulating cold water bath maintained at 4°C. In the present protocol, participants underwent a series of 3 cold pressor tasks, with the first 2 consisting of serial immersions of the right hand for 30 sec, with 2 min between immersions. The 3rd and final cold pressor task involved an immersion of the right hand lasting until a participant reached pain tolerance (or a 3 min maximum). During the first 2 cold pressor trials, we assessed conditioned pain modulation (CPM), a non-invasive test of endogenous pain-inhibitory systems using a heterotopic noxious conditioning stimulation paradigm [46,47]. During each these cold pressor tests, PPTh was assessed on the contralateral trapezius. As in prior studies [42,48] we calculated a CPM Index that reflected the magnitude of change in PPTh during cold pressor relative to baseline. The CPM Index is calculated using the formula: (PPTh during the cold pressor test/baseline PPTh)*100. Scores over 100 indicate positive/effective CPM (i.e., pain threshold increased during the cold pressor test). Finally, ratings of cold pain after-sensations (i.e., lingering pain following withdrawal of the hand from the cold water bath) were obtained 30 seconds after the cold pressor test [42].
Statistical Methods
The primary manuscript assessed whether the high NA and low NA groups differed in the percent improvement in average daily pain during the opioid maintenance period [1]. Here, we examine changes in QST responses from baseline during that opioid maintenance period. As noted in the primary manuscript, sample size calculations were based on a power of 0.80 to test the hypothesis that opioids confer diminished analgesia in the high NA versus the low NA group. Based on our previous data using IV opioid administration [32], 20 subjects each were needed in the low and high groups to find a 33% difference in average percent pain intensity improvement [1]. Because not all subjects were able to undergo QST at baseline and during treatment, we fell somewhat short of this goal, though the primary manuscript analyzed clinical outcome data from over 20 patients per group [1].
The brief QST procedures described above yielded 6 measures of pain responses: PPTh (on the trapezius), temporal summation of mechanical pain, cuff pain threshold, cold pain tolerance, cold pain after-sensations, and conditioned pain modulation. For each of these measures, we performed a 2 (Group) X 2 (Time) mixed factorial ANCOVA to analyze QST changes in the 2 groups (high NA vs. low NA) during opioid treatment. As the high NA group was treated with larger opioid doses, this factor was included as a covariate in the ANCOVA. Significant effects were followed up with group comparisons using non-parametric Mann-Whitney U tests to account for the non-normality of some of the distributions of QST variables. Bivariate associations between variables were assessed using Pearson correlations or partial correlations (e.g., controlling for baseline values when examining change scores). Finally, for QST variables that demonstrated an impact of NA on QST-related changes, we performed a follow-up multiple linear regression analyses comparing the predictive capability of several different measures of distress: the HADS, PCS, NEO Neuroticism Subscale, BDI, and PASS. In these regression analyses, the dependent variable (DV) was the change score for the QST variable, and after the initial steps included the baseline DV value (Step 1) and opioid dose (Step 2), those 5 distress-related measures were included in a stepwise fashion (i.e., only significant factors entered into the regression) in Step 3. Data were analyzed using SPSS version 20.
Results
A total of 31 subjects completed the trial and provided QST data at baseline and during treatment. Trial completers with QST data (n = 31, including 16 low NA and 15 high NA) did not differ from those missing QST data on demographic variables (e.g., age, sex), or key clinical variables (e.g., pain intensity at baseline, HADS, PCS, or BDI scores, etc; all p’s > .4). See Table 1 for descriptive data in this sample.
Table 1.
