Abstract
Untethered robots miniaturized to the length scale of millimeter and below attract growing attention for the prospect of transforming many aspects of health care and bioengineering. As the robot size goes down to the order of a single cell, previously inaccessible body sites would become available for high-resolution in situ and in vivo manipulations. This unprecedented direct access would enable an extensive range of minimally invasive medical operations. Here, we provide a comprehensive review of the current advances in biome dical untethered mobile milli/microrobots. We put a special emphasis on the potential impacts of biomedical microrobots in the near future. Finally, we discuss the existing challenges and emerging concepts associated with designing such a miniaturized robot for operation inside a biological environment for biomedical applications.
Keywords: Biomedical engineering, medical robots, microrobots, minimally invasive surgery
I. INTRODUCTION
One of the highest potential scientific and societal impacts of small-scale (millimeter and submillimeter size) untethered mobile robots would be their healthcare and bioengineering applications. As an alternative to existing tethered medical devices such as flexible endoscopes and catheters, mobile medical milli/microrobots could access complex and small regions of the human body such as gastrointestinal (GI), brain, spinal cord, blood capillaries, and inside the eye while being minimally invasive and could even enable access to unprecedented submillimeter size regions inside the human body, which have not been possible to access currently with any medical device technology [1], [2].
As an alternative to tethered flexible endoscopes used in the GI tract, untethered pill-size, swallowable capsule endoscopes with an on-board camera and wireless image transmission device have been commercialized and used in hospitals (FDA approved) since 2001, which has enabled access to regions of the GI tract that were impossible to access before, and has reduced the discomfort and sedation related work loss issues [3]–[7]. However, capsule endoscopy is limited to passive monitoring of the GI tract via optical imaging as clinicians have no control over the capsule’s position, orientation, and functions. Several groups have been proposing active, robotic capsule endoscopes within the last decade where such devices could be remotely controlled to achieve active imaging and have other medical functions [8]–[13]. In bioengineering, mobile microrobots, due to their ability to manipulate individual biological microentities with high precision repeatedly, could be used as a new scientific study or prototyping tool for tissue engineering (e.g., assembling and controlling the building blocks of regenerated tissues) and cellular biology such as single cell studies by manipulating single non-motile or motile cells.
Reported small-scale biomedical robot sizes use range from tens of micrometers to several centimeters. We can classify such different length scale miniature robots as millirobots and microrobots. We define a mobile microrobot as a mobile robotic system where its untethered mobile component has all dimensions less than 1 mm and larger than 1 μm and its mechanics is dominated by microscale physical forces and effects. Thus, for microrobots, bulk forces such as inertial forces and buoyancy are negligible or comparable to surface area and perimeter related forces such as surface tension, adhesion, viscous forces, friction, and drag. In millirobots, their untethered mobile components have all dimensions less than palm size and larger than 1 mm and macroscale forces such as bulk forces dominate their mechanics. On-board components for milli/microrobots must have overall sizes much smaller than the given robot overall size. Therefore, all onboard robot components such as mechanisms, tools, actuators, sensors, power source, electronics, computation, and wireless communication must be miniaturized down to micron scale. Moreover, for milli/microrobots, such components need to be fabricated by micro/nanofabrication methods, which are different from conventional macroscale machining techniques.
There are two main approaches of designing, building, and controlling mobile medical small-scale robots:
On-board approach: Similar to a typical macroscale mobile robot, the untethered, self-contained and self-propelled miniature robot has all on-board components to operate autonomously or with a remote control.
Off-board approach: The mobile, untethered component of the milli/microrobotic system is externally (off-board) actuated, sensed, controlled, or powered.
Since various commercial on-board components exist for millirobots, on-board approach is possible for millirobots while such components are not readily available for microrobots. Thus, most of the current mobile microrobotics studies in literature have been using the off-board approach, and therefore our microrobotics definition also covers such studies.
In addition to the on-board and off-board approaches, milli/microrobots can be also classified as synthetic and biohybrid. In the former case, the milli/microrobot is made of fully synthetic materials such as polymers, magnetic materials, silicon, composites, elastomers, and metals, while the latter is made of both biological and synthetic materials. biohybrid milli/microrobots are typically integrated with muscle cells such as cardiomyocytes or microorganisms such as bacteria, algae, spermatozoids, and protozoa, and powered by the chemical energy inside the cell or in the environment [14]. They harvest the efficient and robust propulsion, sensing, and control capabilities of biological cells or tissues. Such cells could propel the robot in a given physiologically compatible environment, and sense environmental stimuli to control the robot motion by diverse mechanisms such as chemotaxis, magnetotaxis, galvanotaxis, phototaxis, thermotaxis, and aerotaxis.
