Abstract
Background
Early identification of carotid and vertebral artery dissections has been advocated to reduce stroke among trauma patients. We sought to characterize trends in the diagnosis of traumatic carotid and vertebral artery dissections and association changes in stroke rate among Medicare beneficiaries.
Methods
Using Medicare claims, we created a cohort of 5,961 beneficiaries admitted with a new traumatic carotid or vertebral artery dissection from 2001 to 2012. We calculated rates of stroke during hospitalization and 90 days of discharge. We calculated rates of carotid imaging using computed tomography-angiography, carotid duplex, and plain angiography index hospitalization. To study concurrent secular trends, we created a secondary cohort of patients admitted after any traumatic injury from 2001 to 2012 and determined rates of stroke and carotid imaging within this cohort.
Results
From 2001 to 2012, incidence of traumatic carotid dissection increased 72% among Medicare beneficiaries (1.1–1.76 per 100,000 patients; rate ratio [RR], 1.72; 95% CI, 1.6–1.9, P < 0.001). Among patients diagnosed with traumatic carotid or vertebral artery dissections, the combined in-hospital and 90-day stroke rate did not change significantly (4.9% in 2001; 5.2% in 2012; RR, 1.06; 95% CI, 0.93–1.20; P = 0.094). Likewise, there was little change in mortality (10.3%; RR, 1.01; 95% CI, 0.95–1.06; P = 0.88). Among all trauma patients, the use of computed tomography angiography has increased 16-fold (2–35 per 100,000 patients; RR, 16.7; 95% CI, 13–19; P < 0.0001).
Conclusions
Despite increased diagnosis of carotid or vertebral artery dissection, there has been little change in stroke risk among trauma patients. Efforts to more effectively target imaging and treatment for these patients are necessary.
BACKGROUND
Dissection of the carotid or vertebral artery, whether traumatic or nontraumatic in origin, has the potential to result in stroke or death.1,2 To prevent this consequence, professional societies have called for early diagnosis in high risk trauma patients. Although initially diagnosed primarily through invasive angiography, the advent of high-resolution computed tomography has made it a faster, easier, and less-invasive process to diagnose a carotid or vertebral dissection.3–5
However, it is unclear whether identifying traumatic carotid or vertebral dissections earlier can result in fewer strokes. First, stroke is often the presenting symptom of carotid or vertebral dissection, up to 40% of patients with traumatic etiologies of dissection present with stroke and imaging may only serve to identify a stroke that has already occurred.6–9 Second, patients who experience a carotid or vertebral dissection are heterogeneous and often have multiple competing injuries that place them at higher stroke risk. This heterogeneity can make it difficult to understand the risk of stroke.10 For these reasons, we hypothesized that more aggressive use of high-resolution imaging over the past decade has identified more dissections but has had little association with a change in stroke risk among patients with carotid or vertebral artery dissections.
To test this hypothesis, we examined trends in the use of noninvasive and invasive imaging along with overall stroke rates among Medicare beneficiaries admitted with traumatic carotid or vertebral artery dissections from 2001 to 2012. We examined stroke risk and imaging use among all beneficiaries admitted with a trauma diagnosis to account for secular trends.
METHODS
To explore both imaging use among trauma patients and the diagnosis and outcome of traumatic carotid or vertebral artery dissection, we created 2 distinct patient populations: patients admitted with any traumatic injury and patients admitted with a traumatic carotid or vertebral artery dissection.