Descriptive baseline data
| Variable | Low NA (n = 16) | High NA (n = 15) | p |
|---|---|---|---|
| Age (years) | 54 ± 11 | 49 ± 10 | .15 |
| Female sex (%) | 69% | 67% | .83 |
| % Working | 50% | 13% | .02 |
| % Married | 38% | 47% | .45 |
| % Taking opioids pre-enrollment | 31% | 40% | .41 |
| Baseline pain intensity | 7.1 ± 1.3 | 7.6 ± 1.8 | .38 |
| Pain duration | 5.9 ± 4.7 | 6.9 ± 5.1 | .41 |
| % Substance abuse history | 19% | 20% | .87 |
| % Comorbid major depression | 6% | 73% | <.001 |
| HADS Depression | 3.9 ± 2.1 | 11.9 ± 4.2 | <.001 |
| HADS Anxiety | 4.2 ± 2.6 | 12.2 ± 2.2 | <.001 |
| Pain catastrophizing scale | 16.6 ± 7.1 | 32.2 ± 9.1 | <.001 |
| QST Variables at Baseline | |||
| PPTh (Trapezius) | 332.8 ± 197.1 | 306.0 ± 160.6 | .92 |
| PPTh during cold pressor test | 431.6 ± 225.7 | 417.2 ± 251.1 | .81 |
| Mechanical TSP | 24.5 ± 20.3 | 19.2 ± 12.0 | .33 |
| Cuff pain threshold | 146.9 ± 84.8 | 140.9 ± 72.2 | .93 |
| Cold pain tolerance | 56.8 ± 44.8 | 24.6 ± 32.1 | .20 |
| Cold pain after-sensations | 18.5 ± 24.7 | 31.7 ± 29.1 | .42 |
*p < .05.
**p < .01.
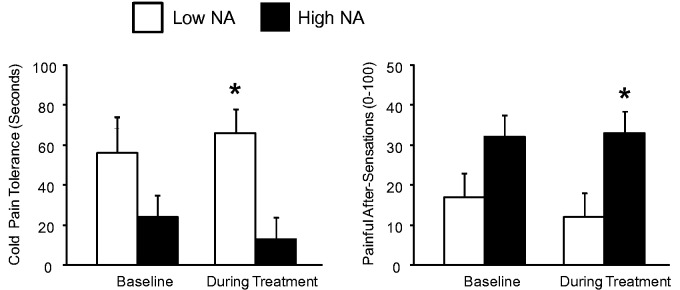
The ANCOVA examining PPTh on the trapezius revealed no main effect of Time [F (1,28) = .10, p = .76], no main effect of Group [F (1,28) = .1, p = .83], and no interaction [F (1,28) = .4, p = .54]. Similar findings were evident for the measure of mechanical cuff pain threshold on the calf (all p’s > .4). The ANCOVA for cold pain tolerance yielded a significant main effect of Group [F (1,28) = 6.3, p = .02], no main effect of Time (p = .90) and a trend for a Group X Time interaction [F (1,28) = 3.9, p = .06]. The high NA group showed a decrease in cold pain tolerance during opioid treatment, while cold pain tolerance increased with opioid treatment in the low NA group. Mann-Whitney U tests revealed no significant group difference at baseline, but significantly higher tolerance in the low NA group during treatment (p = .004). See Figure 1. A similar effect was evident on the measure of cold pain after-sensations. The ANOVA indicated that painful after-sensation ratings in the high NA group were elevated compared to the low NA group (p-value for the main effect of Group = .04), with no main effects of Time (p = .89) and no interaction (p = .48). Mann-Whitney U tests indicated that the low NA group had significantly lower (p = .01) ratings of after-sensation pain during opioid treatment. See Figure 1.
Figure 1.
Cold pain responses in low- and high-NA groups. Data presented as group means ± SEM. *Groups differ at p < .05 on non-parametric Mann-Whitney U tests.
Significant Group X Time interactions were observed for the measures of pain modulation. The ANCOVA for mechanical (probe) temporal summation showed no main effects of Time or Group (p’s > .3), but the interaction was significant [F (1,28) = 6.4, p = .02]. As is evident in Figure 2, the low NA group showed a reduction in temporal summation, while a slight increase was evident for the high NA group. However, Mann-Whitney U tests failed to achieve significance for the direct comparison of groups. Similarly, the ANCOVA for CPM produced no significant main effects (p’s > .6), but the Group X Time interaction was significant at F (1,28) = 4.1, p = .05. The high NA group showed a large decrease in CPM during treatment, while CPM increased slightly in the low NA group of participants. Mann-Whitney U tests indicated that while the groups did not differ at baseline, the low NA group had a significantly higher (p = .02) CPM Index during opioid treatment. See Figure 3.