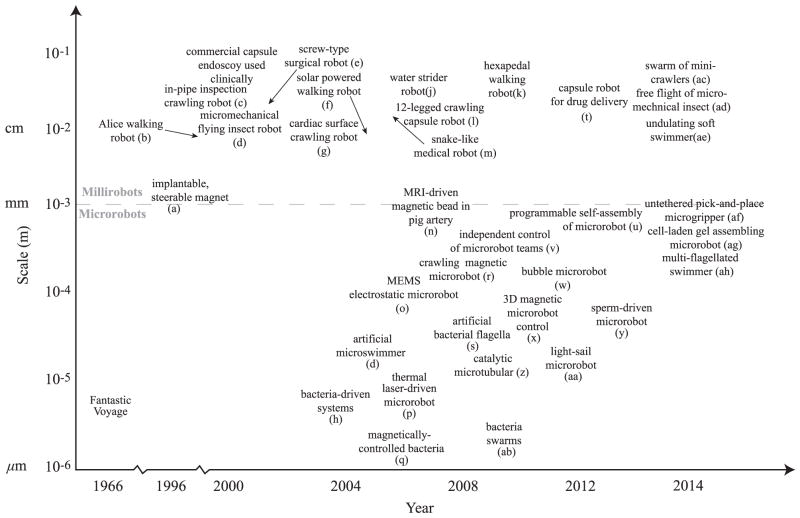
Advances in and increased use of microelectromechanical systems (MEMS) since the 1990s have driven the development of untethered milli/microrobots. MEMS fabrication methods allow for precise features to be made from a wide range of materials, which can be useful for functionalized microrobots. There has been a surge in microrobotics work in the past few years, and the field is relatively new and is growing fast [1], [15]. Fig. 1 presents an overview of a few of the new microrobotic technologies, which have been published, along with their approximate overall size scale.
Fig. 1.
Approximate timeline showing the emerging new milli/microrobot systems with their given overall size scale as significant milestones. (a) Implantable tiny permanent magnet steered by external electromagnetic coils [16]. (b) Alice 1 cm3 walking robot [17]. (c) In-pipe inspection crawling robot [18]. (d) Micromechanical flying insect robot [19]. (e) Screw-type surgical millirobot [20]. (f) Solar powered walking robot [21]. (g) Cardiac surface crawling medical robot [22]. (h) Bacteria-driven biohybrid microrobots [23]. (i) biohybrid magnetic microswimmer [24]. (j) Water strider robot [25]. (k) Hexapedal compliant walking robot [26]. (l) 12-legged crawling capsule robot [27]. (m) Snake-like medical robot [28]. (n) Magnetic bead driven by a Magnetic Resonance Imaging device in pig artery [29]. (o) MEMS electrostatic microrobot [30]. (p) Thermal laser-driven microrobot [31]. (q) Magnetically controlled bacteria [32]. (r) Crawling magnetic microrobot [33]. (s) Magnetic microswimmer inspired by bacterial flagella [34], [35]. (t) Flexible capsule endoscope with drug delivery mechanism [36]. (u) Programmable self-assembly of microrobots [37]. (v) Independent control of microrobot teams [38]. (w) Bubble microrobot [39]. (x) 3D magnetic microrobot control [40]. (y) Sperm-driven biohybrid microrobot [41]. (z) Catalytic microtubular [42]. (aa) Light-sail microrobot [43]. (ab) Bacteria swarms as microrobotic manipulation systems [44]. (ac) Swarm of mini-crawlers [45]. (ad) Free flight of micromechanical insect [46]. (ae) Undulating soft swimmer [47]. (af) Untethered pick-and-place microgripper [48]. (ag) Cell-laden gel assembling microrobot [49]. (ah) Multiflagellated swimmer [50].
The first miniature machines were conceived by Feynman in his lecture on “There’s Plenty of Room at the Bottom” in 1959. In popular culture, the field of milli/microrobotics is familiar to many due to the 1966 sci-fi movie Fantastic Voyage, and later the 1987 movie Inner-space. In these films, miniaturized submarine crews are injected inside the human body and perform noninvasive surgery. The first studies in untethered robots using principles which would develop into milli/microrobot actuation principles were only made recently, such as a magnetic stereotaxis system [16] to guide a tiny permanent magnet inside the human body and a magnetically driven screw which moved through tissue [20]. At the millimeter and centimeter size scale, advances in such millirobots have brought crawling, flying, and swimming devices with increased interest over the last decade. While many developments in millirobots are not directly relevant to biomedical applications, the technologies developed can be used in biomedical millirobots. One major milestone was the creation of centimeter-scale crawling robot with onboard power and computation in 1999 [51]. Micromechanical flying insect robots were first introduced in 2000 [19]. A solar powered crawling robot was introduced in 2004 [21]. Centimeter-scale compliant running robots with onboard power, actuation, and control were advanced with compliant mechanisms in 2008 [26]. Free flight (but with off-board power delivered via wires) mechanical insect-inspired robot was demonstrated in 2013 [46]. The first capsule endoscopes for medical use were used clinically in 2001 under FDA approval. Additional milestones for capsule endoscopy has been the introduction of a crawling mechanism [52] and the introduction of on-board drug delivery mechanism [53].