Primary Cohort Definition: Patients with Traumatic Carotid and Vertebral Artery Dissections
Medicare claims data (2001–2012) were used to identify patients admitted to the hospital with traumatic carotid or vertebral artery dissection and calculate the population-based incidence rates of the injury, use of imaging modalities, and stroke rates among these patients over time. We used International Classification of Diseases, version 9 (ICD-9) codes to identify patients admitted with a traumatic carotid or vertebral artery dissection. We included patients admitted with a new concurrent diagnosis of traumatic injury and carotid or vertebral artery dissection. Due to the rarity of the injury, patients aged 20–99 years were included within this cohort, who were enrolled in fee-for-service Medicare. Only the first admission for a carotid or vertebral dissection within a 90-day window was included. Population-based rates were calculated using midyear Medicare enrollees. Comorbidities; including hypertension, diabetes, coronary artery disease, renal insufficiency, malignancy, and congestive heart failure; were recorded both individually and in aggregate using the Charlson comorbidity score. Injury severity score was calculated using commercially available freeware ICD Programs for Injury Categorization (ICDPIC), a validated program which uses ICD-9 code to map injury to area of the body to calculate Abbreviated Injury Score and then calculates the square of the highest 3 scores to determine injury severity score.11 Please see Appendix 1 for codes used in the analysis.
Second Cohort Definition: Patients with Traumatic Injury
To study patterns of treatment among all injured patients—not just those with dissection, a traumatic injury cohort was created. Patients were identified using ICD-9 codes indicative of traumatic injury at the time of hospital admission, excluding codes indicative of superficial injury. Patients younger than 65 years, older than 99 years or not enrolled in fee for service Medicare were excluded. One admission for a traumatic injury was allowed every 90 days. Population-based rates were calculated using midyear Medicare enrollees. Cohort was similarly described using both Charlson comorbidity score and its components and ISS scores using ICDPIC.11 Patients with a carotid or vertebral artery injury were present within this cohort.
To comprehensively describe temporal trends in traumatic admissions, we performed additional exploratory analysis examining emergency room admissions for traumatic injury. Using the same ICD-9 codes, we identified all patients seen in emergency rooms from 2007 to 2012 and calculated the proportion of patient admitted from the emergency room over time. We found few clinically important differences in both the rate of Medicare beneficiaries admitted over time and the proportion of patient admitted from the Emergency Department over time.
Calculating Imaging Rates
Among patients admitted with a carotid or vertebral artery dissection, procedural codes were used to identify computed tomography (CT) angiograms, plain angiograms, and duplex studies of the carotid or vertebral arteries. We included the first code for each of these imaging modalities per day, if multiple codes were present. This information was used to calculate the percentage of patients who received each imaging modality during their index admission. The same technique was used within patients admitted with any traumatic injury.
Calculating Stroke Rate
Stroke was the primary outcome. The outcome of stroke was defined by the presence of ICD-9 codes indicative of ischemic stroke, using coding algorithms identified in prior studies. It was defined at 2 time points: in-hospital if it occurred during the index hospitalization or in initial transfer and 90 days of discharge after index hospitalization. If a patient was readmitted more than 1 time for a stroke, we included only the first within our 90-day follow-up period. We analyzed stroke rates over time using combined (in-hospital and 90 days) and separated rates.
Assessment of Secondary Outcomes
We identified 2 secondary outcomes: readmission and mortality. Readmission was measured from discharge to 90 days postdischarge and included readmission to any hospital for any reason. Transfers from the index hospitalization were not considered readmissions. We included only the first readmission for a patient. Mortality was measured during the index hospitalization and within 90 days postdischarge. Mortality rates were analyzed using both combined (in-hospital and 90 days) and separated rates.
Statistical Analyses
Except where noted previously mentioned, unit of analysis was the patient. All analyses were performed using SAS (Cary, NC) and Microsoft Excel (Redmond, WA). Absolute changes were calculated using standard relative rates and t-tests for 95% significance. Simple linear regression models were used to calculate tests of trend.
The Geisel School of Medicine Center for the Protection of Human Subjects granted Institutional Review Board exemption for this study. The patient consent was not obtained as all conditions for waiver of consent as outlined by the policy of protection of human research subjects were met.
RESULTS
Demographics of Patients Admitted with Carotid or Vertebral Artery Dissection
We identified 5,961 Medicare beneficiaries admitted to hospitals with a traumatic carotid or vertebral artery dissection from 2001 to 2012. There were few clinically significant differences in patients over time when we compared patients admitted earlier in the study period (2001–2007) to those admitted later (2008–2012; Table I). However, the proportion of patients younger than 65 years and with peripheral vascular disease has increased from 2001 to 2012.