Figure 2.
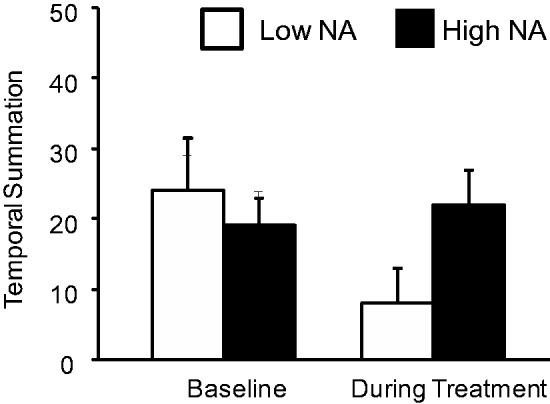
Mechanical temporal summation (pain rating of 10th stimulus minus pain ratings of 1st stimulus) in low- and high-NA groups. Data presented as group means ± SEM.
Figure 3.
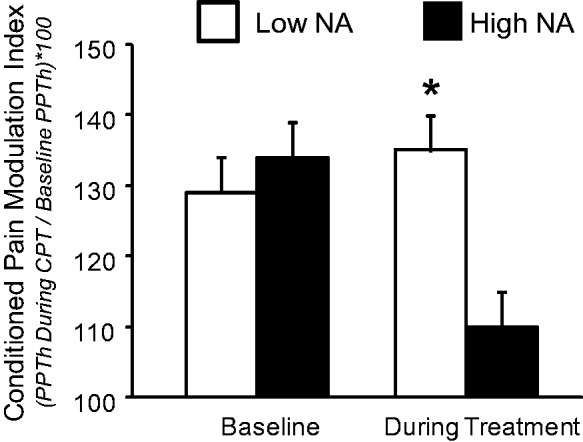
Conditioned pain modulation in low- and high-NA groups. Data presented as group means ± SEM. *Groups differ at p < .05 on non-parametric Mann-Whitney U tests.
Partial correlations (controlling for baseline values of both variables) between changes in QST parameters and the percentage decrease in pain intensity (i.e., the primary outcome for the trial) revealed no significant associations (all p’s > .2). That is, the degree of analgesia obtained by a patient was unrelated to the degree change in pain threshold, tolerance, or modulation. Similar null findings (i.e., no significant correlations) were evident between opioid dose and changes in QST parameters (all p’s > .1). On the other hand, changes in cold pain responses were significantly or near-significantly related to opioid misuse (see Refs. 1 and 41 for a full description of the Drug Misuse Index, DMI). ANCOVAs (controlling for NA group and for baseline values of the QST variable) showed a significant difference in treatment-related changes in cold pain after-sensations: the group that was positive for opioid misuse (i.e., positive DMI) demonstrated an increase of +14.0 ± 6.7 in cold pain after-sensations, while patients who were negative for misuse had a mean reduction of −2.7 ± 3.5 [F (1,28) = 4.7, p = .04]. Similarly, the ANCOVA (again, controlling for NA group and for baseline cold pain tolerance) for cold pain tolerance suggested a near-significant difference between positive DMI patients (who showed a −10.0 ± 9.2 reduction in cold pain tolerance) and negative DMI patients (who had a +3.3 ± 5.2 increase in cold pain tolerance).