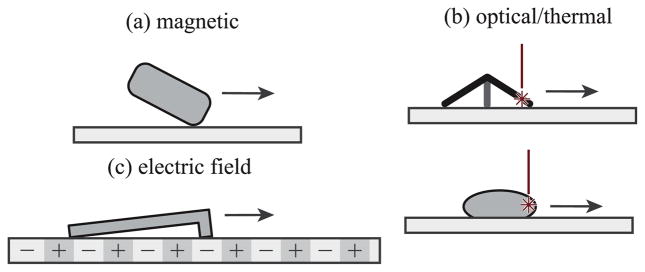
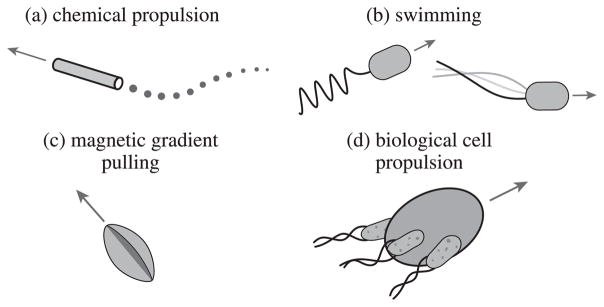
At the submillimeter scale, other significant milestone studies in untethered microrobotics include a study on bacteria-inspired swimming propulsion [54], bacteria-propelled beads [23], [55], steerable electrostatic crawling microrobots [30], catalytic self-propelled microtubular swimmers [24], laser-powered microwalkers [31], magnetic resonance imaging (MRI) device-driven magnetic beads [29], and magnetically driven millimeter-scale nickel robots [56]. These first studies have been followed by other novel actuation methods such as helical propulsion [34], [57], stick-slip crawling microrobots [33], magnetotactic bacteria swarms as microrobots [58], optically driven bubble microrobots [39], and microrobots driven directly by the transfer of momentum from a directed laser spot [43], among others. Figs. 2 and 3 shows a number of the existing approaches to microrobot mobility in the literature for motion in two-dimensions (2D) and three-dimensions (3D). Most of these methods belong to the off-board (remote) microrobot actuation and control approach, and will be discussed in detail later. It is immediately clear that actual microrobots do not resemble the devices shrunk down in popular microrobotics depictions.
Fig. 2.
Some existing off-board approaches to mobile microrobot actuation and control in 2D. (a) Magnetically driven crawling robots include the Mag-μBot [33], the Mag-Mite magnetic crawling microrobot [59], the magnetic microtransporter [60], rolling magnetic microrobot [61], the diamagnetically-levitating mm-scale robot [62], the self-assembled surface swimmer [63], and the magnetic thin-film microrobot [64]. (b) Thermally driven microrobots include the laser-activated crawling microrobot [31], microlight sailboat [43], and the optically controlled bubble microrobot [39]. (c) Electrically driven microrobots include the electrostatic scratch-drive microrobot [65] and the electrostatic microbiorobot [60]. Other microrobots which operate in 2D include the piezoelectric-magnetic microrobot MagPieR [66] and the electrowetting droplet microrobot [67].
Fig. 3.
Some existing off-board and on-board approaches to mobile milli/microrobot actuation and control in 3D. (a) Chemically propelled designs include the microtubular jet microrobot [42] and the electro-osmotic swimmer [68]. (b) Swimming milli/microrobots include the colloidal magnetic swimmer [24], the magnetic thin-film helical swimmer [69], the micron-scale magnetic helix fabricated by glancing angle deposition [35], the microhelix microrobot with cargo carrying cage, fabricated by direct laser writing [70] and the microhelix microrobot with magnetic head, fabricated as thin-film and rolled using residual stress [34]. (c) Milli/microrobots pulled in 3D using magnetic field gradients include the nickel microrobot capable of five-degrees-of-freedom (DOF) motion in 3D using the OctoMag system [40] and the MRI-powered and imaged magnetic bead [71]. (d) Cell-actuated biohybrid approaches include the artificially-magnetotactic bacteria [72], the cardiomyocyte driven microswimmers [73], the chemotactic steering of bacteria-propelled microbeads [74], sperm-driven and magnetically steered microrobots [41], and the magnetotactic bacteria swarm manipulating microscale bricks [44].