Table I.
Characteristics of patients admitted with traumatic carotid and vertebral artery dissections, 2001–2012
| Total all years | Total | 2001–2007 | 2008–2012 | P valuea |
|---|---|---|---|---|
| Total number | 5,961 | 3,105 | 2,856 | |
| Age, years (mean) | 70.6 | 71.2 | 69.9 | 0.003 |
| Sex (% female) | 42.9 | 43.4 | 42.4 | 0.05 |
| Race (% black) | 11.7 | 12.2 | 11.1 | 0.39 |
| Medicaid eligible (%) | 24.7 | 24.3 | 28.8 | 0.3 |
| Injury Severity Score (mean) | 11.1 | 10.9 | 11.4 | 0.34 |
| Charleston Comorbidity Score (mean) | 1.1 | 1.1 | 1.1 | 0.07 |
| Diabetes mellitus (%) | 9.9 | 10.3 | 9.3 | 0.74 |
| Congestive heart failure (%) | 10.5 | 11.6 | 9.0 | 0.007 |
| Peripheral vascular disease (%) | 12.2 | 7.9 | 16.9 | <0.0001 |
| Chronic renal failure (%) | 6 | 5.0 | 7.1 | 0.04 |
| Aged less than 65 (%) | 26.4 | 24.3 | 28.8 | 0.0001 |
| Distance to hospital, medianb (miles) | 65 | 53.3 | 76.8 | 0.002 |
| Admitted from emergency room (%) | 84 | 83.5 | 83.4 | 0.94 |
| Median household income (US $) | 54,093 | 52,882 | 55,408 | <0.0001 |
| Povertyc (%) | 24 | 24.7 | 23.3 | 0.001 |
| Primary diagnosis of carotid or vertebral dissection (%) | 28 | 31.1 | 24.4 | <0.0001 |
Calculated using linear test of trend, by year.
Straight line distance.
Defined as less than 150% of poverty level.
Diagnosis of Traumatic Carotid or Vertebral Artery Dissection and Stroke
Among patients admitted to the hospital as a result of trauma, the diagnosis of carotid or vertebral dissection has increased 72% during the study period (1.1–1.76 per 100,000 beneficiaries; rate ratio [RR], 1.72; 95% CI, 1.6–1.9; P < 0.0001). The diagnosis remains rare overall that we identified only 370 patients in 2001 and 1,383 in 2011 admitted with the diagnosis.
Trends in Imaging Use among Patients Admitted with Traumatic Carotid or Vertebral Artery Dissection
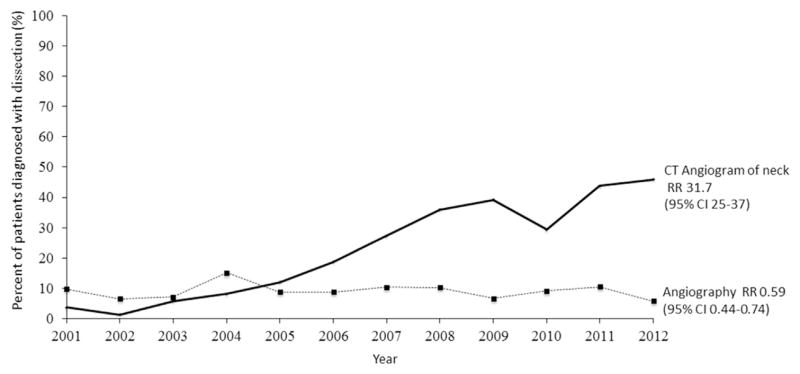
The use of noninvasive carotid and vertebral artery imaging (CT angiogram of the neck and brain) has increased from 2001 to 2012 for patients with traumatic carotid or vertebral dissections. CT angiography has increased 32 times among patients with a carotid or vertebral artery dissection (1.2–40%; RR, 31.7; 95% CI for the rate 24.8–37.2; P = 0.001). This increased use of noninvasive imaging has been associated with a nonsignificant decline in invasive angiography (9–6%; RR, 0.59; 95% CI, 0.7–0.8; P = 0.63) of the carotid or vertebral artery (Fig. 1). The use of carotid duplex was uncommon but declined slightly over this time period (6–4.5%; RR, 0.75; 95% CI, 0.5–0.9; P = 0.94).