Finally, we performed multiple linear regression analyses on the cold pain tolerance, CPM, and temporal summation data (as these were the 3 QST measures that yielded significant or near-significant Group X Time interactions), comparing the predictive capability of several different measures of distress. None of the individual distress-related measures emerged as predictors of changes in CPM after controlling for baseline CPM and opioid dose. For temporal summation, PCS scores emerged as a significant predictor, explaining 18% of the variance in the degree of change in temporal summation. Lower baseline PCS scores were associated with a decrease in temporal summation during opioid treatment (see Table 2). For change in cold pain tolerance, HADS scores emerged as an inverse predictor, explaining 12% of the variance. Lower baseline HADS scores were associated with an increase in pain tolerance during opioid treatment (see Table 3).
Table 2.
Hierarchical linear regression predicting change in temporal summation
| Variable | Step R2 | β | p |
|---|---|---|---|
| Step 1: Baseline Temporal Summation | .30** | −.55 | <.001 |
| Step 2: Opioid Dosage | .04 | −.21 | .11 |
| Step 3: Pain Catastrophizing Scale | .18** | .45 | .001 |
*p < .05.
**p < .01.
Table 3.
Hierarchical linear regression predicting change in cold pain tolerance
| Variable | Step R2 | β | p |
|---|---|---|---|
| Step 1: Baseline Temporal Summation | .01 | −.01 | .89 |
| Step 2: Opioid Dosage | .04 | −.21 | .23 |
| Step 3: Hospital Anxiety & Depression Scale | .12* | −.38 | .04 |
*p< .05.
Discussion
In this prospective cohort study, patients with discogenic pain were phenotyped into distinct groups with differing levels of negative affect. Collectively, despite being administered higher opioid doses, the high NA group experienced substantially less analgesia [1], a finding that confirms past observations in other samples [31,32]. In the present report, an adjunctive QST study, we report evidence that, despite a reduced sample size and potentially limited power, the high NA group also experienced more maladaptive sensory changes during opioid treatment. Compared with the low NA group, high NA patients exhibited reductions in pain tolerance and endogenous pain inhibition, and they did not exhibit a decrease in temporal summation of pain. We also found that elevated baseline levels of catastrophizing were associated with enhanced temporal summation over the course of the study. In considering the pattern of results, it is interesting to note that the present findings highlight the selectivity of OIH-like changes in pain responses over the course of sustained opioid treatment. There were no main effects of time in this study, and the QST parameters we examined did not all exhibit the same pattern of changes. A study of OIH that examined a single pain modality and evaluated group-level changes over the course of treatment might well conclude that opioid administration does not alter pain sensitivity, which has been the conclusion of some previous trials [19]. In contrast, this study suggests that differential effects of opioids on QST may occur as a function of the parameter tested and of the patients’ psychological state, with those highest in distress and negative affect being at greater risk for reductions in cold pain tolerance and endogenous pain inhibition. It is interesting to note that the high NA group received higher doses of opioid over the course of this study, which may have contributed to the maladaptive sensory changes observed in this group. While our analyses did control for opioid dose, we cannot rule out the possibility that dose is an important contributor to OIH effects, and the intersection between affect, opioid dose, and decrements in pain modulation appears to be a fruitful area for future research on this topic.
Additional work will be required to fully assess the clinical relevance of these changes. A recent secondary analysis of a non-randomized opioid trial reported a moderate inverse relationship between changes in thermal pain sensitivity and degree of self-reported hydromorphone analgesia (i.e., those who showed an increase in pain sensitivity reported the least benefit from opioids [17]). In the present prospective trial, the high NA group did obtain the least opioid analgesia and exhibited more maladaptive changes in pain sensitivity and modulation. However, there was not a significant linear association between QST changes and changes in clinical pain intensity; that is, OIH-like changes in pain sensitivity and modulation were distinct from analgesic responses to chronic opioid therapy (including, presumably, the development of opioid tolerance). In general, though, indices of negative affect and pain-related catastrophizing have previously been associated with putative deficits in opioid-mediated pain inhibition [49, 50]. For example, catastrophizing predicts greater post-operative use of opioid analgesics after a painful surgical procedure [51], as well as reduced acute analgesic benefit of opioids in a laboratory setting [52], similar to what our group has observed in prior studies of NA [32]. Functional neuroimaging studies have also suggested that elevated catastrophizing and negative mood are related to enhanced activation of pain-processing brain areas such as anterior cingulate cortex and amygdala during the administration of calibrated noxious stimuli [53–55], potentially reflecting a deficit in endogenous opioid-mediated pain inhibition.