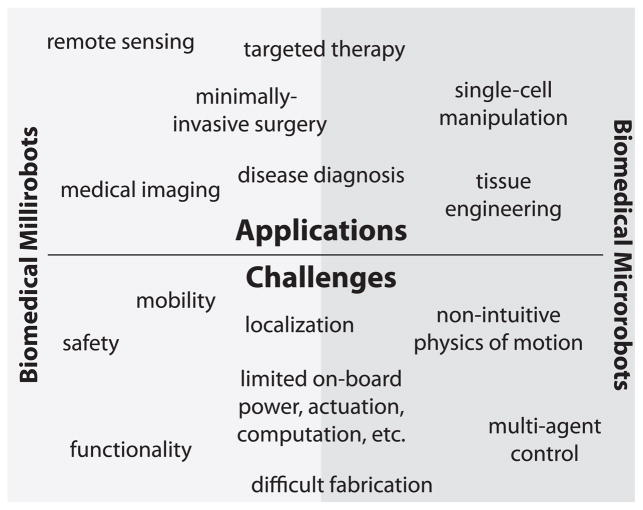
In this review paper, first, existing and potential biomedical applications of mobile millirobots and microrobots are described including a brief case study in each application category, if available. Next, challenges and emerging concepts in miniaturized biomedical robots are presented. Finally, Section IV provides the conclusions and future directions. The material covered in the paper is outlined in schematic form in Fig. 4.
Fig. 4.
Applications and challenges for biomedical milli/microrobots.
II. CURRENT AND POTENTIAL BIOMEDICAL APPLICATIONS OF MILLI/MICROROBOTS
A. Active Visual Imaging for Disease Diagnosis
Active visual (optical) imaging such as endoscopic and laparoscopic techniques is one of the most significant methods to diagnose diseases. While flexible endoscopes and catheters provide visual disease diagnosis currently, they can be invasive and are only for short duration screening purposes. For minimally invasive and implantable (long-duration) visual imaging and accessing small spaces that were not possible to reach before (e.g., small intestines), existing pill-size capsule endoscopes have been becoming a significant alternative [4], [7], [75], [76]. Such commercial pill-size capsule cameras have an on-board camera, a wireless transmission device, and a battery to just take images and send them to an external recording device. Turning such passive imaging devices into capsule millirobots would enable untethered active imaging of hard-to-access areas minimally invasively and for long durations. Therefore, many groups have been proposing robotic capsule millirobots for active imaging using different approaches. Using an on-board actuation approach, miniature motors based on leg or fin mechanisms were used to propel capsule robots inside the GI tract in a controlled manner. Through off-board actuation approach, many groups used remote magnetic actuation to stop, propel, or navigate capsule millirobots in the GI tract [77]. The former approach does not require bulky external devices for actuation while motors consume too much power compared to imaging, which reduces the imaging duration from hours to several minutes. However, external actuation or power transfer does not have such issue while they require bulky equipment around the patient, which would limit her/his motion capability and could be more expensive.
During active imaging, it is important to know the exact 3D location (and orientation) of the millirobot to enable more localized diagnosis and new advanced methods such as 3D visual mapping of the GI tract such as stomach by combining the 3D position information with the 2D camera images. For the localization of millirobots inside the GI tract, as the first approach, medical imaging devices such as fluoroscopy, which uses low-dose X-rays to image the capsule region at 1–2 frames per second [77] ultrasonic imaging [77]–[80], positron emission tomography (PET) [77], [81] and magnetic resonance imaging (MRI) [77]. As an alternative approach, a radio transmitter has been placed on the commercial passive capsule endoscopes, and by placing multiple receiver antennas around the patient, an average position error of approximately 38 mm has been realized [77]. Moreover, by placing a small magnet inside the millirobot, hall-effect sensor arrays outside the patient have been used to localize the device [77], [82]. However, for magnetically actuated capsule robots, hall-effect sensor-based methods get more challenging due to the interference of the magnetic field from the actuating external magnet or electromagnetic coils and the magnet on the capsule robot on the sensor. Several studies addressed this problem and could still enable 3D localization using hall-effect sensors on the capsule [83] or outside the patient’s body [84]. Also, magnetically actuated soft capsule robots with a hall-effect sensor could be localized using the shape change information of the capsule due to the external magnet position [85].