Fig. 1.
Rate of imaging among Medicare beneficiaries diagnosed with traumatic carotid or vertebral artery dissections, 2001–2012.
Stroke Risk among Patients with Traumatic Carotid or Vertebral Artery Dissection
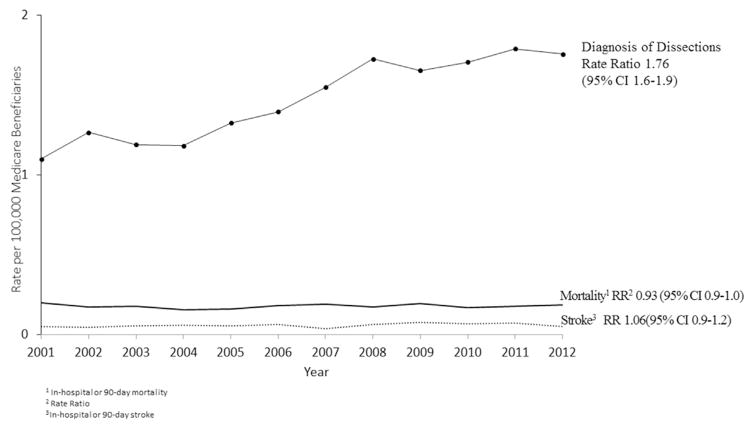
Among patients with a traumatic carotid or vertebral dissection, the percentage experiencing stroke (either while in-hospital or within 90 days of discharge) has not changed significantly (4.9% in 2001 to 5.2% in 2012; RR, 1.06; 95% CI, 0.93–1.20; P = 0.094; Fig. 2).
Fig. 2.
Diagnosis of traumatic carotid or vertebral artery dissections and rate of stroke or death among Medicare beneficiaries, 2001–2012.
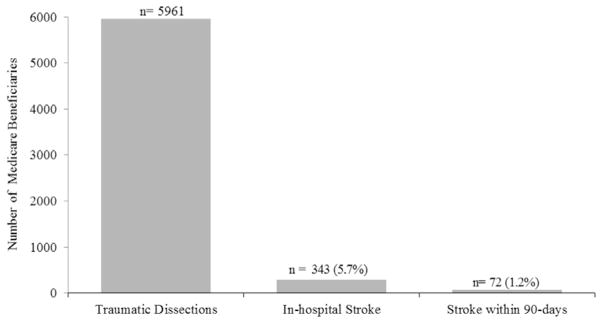
Overall, patients admitted with a traumatic carotid or vertebral dissection had a risk of in-hospital stroke of 5.7%, and few patients (1.2%) experienced stroke in the 90 days after hospital discharge (Fig. 3).
Fig. 3.
In-hospital and 90-day stroke rates among Medicare beneficiaries with traumatic carotid or vertebral artery dissection, 2001–2012.
Secondary Outcomes: Mortality and Readmission
In-hospital mortality among patients has changed little for patients with traumatic carotid or vertebral dissection over the study period. In-hospital mortality has remained at 10.3% from 2001 to 2012 (RR, 1.01; 95% CI, 0.95–1.06; P=0.88). When including data to 90 days (Fig. 1), rates have declined slightly (20–18.5%; RR, 0.93; 95% CI, 0.9–1; P = 0.97). Readmission rates have declined slightly from 20% to 18% in traumatic dissections (RR, 0.88; 95% CI, 0.9–1; P = 0.62). Neither declines in mortality or readmission were statistically significant by either relative rate or linear tests of trend.