It is important to emphasize that the present QST data do not bear directly on the question of when opioids should be prescribed, for whom, and for how long. But they do suggest that subgroups of patients may be at relatively greater risk for maladaptive changes in pain sensitivity and pain modulation. The findings of the primary analysis indicate that patients with high levels of negative affect may derive reduced analgesic benefit from opioids, and if these patients also experience decrements in CPM and reduced pain tolerance, they may be at elevated risk for the development of other pain-related sequelae or other persistent pain conditions (post-surgical pain, for example [27,56,57]). Moreover, the present results hint that some opioid-induced sensory changes (in cold pain tolerance and cold pain after-sensations) may also be associated with indices of opioid misuse. Because misuse was measured during the same time frame as QST changes, the temporal aspects of this association are unclear; additional work in this area may play a valuable role in determining whether QST-assessed changes in pain processing may serve as a signal of, or risk factor for, impending misuse of opioids (or conversely, whether they may be a consequence of that misuse). Collectively, it is possible that if symptoms of NA are identified and effectively treated early in the course of LBP, this might help to reduce negative pain-related sequelae such as OIH, improve the outcomes from subsequent (opioid) management, and potentially even reduce the incidence of opioid misuse.
Several limitations should temper the interpretation of these findings. First, with no control group, we cannot be certain that any changes we observe in QST responses are definitively opioid-related. Many QST studies have demonstrated the strong temporal stability of patients’ responses on these measures [58], suggesting that the observed changes in the present study may well be due to medication effects, but we cannot rule out the possibility of other sensitization- or habituation-producing processes. Second, we do not have detailed data on medical comorbidities (e.g., fibromyalgia) that may be associated with alterations in pain-modulatory processes [28]. Additionally, the sample size for this analysis is fairly small, and we were unable to perform QST on all of the study participants utilized in the primary analyses, which likely limited our power to detect some of these effects. Finally, we were unable to obtain serial QST measurements over the course of opioid treatment, which would have helped to shed light on the time course and duration of opioid-induced sensory changes. Yet despite these limitations, this study is the first to document psychologically-defined subgroup differences in the effects of opioid treatment on pain sensitivity and modulation. As such, it suggests the possibility that, in addition to being at risk for reduced opioid analgesic benefits, chronic pain patients high in negative affect and pain-related distress may also be at elevated risk for reduced pain inhibition and pain tolerance. The further study of such effects in randomized, controlled trials of opioids for chronic pain appears to be a fruitful area for investigation.
Funding source: This study was supported by the National Institute of Drug Abuse (NIDA) of the National Institutes of Health (K23 DA020682, Wasan, PI) and the Arthritis Foundation (Investigator Award; Wasan, PI), as well as by grants R01 AG034982 (Edwards) and R21 AR057920 (Edwards).
Conflicts of interest: The authors have no conflicts of interest to declare.