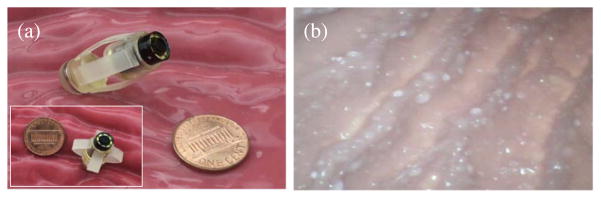
As an example, capsule millirobot, magnetically actuated soft capsule endoscope (MASCE) with an integrated CMOS camera (see Fig. 5 and Table 1) was proposed to actively image stomach type of 3D surfaces using remote magnetic control [53]. Soft design of the capsule body enabled safe operation (i.e., no damage to the tissue due to high stresses), extra degree-of-freedom actuation, and shape changing capability. After swallowing the MASCE and reaching to stomach in several seconds, an external magnet was used to roll it inside stomach for navigation and position control via the two tiny permanent magnets embedded inside it. Several localization methods [84], [85] were proposed to know the 3D position and 2D orientation of the robot precisely during imaging. Inside a surgical phantom stomach model, the feasibility of active imaging using such millirobot was demonstrated in vitro.
Fig. 5.
(a) Photograph of the prototype (left picture) of an example magnetically actuated capsule millirobot for active imaging inside stomach. A CMOS camera and LED lighting were integrated to the soft capsule robot, which can axially deform due to external magnetic actuation control. (b) An active imaging example (right picture) snapshot of the surgical stomach model from the CMOS camera during its active orientation control by an external magnet.
Table 1.
Specifications of the Example Magnetically Actuated Soft Capsule Millirobot Shown in Fig. 5
| Diameter | 10 mm |
| Length (min/max) | 24 mm/30 mm |
| Weight | 6.1 g |
| Internal magnets | 8 mm diameter × 1 mm long cylindrical NdFeB |
| Body material | Polyurethane elastomer |
Since the currently available smallest CMOS camera with its lens from Awaiba GmbH with reasonable resolution (62,500-pixels) is 1 mm × 1 mm × 1 mm current active imaging functions are only [86] available for milliscale medical robots. Future lower resolution smaller cameras with integrated lighting and lens could enable mobile microrobots to actively image new smaller spaces inside the human body such as bile duct, spinal cord fluid, and brain lobes.
B. Mobile In Situ Sensing for Disease Diagnosis and Health Monitoring
Current passive biomedical sensors can be implanted inside or located outside the human body for continuous monitoring of a patient’s or healthy person’s health condition. Such sensors could measure or detect glucose, pH, temperature, oxygen, viral or bacterial activity, body motion (inertia), balance, blood pressure, respiration, muscle activity, neural activity, pulse rate, etc., in situ to diagnose and inform any abnormal medical condition or pathological activity. Adding remote or on-board mobility and control capability to such sensors by having them on medical milli/microrobots could enable a future mobile medical sensor network inside the human body for active health monitoring. Thus, various mobile sensors could be concurrently deployed with the purpose of patrolling inside the different body parts in a minimally invasive manner. Such important biomedical application of milli/microrobots (other than visual monitoring as given in Section II-A) has not been explored much yet. As a preliminary study, Ergeneman et al. [87] proposed a magnetically controlled untethered magnetic microrobot that could achieve optical oxygen sensing for intraocular measurements inside the eye.
C. Targeted Therapy
Targeted therapy is able to enrich the local concentration of therapeutics such as drugs, mRNA, genes, radioactive seeds, imaging contrast agents, stem cells, and proteins in a specific targeted region inside the body while maintaining minimal side effects in the rest of the body. Moreover, controlling the release kinetics can also modulate the concentration of the drug at the therapeutic window, and thereby prolonging the effect of single dose administration. Mobile milli/microrobots can release such therapeutic biological and chemical substances in a specific target location in precise and controlled amounts so that potential side effects are minimized and stronger amounts of the substances could be delivered for faster and better recovery.
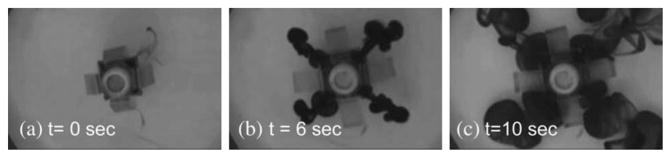
As the main targeted therapy application, small-scale mobile robots have been used for targeted drug delivery in the GI tract, blood vessels, etc. At the millimeter scale, active capsule endoscopes have been used to deliver drugs in the GI tract using passive or active drug release methods [8]. Typical drug delivery capsules use a remotely controlled triggering to move a mechanism that could eject the drug actively for one time in a controlled amount into the target location. Triggering of the drug release mechanism can be achieved by visible light, near-infrared light, ultrasound, or magnetic fields [88]. Also, the Joule electrical heating of a shape memory alloy wire could be used to trigger a drug mechanism [89]. A piston mechanism in a capsule robot was moved by a micromotor based actuation method [90] and by a remotely triggered ignition of the propellant based microthruster [91] for single-use ejection of drugs. An axial compression of a magnetically actuated soft capsule millirobot also enabled controlled ejection of liquid drugs for multiple times inside stomach [53], [92]. Moreover, the same soft capsule robot could change into a spherical like shape inside stomach so that it could stay there for a long time to deliver drugs by passive diffusion [36] as a semi-implantable drug delivery platform. After the drug delivery operation was over, the capsule was taken out by changing back its shape from a spherical shape to a cylindrical one, which enables its disposal naturally by peristalsis. As a specific example, Fig. 6 shows the active ejection of a liquid drug from the soft capsule robot using remote magnetic actuation control.