Comparison of Stroke Risk between Patients with Traumatic Carotid or Vertebral Dissection and All Admitted Trauma Patients
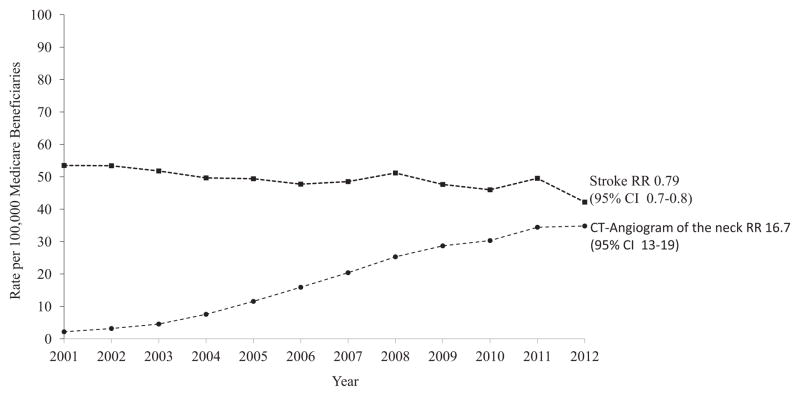
To characterize temporal trends in stroke treatment in trauma patients during this time, we examined the stroke rate among all Medicare beneficiaries admitted after traumatic injury. As with patients with carotid or vertebral dissection, significant increase was found in the use of CT angiography of the neck within this group over the same time period (2–35 per 10,000; RR, 16.7; 95% CI, 13–19; P < 0.0001). However, unlike patients specifically identified with carotid or vertebral artery dissection, where there has been little decline in stroke risk despite a large increase in CT imaging, the increase in cross-sectional neck imaging among all trauma patients has been temporally associated with a 20% decline in stroke rates (54–32 per 100,000; RR, 0.79; 95% CI, 0.7–0.8; P = 0.002; Fig. 4).
Fig. 4.
Trends in imaging and stroke among Medicare beneficiaries admitted with any traumatic injury, 2001–2012.
DISCUSSION
In this study, we found that Medicare patients involved in trauma undergo CT angiography of the neck and brain more than 16 times more commonly than they did a decade ago and are nearly twice as likely to be found to have a carotid or vertebral artery dissection. However, while the rate of stroke has declined nearly 20% overall among all Medicare patients admitted for trauma, little change has occurred in stroke rates for patients with traumatic carotid or vertebral dissections.
Uncertainty surrounding carotid or vertebral dissections has been previously studied. For example, estimates of the prevalence of carotid and vertebral artery dissection among patients admitted for a trauma diagnosis have varied from 0.03% to 4.8%.12 The prevalence of arterial injury in our cohort fell within the lower end (0.08%) of this range, likely due to the use of claims data rather than trauma registries. Furthermore, estimates of stroke rates for this population vary; early reports noted 50–60%,13 but the recent literature quotes the range at 3.6–15%.8 Although most dissection-related strokes occur while in the hospital, the risk of stroke can remain for 3–6 months as the dissection heals.14 Our study found that the stroke rate among patients with traumatic carotid or vertebral artery dissection was around 5%, and only 1% of patients experienced a stroke after hospital discharge. We suspect the decline in stroke rates among all trauma patients mirrors similar declines in the general US population and reflects improved control of stroke-related risk factors.15
Prior studies have suggested that looking harder for carotid or vertebral dissection—especially in trauma patients—would help to limit stroke. For example, published reports of single-institution centers that have noted a marked decrease of stroke in patients diagnosed with these injuries with the implementation of aggressive screening criteria.16,17
However, our national observational analysis failed to replicate these findings. Although imaging use and diagnosis of injury increased, stroke rate did not change. The cause of these differences could be due to our use of population-based data. Our cohort included patients admitted to all levels of hospital not just tertiary level trauma centers. Other population-based sources show similar increases in diagnosis: a weighted national estimate from Healthcare Cost and Utilization Project the diagnosis of carotid or vertebral dissection (traumatic or nontraumatic) has increased almost 9 times since 2002 (2,061–14,030).18 Further analysis could reveal whether stroke rates have remained similarly stagnant in the non-Medicare population. Second, due to the use of claims data, the average age of patients was older than that found in previously published studies. Although younger patients are more likely to experience a traumatic carotid or vertebral artery dissection, the risk of stroke after a traumatic dissection is most closely tied to the degree of vessel injury rather than age. Older patients are more likely to have a low-impact injury, higher mortality, and increased risk of recidivism, and may not benefit from aggressive stroke prevention.19,20