Reference
- 1.Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain 2012;13:715–24. [DOI] [PubMed] [Google Scholar]
- 2.Jena AB, Goldman D, Weaver L, Karaca-Mandic P. Opioid prescribing by multiple providers in Medicare: Retrospective observational study of insurance claims. BMJ 2014;348:g1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011;305:1315–21. [DOI] [PubMed] [Google Scholar]
- 4.Bonar EE, Ilgen MA, Walton M, Bohnert AS. Associations among pain, non-medical prescription opioid use, and drug overdose history. Am J Addict 2014;23:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohnert AS, Ilgen MA, Trafton JA, et al. Trends and regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain 2014;30: 605–12. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol 2008;16:405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: A cohort study. Ann Intern Med 2010;152:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edlund MJ, Martin BC, Devries A, et al. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: The TROUP study. Clin J Pain 2010;26:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crofford LJ. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat Rev Rheumatol 2010;6:191–7. [DOI] [PubMed] [Google Scholar]
- 10.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: A systematic review and literature synthesis. Clin J Pain 2008;24:497–508. [DOI] [PubMed] [Google Scholar]
- 11.Tompkins DA, Campbell CM. Opioid-induced hyperalgesia: Clinically relevant or extraneous research phenomenon? Curr Pain Headache Rep 2011;15:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raffa RB, Pergolizzi JV., Jr Opioid-induced hyperalgesia: Is it clinically relevant for the treatment of pain patients? Pain Manag Nurs 2013;14:e67–83. [DOI] [PubMed] [Google Scholar]
- 13.Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans: Molecular mechanisms and clinical considerations. Clin J Pain 2008;24:479–96. [DOI] [PubMed] [Google Scholar]
- 14.Colvin LA, Fallon MT. Opioid-induced hyperalgesia: A clinical challenge. Br J Anaesth 2010;104:125–7. [DOI] [PubMed] [Google Scholar]
- 15.Hay JL, White JM, Bochner F, et al. Hyperalgesia in opioid-managed chronic pain and opioid-dependent patients. J Pain 2009;10:316–22. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Malarick C, Seefeld L, et al. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain 2009;143:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzan E, Eisenberg E, Treister R, Haddad M, Pud D. A negative correlation between hyperalgesia and analgesia in patients with chronic radicular pain: Is hydromorphone therapy a double-edged sword? Pain Physician 2013;16:65–76. [PubMed] [Google Scholar]
- 18.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: A preliminary prospective study. J Pain 2006;7:43–8. [DOI] [PubMed] [Google Scholar]
- 19.Chu LF, D'Arcy N, Brady C, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: A double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain 2012;153:1583–92. [DOI] [PubMed] [Google Scholar]
- 20.Riley JL, III, Hastie BA. Individual differences in opioid efficacy for chronic noncancer pain. Clin J Pain 2008;24:509–20. [DOI] [PubMed] [Google Scholar]
- 21.Fishbain DA, Cole B, Lewis JE, Gao J, Rosomoff RS. Do opioids induce hyperalgesia in humans? An evidence-based structured review. Pain Med 2009;10: 829–39. [DOI] [PubMed] [Google Scholar]
- 22.Jensen KB, Lonsdorf TB, Schalling M, Kosek E, Ingvar M. Increased sensitivity to thermal pain following a single opiate dose is influenced by the COMT val(158)met polymorphism. PLoS One 2009;4:e6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards RR, Wasan AD, Michna E, et al. Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. J Pain 2011;12:953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ram KC, Eisenberg E, Haddad M, Pud D. Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain — new perspective of opioid-induced hyperalgesia. Pain 2008;139:431–8. [DOI] [PubMed] [Google Scholar]
- 25.Backonja MM, Walk D, Edwards RR, et al. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain 2009;25:641–7. [DOI] [PubMed] [Google Scholar]
- 26.Backonja MM, Attal N, Baron R, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain 2013;154:1807–19. [DOI] [PubMed] [Google Scholar]
- 27.Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: Between pro- and anti-nociception. Pain 2014 Apr;155(4):663–5. [DOI] [PubMed] [Google Scholar]
- 28.Clauw DJ. Fibromyalgia and related conditions. Mayo Clin Proc 2015;90:680–92. [DOI] [PubMed] [Google Scholar]
- 29.Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care 2015;9:131–7. [DOI] [PubMed] [Google Scholar]
- 30.Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain 2009;10:556–72. [DOI] [PubMed] [Google Scholar]