Fig. 6.
Active drug delivery demonstration of a soft capsule millirobot (see Table 1 for its specifications) inside stomach. (a)–(c) Time snapshots of the drug diffusion during the active compression of the drug chamber with the remote magnetic actuation [92].
At the micron scale, there have been some preliminary studies to use untethered mobile microrobots to deliver drugs or other agents in the vascular system and eye [93]. In relatively larger human arteries with a dimension from 4 to 25 mm with a blood flow velocity from 100 to 400 mm/s, milliscale robots can be pulled or pushed around using magnetic field gradients [94]. Martel et al. proposed the magnetic resonance navigation to actuate a 1.5 mm diameter spherical magnet in swine carotid artery [29]. And the similar system was later used by Pouponneau et al. to deliver doxorubicin through rabbit hepatic artery [95]. In contrast to the system actuated by the spherical permanent magnet, this magnetic navigation system has larger switching rate enabling a closed-loop control [94].
To be able to access to the vessels smaller than arterioles (< 150 μm), rotating magnetic microswimmers with a helical tail, inspired by flagella swimming of bacteria, were proposed for efficient swimming locomotion in low Reynolds number [35], [50], [57]. Such microswimmers can be coated with drugs and deliver them in a target location using passive diffusion [96] or potentially by an active release mechanism. Moreover, several studies proposed biohybrid microrobots where bacteria attached to a cargo such as drug particles or molecules transported the cargo to a desired location [14], [93] by remote control or bacterial sensing of the environment. Here, bacteria behave as on-board microactuators using the chemical energy inside the cell or in the environment and also as on-board microsensors detecting chemical, pH, oxygen, and temperature gradients in the environment [17]. A magnetotactic unipolar MC-1 bacterium could transport up to 70 sub-200 nm diameter liposomes, which encapsulate drugs, without a significant impact to the bacteria’s swimming velocity using the remote magnetic steering control [97]. Also, Carlsen et al. [98] used many chemotactic bacteria to transport potential drug microparticles with embedded superparamagnetic nanoparticles while using remote magnetic fields to control the motion direction of the microparticles to reach to targeted regions before releasing the potential drug cargo. Swimming speed of such bacteria-propelled microparticles with 6 μm diameter was up to 7.3 μm/s under homogenous < 10 mT magnetic fields. Such biohybrid microrobots could be manufactured in large numbers cost effectively and fast, which could enable future targeted drug delivery applications using microrobot swarms (see Fig. 7).
Fig. 7.
Conceptual sketch of a bacteria-propelled biohybrid microrobot swarm, as a dense stochastic network, transporting and delivering drugs on targeted regions inside the stagnant fluid regions of the human body.
D. Minimally Invasive Surgery
In addition to diagnostic and therapeutic applications of milli/microrobots, next level of their medical use could be minimally invasive surgery inside the body. Such surgical operations or functions could be opening clogged vessels or other channels, cauterization, hyperthermia, biopsy, occlusion, electrical stimulation, injection, cutting, drilling, biomaterial removal, or addition at a given target, etc. Only several of these potential applications have been studied before. Many groups proposed integrated biopsy tools for capsule millirobots to collect tissue samples for further disease diagnosis. Kong et al. designed a rotational biopsy device designed to scratch the epithelial tissue [99]. Park et al. proposed a spring-driven biopsy microdevice with microspikes [100], [101]. Simi et al. created a biopsy capsule with a rotational razor that can be activated by a magnetic torsion spring mechanism [102]. These preliminary biopsy capsules have common drawbacks of inaccurate targeting of a certain area and inability of conducting biopsy for multiple times. On the other hand, in their soft capsule millirobot, Yim et al. [12] could release hundreds of untethered microgrippers that could grab tissue stochastically by self-folding due to the increased body temperature, and retrieve the microgrippers with their grabbed tissues inside stomach ex vivo for further genetic analysis. Next, inside the eye, Ullrich et al. tried to puncture a blood vessel close to the retina using the rotational motion of a magnetic millirobot with a sharp tip [103]. Yu et al. [104] and Miloro et al. [105] proposed magnetic millirobots that could be spun remotely by remote rotating magnetic fields to potentially open clogs in blood vessels. In overall, there are only few preliminary minimally invasive surgery studies, which could be extended significantly with many new potential applications inside the circulatory system, brain, spinal cord, and other organs.