Aggressive use of CT-based imaging is not without cost. Using 2012 Medicare reimbursement schedules, we estimated that $30,576 was spent on imaging per diagnosed dissection in 2002, while in 2012, $117,011 was spent (almost a 4-fold increase). However, the cost of increased imaging is not merely financial but also societal. While Kaye et al.21 noted the use of CT angiography for early diagnosis and prevention of carotid or vertebral artery dissection-related stroke to be cost-effective, this finding assumes that dissection-related strokes are preventable. Mayberry et al. conducted a decade-long retrospective review at 2 level-1 trauma centers without screening protocols and found that while his stroke rate among patient who had a carotid artery dissection was 65%, with 40% of dissection-related strokes occurring before admission. He suggested that screening protocols based on injuries had a low potential for stroke prevention.22 Likewise, Stein et al.8 found in a review of 200 injuries at a level-1 trauma center with an aggressive screening protocol that 44% had infarcts at admission and 30% had contraindications to therapy. Aggressive screening protocols also tend to identify lower-grade asymptomatic injuries that may heal spontaneously and never pose significant risk of stroke. For example, in studies comparing angiography to CTA-based screening guidelines, Goodwin et al. and Paulus et al. noted that most injuries identified were low-grade (39% grade 1, 27% grade 2, 59.4% grade 1, and 18.8% grade 2).5,23 In his initial work, Biffl et al.24 notes that 63% of grade 1 injuries heal regardless of therapy. Higher-grade injuries are more likely to be symptomatic, and aggressive screening protocols may do little to identify injuries that would benefit from treatment.
Our results, while observational, suggest that the incidental findings of carotid or vertebral artery dissection in trauma patients has been not been associated with changes in clinical outcomes, and therefore, protocols to specifically search for these injuries may be unwarranted. The unchanged stroke rates within this cohort is the more notable in light of decreasing stroke rates within the trauma population overall. Strokes due to carotid or vertebral artery dissection represent only a small fraction of strokes within the trauma population, yet do not seem to be as treatable. Future work is needed to better understand which patients need screening for carotid or vertebral dissection, and perhaps which patients might not benefit from the excess radiation, imaging, and cost.
Limitations
Our study has several limitations. First, due to our use of administrative data, we were unable to distinguish strokes that occurred on presentation or en route from those that occurred during hospitalization. As in all retrospective analyses, the timing of the event is unknown, and the patients could have experienced dissection after an injury or experience injury as a result of dissection-related stroke. Second, we were unable to discern which patient underwent treatment for their carotid or vertebral artery dissection. Because most patients undergo medical treatment, these data are unreliable within the Medicare data set. Further research is needed to elucidate whether more patients are unable to receive treatment or whether the lack of decline in the stroke rate is despite the use of the best medical treatment. Third, the degree or grade of dissection was unknown within these patients. Finally, due to our use of Medicare data, our population is older than previously studied trauma populations. However, older adults represent close to 23% of known traumatic injuries and are the fastest growing of the trauma population.20,25–27 While granular data on patients of all ages would be available through the National Trauma Databank, we chose to use Medicare claims to allow for patient-level follow-up to more fully characterize long- and short-term stroke risk. Further studies using national trauma databases linked with longer-term follow-up data could better clarify the role of these clinical details.