- 31.Jamison RN, Edwards RR, Liu X, et al. Relationship of negative affect and outcome of an opioid therapy trial among low back pain patients. Pain Pract 2013;13:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasan AD, Davar G, Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain 2005;117:450–61. [DOI] [PubMed] [Google Scholar]
- 33.Wasan AD, Kaptchuk TJ, Davar G, Jamison RN. The association between psychopathology and placebo analgesia in patients with discogenic low back pain. Pain Med 2006;7:217–28. [DOI] [PubMed] [Google Scholar]
- 34.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69–77. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and Validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- 36.McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): Preliminary development and validity. Pain Res Manag 2002;7:45–50. [DOI] [PubMed] [Google Scholar]
- 37.Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain 2007;130:144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu SM, Compton P, Bolus R, et al. The addiction behaviors checklist: Validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain Symptom Manage 2006;32:342–51. [DOI] [PubMed] [Google Scholar]
- 39.Michna E, Jamison RN, Pham LD, et al. Urine toxicology screening among chronic pain patients on opioid therapy: Frequency and predictability of abnormal findings. Clin J Pain 2007;23:173–9. [DOI] [PubMed] [Google Scholar]
- 40.Jamison RN, Ross EL, Michna E, et al. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: A randomized trial. Pain 2010 Sep;150(3):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Attal N, Bouhassira D, Baron R, et al. Assessing symptom profiles in neuropathic pain clinical trials: Can it improve outcome? Eur J Pain 2011;15:441–3. [DOI] [PubMed] [Google Scholar]
- 42.Edwards RR, Mensing G, Cahalan C, et al. Alteration in pain modulation in women with persistent pain after lumpectomy: Influence of catastrophizing. J Pain Symptom Manage 2013;46:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martel MO, Wasan AD, Edwards RR. Sex Differences in the stability of conditioned pain modulation (CPM) among patients with chronic pain. Pain Med 2013;14:1757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jespersen A, Dreyer L, Kendall S, et al. Computerized cuff pressure algometry: A new method to assess deep-tissue hypersensitivity in fibromyalgia. Pain 2007;131:57–62. [DOI] [PubMed] [Google Scholar]
- 45.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L. Spatial and temporal aspects of deep tissue pain assessed by cuff algometry. Pain 2002;100:19–26. [DOI] [PubMed] [Google Scholar]
- 46.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, et al. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010;14:339. [DOI] [PubMed] [Google Scholar]
- 47.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 2010;23:611–5. [DOI] [PubMed] [Google Scholar]
- 48.Edwards RR, Grace E, Peterson S, et al. Sleep continuity and architecture: Associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain 2009;13:1043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell CM, Edwards RR. Mind-body interactions in pain: The neurophysiology of anxious and catastrophic pain-related thoughts. Transl Res 2009;153:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol 2011;7:216–24. [DOI] [PubMed] [Google Scholar]
- 51.Jacobsen PB, Butler RW. Relation of cognitive coping and catastrophizing to acute pain and analgesic use following breast cancer surgery. J Behav Med 1996;19:17–29. [DOI] [PubMed] [Google Scholar]
- 52.Fillingim RB, Hastie BA, Ness TJ, et al. Sex-related psychological predictors of baseline pain perception and analgesic responses to pentazocine. Biol Psychol 2005;69:97–112. [DOI] [PubMed] [Google Scholar]
- 53.Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004;127: 835–43. [DOI] [PubMed] [Google Scholar]
- 54.Strigo IA, Simmons AN, Matthews SC, Craig AD, Paulus MP. Increased affective bias revealed using experimental graded heat stimuli in young depressed adults: Evidence of “emotional allodynia”. Psychosom Med 2008;70:338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berna C, Leknes S, Holmes EA, et al. Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biol Psychiatry 2010;67:1083–90. [DOI] [PubMed] [Google Scholar]
- 56.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006;367:1618–25. [DOI] [PubMed] [Google Scholar]
- 57.Granovsky Y, Yarnitsky D. Personalized pain medicine: The clinical value of psychophysical assessment of pain modulation profile. Rambam Maimonides Med J 2013;4:e0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geber C, Klein T, Azad S, et al. Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): A multi-centre study. Pain 2011;152:548–56. [DOI] [PubMed] [Google Scholar]