E. Tissue Engineering
Many diseases could be treated by precisely delivering the differentiated stem cells and regenerating tissues at the pathological sites [106]. Preliminary research has been done by Kim et al. who designed a cage shape microrobot which is fabricated by stereolithography of negative tone photoresist [107]. Coating the developed polymer structures with Ni/Ti bilayer rendered the microrobot steerable by the magnetic field. By coating the microrobot further with poly-L-lysine, the author could culture human embryonic kidney cell (HEK293) in 3D inside the microrobot, showing the possibility of using it as bio-scaffold to support tissue regeneration [2]. Alternatively, artificial tissues can also be constructed in vitro first and then replace its malfunction in vivo counterparts, and thereby provide a new source for medical transplantation [108]–[110]. One way to achieve artificial tissues is by arranging microscale hydrogels (microgel) laden with different cells into predefined geometries [111]–[113]. For example, Tasoglu et al. [114] functionalized microgel with radical solution in a high magnetic gradient to make it paramagnetic. This enables microgels to be self-assembled into desired shapes under the influence of a uniform magnetic field. After the assembly, the magnetization of microgel could be disabled by vitamin E, so that the free radicals could be eliminated to ensure the proliferation of cells throughout the hydrogel scaffold [114].
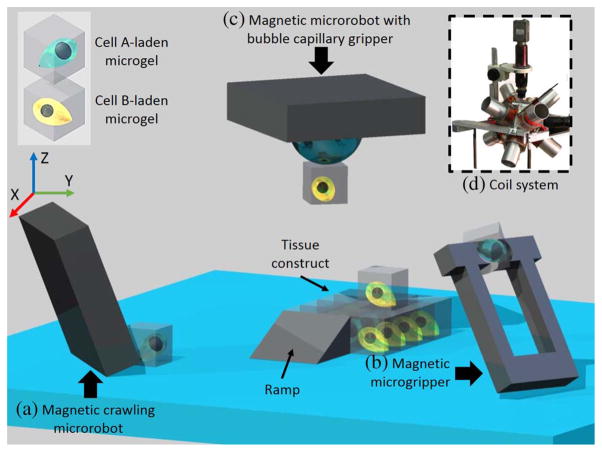
As a more general way, the microrobot can also directly manipulate the non-functionalized microgels into desired geometry. For example, Tasoglu et al. [49] used a crawling magnetic microrobot (750 × 750 × 225 μm3) to push cell laden microgels made of either polyethylene glycol dimethacrylate (PEGDMA) or gelatin methacrylate (GelMA). As shown in Fig. 8(b), the assembly on the upper layer was aided by a microfabricated ramp to elevate the microrobot. In contrast to the conventional manipulation by optical tweezers [115] and dielectrophoresis force [116], this microrobotic approach distinguishes itself by minimally relying on the property of the microobjects. Thus, many different materials could be transported and integrated into tissue construct [49]. This is especially helpful in testing various combinations of different materials to figure out the optimal solution for constructing a specific tissue.
Fig. 8.
3D assembly of cell-laden microgels by different microrobots. (a) Magnetic crawling microrobot [49]. The microgel is pushed by the microrobot to the desired position. A microfabricated ramp is used to elevate the microrobot to higher layer of the tissue constructs. (b) Magnetic microgripper [48]. The jaw is opened and closed by external magnetic field to pick up and release the microobject. (c) Magnetic microrobot with bubble capillary gripper [117]. Changing the pressure inside the working environment can extend and retract the bubble to pick up and release the microobject. (d) Magnetic coil system for microrobot control.
However, it has to be noticed that the microfabricated ramp used could limit the maximum layers of the assembly. To interface microrobot with the conventional tissue culturing dish with flat bottom, the microrobot has to pick up and drop the microgel on top of each other. Diller et al. addressed this by reshaping the magnetic microrobot into a gripper, as shown in Fig. 8(c) [48]. The microgripper jaw was remotely controlled by the magnetic field to clamp and release the microgel. In another work by Giltinan et al. [117], the microobject was picked up by the capillary force on a microbubble nested in the cavity of the magnetic microrobot, as shown in Fig. 8(d). Increasing the pressure inside the working environment could retract the bubble and release the microgel.