CONCLUSIONS
In summary, our study finds that cross-sectional vascular imaging is used much more often in recent years for Medicare patients admitted to the hospital with traumatic injuries, and the incidence of carotid and vertebral artery dissection has increased by 75% over the past decade. However, the risk of stroke risk has changed little for these patients. This observational evidence suggest that a risk-stratified approach to searching for carotid artery dissection in trauma patients may be beneficial and minimize potential overdiagnosis of carotid dissections in patients who may not benefit from treatment.
Supplementary Material
Acknowledgments
Funding: This study was partially funded through K-08 funding (Philip Goodney, PI), grant number: K08 (NHLBI K08HL05676).
Footnotes
This study was a poster at the Interactive Poster Session (titled: Traumatic carotid and vertebral artery dissections among Medicare beneficiaries: 2001–2012) at the Society for Vascular Surgery Vascular Annual Meeting, June 17–20, 2015.
Authors’ contributions: Design and interpretation of data were done by P.P.G., K.N., and D.H.S. Data acquisition and data analysis were carried out by D.J.G. and K.N.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.avsg.2016.06.001.
References
- 1.Biffl WL, Moore EE, Ryu RK, et al. The unrecognized epidemic of blunt carotid arterial injuries–early diagnosis improves neurologic outcome. Ann Surg. 1998;228:462–9. doi: 10.1097/00000658-199810000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cogbill TH, Moore EE, Meissner M, et al. The spectrum of blunt injury to the carotid-artery–a multicenter perspective. J Trauma. 1994;37:473–9. doi: 10.1097/00005373-199409000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Utter GH, Hollingworth W, Hallam DK, et al. Sixteen-slice CT angiography in patients with suspected blunt carotid and vertebral artery injuries. J Am Coll Surg. 2006;203:838–48. doi: 10.1016/j.jamcollsurg.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Anaya C, Munera F, Bloomer CW, et al. Screening multidetector computed tomography angiography in the evaluation on blunt neck injuries: an evidence-based approach. Semin Ultrasound CT MR. 2009;30:205–14. doi: 10.1053/j.sult.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin RB, Beery PR, 2nd, Dorbish RJ, et al. Computed tomographic angiography versus conventional angiography for the diagnosis of blunt cerebrovascular injury in trauma patients. J Trauma. 2009;67:1046–50. doi: 10.1097/TA.0b013e3181b83b63. [DOI] [PubMed] [Google Scholar]
- 6.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344:898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- 7.Biousse V, D’Anglejan-Chatillon J, Touboul PJ, et al. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995;26:235–9. doi: 10.1161/01.str.26.2.235. [DOI] [PubMed] [Google Scholar]
- 8.Stein DM, Boswell S, Sliker CW, et al. Blunt cerebrovascular injuries: does treatment always matter? J Trauma. 2009;66:132–43. doi: 10.1097/TA.0b013e318142d146. discussion 43–44. [DOI] [PubMed] [Google Scholar]
- 9.Edwards NM, Fabian TC, Claridge JA, et al. Antithrombotic therapy and endovascular stents are effective treatment for blunt carotid injuries: results from longterm followup. J Am Coll Surg. 2007;204:1007–13. doi: 10.1016/j.jamcollsurg.2006.12.041. [DOI] [PubMed] [Google Scholar]
- 10.Ramadan F, Rutledge R, Oller D, et al. Carotid-artery trauma–a review of contemporary trauma center experiences. J Vasc Surg. 1995;21:46–56. doi: 10.1016/s0741-5214(95)70243-1. [DOI] [PubMed] [Google Scholar]
- 11.Clark DE, Osler T, HahnI DR. Statistical Software Components S457028. Boston College Department of Economics; 2010. CDPIC: Stata module to provide methods for translating International Classification of Diseases (Ninth Revision) diagnosis codes into standard injury categories and/or scores. [Google Scholar]
- 12.Harrigan MR, Falola MI, Shannon CN, et al. Incidence and trends in the diagnosis of traumatic extracranial cerebrovascular injury in the nationwide inpatient sample database, 2003–2010. J Neurotrauma. 2014;31:1056–62. doi: 10.1089/neu.2013.3309. [DOI] [PubMed] [Google Scholar]
- 13.Miller PR, Fabian TC, Croce MA, et al. Prospective screening for blunt cerebrovascular injuries: analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236:386–93. doi: 10.1097/01.SLA.0000027174.01008.A0. discussion 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redekop GJ. Extracranial carotid and vertebral artery dissection: a review. Can J Neurol Sci. 2008;35:146–52. doi: 10.1017/s0317167100008556. [DOI] [PubMed] [Google Scholar]
- 15.Koton S, Schneider AC, Rosamond WD, et al. Stroke incidence and mortality trends in us communities, 1987 to 2011. JAMA. 2014;312:259–68. doi: 10.1001/jama.2014.7692. [DOI] [PubMed] [Google Scholar]
- 16.Burlew CC, Biffl WL, Moore EE, et al. Blunt cerebrovascular injuries: redefining screening criteria in the era of noninvasive diagnosis. J Trauma Acute Care Surg. 2012;72:330–5. doi: 10.1097/TA.0b013e31823de8a0. discussion 6–7, quiz 539. [DOI] [PubMed] [Google Scholar]
- 17.Eastman AL, Muraliraj V, Sperry JL, et al. CTA-based screening reduces time to diagnosis and stroke rate in blunt cervical vascular injury. J Trauma. 2009;67:551–6. doi: 10.1097/TA.0b013e3181b84408. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 18.HCUP National Inpatient Sample (NIS) Healthcare Cost and Utilization Project (HCUP) MD: Agency for Healthcare Research and Quality R; 2012. www.hcup-us.ahrq.gov/nisoverview.jsp. [Google Scholar]
- 19.Sterling DA, O’Connor JA, Bonadies J. Geriatric falls: injury severity is high and disproportionate to mechanism. J Trauma. 2001;50:116–9. doi: 10.1097/00005373-200101000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Gubler KD, Maier RV, Davis R, et al. Trauma recidivism in the elderly. J Trauma. 1996;41:952–6. doi: 10.1097/00005373-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kaye D, Brasel KJ, Neideen T, et al. Screening for blunt cerebrovascular injuries is cost-effective. J Trauma. 2011;70:1051–6. doi: 10.1097/TA.0b013e318211857d. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 22.Mayberry JC, Brown CV, Mullins RJ, et al. Blunt carotid artery injury–the futility of aggressive screening and diagnosis. Arch Surg. 2004;139:609–12. doi: 10.1001/archsurg.139.6.609. [DOI] [PubMed] [Google Scholar]
- 23.Paulus EM, Fabian TC, Savage SA, et al. Blunt cerebrovascular injury screening with 64-channel multidetector computed tomography: more slices finally cut it. J Trauma Acute Care Surg. 2014;76:279–83. doi: 10.1097/TA.0000000000000101. discussion 84–85. [DOI] [PubMed] [Google Scholar]
- 24.Biffl WL, Moore EE, Offner PJ, et al. Blunt carotid arterial injuries: implications of a new grading scale. J Trauma. 1999;47:845–53. doi: 10.1097/00005373-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Dimitriou R, Calori GM, Giannoudis PV. Polytrauma in the elderly: specific considerations and current concepts of management. Eur J Trauma Emerg Surg. 2011;37:539–48. doi: 10.1007/s00068-011-0137-y. [DOI] [PubMed] [Google Scholar]
- 26.Calland JF, Ingraham AM, Martin N, et al. Evaluation and management of geriatric trauma: an eastern association for the surgery of trauma practice management guideline. J Trauma Acute Care Surg. 2012;73:S345–50. doi: 10.1097/TA.0b013e318270191f. [DOI] [PubMed] [Google Scholar]
- 27.Maxwell CA, Miller RS, Dietrich MS, et al. The aging of America: a comprehensive look at over 25,000 geriatric trauma admissions to United States hospitals. Am Surg. 2015;81:630–6. doi: 10.1177/000313481508100630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.