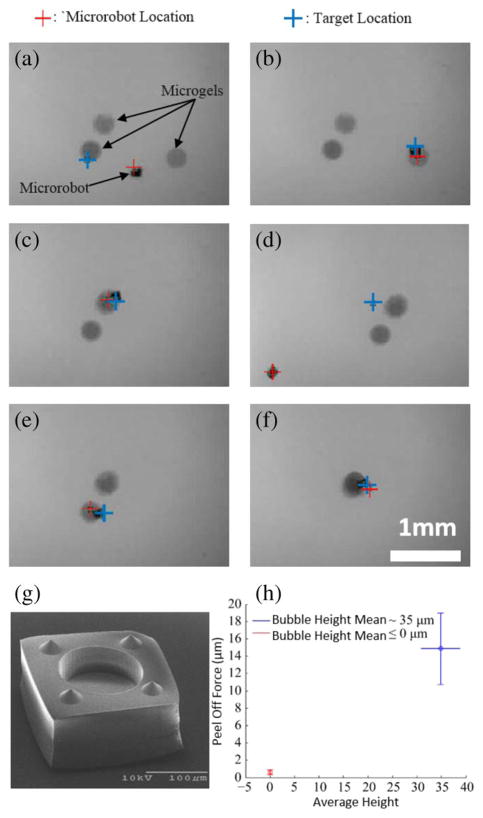
As a specific example, a top-down view of the microrobot manipulating microgel into a stack is shown in Fig. 9(a)–(f). Here, the force required to peel off a silicon substrate using magnetic torque was used as the metric of effectiveness for the capillary gripping magnetic microrobot. While many variables can affect the force required to peel the bubble from the test substrate, Fig. 9(h) shows the peel off forces when the bubble height, measured before the experiment, is less than 0, indicating the bubble is in the cavity, and when the bubble height is approximately 35 μm for a cavity radius of 75 μm. The minimum peel off force average of 0.6 μN and maximum peel off force of 14.9 μN indicate a switching ratio of approximately 25 : 1. The peel off force minimization was aided by surface contact minimizing features, shown on an example microrobot in Fig. 9(g).
Fig. 9.
(a)–(f) Capillary gripping microrobot manipulating hydrogels into a stack as shown from the top-down view. (a) The microrobot position is given by the red cross and the desired position is given by the blue cross. microrobot position control is achieved by a PID controller used to determine the applied magnetic force. The hydrogels are the three circular disks (diameter ~ 350 μm) and the microrobot is a capillary gripping microrobot with a side dimension of 150 μm. (b) The microrobot is directed above the hydrogel and the bubble is drawn out of the cavity by a negative applied pressure in the microrobot workspace. The microrobot is then lowered onto the hydrogel. (c) The microrobot with the hydrogel positions itself over the center hydrogel and comes into contact. (d) The microrobot detaches from the stack of two hydrogels. (e), (f) The process is repeated for the left hydrogel, resulting in a three-hydrogel stack. Scale bar is 1 mm. (g) Example magnetic microrobot with a cavity for bubble-based capillary gripping. The four cones ensure surface contact is minimized when releasing parts. (h) Peel off force versus the average bubble height. The peel off force is calculated as the equivalent force acting on the center of the microrobot due to the applied magnetic torque. The magnetic torque is calculated from the applied uniform magnetic field and the known magnetization of the microrobot. The bubble height is measured from the cavity opening to the highest point of the bubble when it is not in contact with the test substrate. The height of 0 indicates the bubble is completely inside the cavity and there should be no capillary attachment force and is considered to be in the “release” state. Any positive non-zero bubble height will be considered the “pick” state. On a test silicon substrate, the best current work shows an attachment switching ratio of peel off force in the “pick” state to the peel off force in the “release” state of 25 : 1.
F. Cell Manipulation
The biomedical analysis of single cells can differentiate genetic, metabolic and behavior heterogeneity, which pushes the microbiology research to an unprecedented resolution [118], [119]. The single cell manipulation is conventionally done by a micromanipulator, which is a microscale end effector connected to macroscale actuator. This design restricts its access to open channels such as a petri dish [120]. In contrast, untethered microrobots can manipulate cells in enclosed spaces such as microfluidic or other biological chips. Up till now, many different single cell manipulation tasks have been realized by untethered microrobots and are summarized in Table 2. Among these manipulations, microtransportation is the most common operation. Through microtransportation, either single cell can be isolated from its culture for later analysis [4] or drugs can be precisely delivered to a cell network to modulate the intracellular communication [121]. Moreover, random distributed cells can also be re-arranged into desired spatial geometry for the research such as observation of the cancer cell progression [122].
Table 2.
Single Cell Manipulation Studies Conducted by Untethered Microrobots
| Reference | Microrobot Agent | Target Cell | Application |
|---|---|---|---|
| Steager et al. [121] | Magnetic microstructure | Mouse embryo Trypsinized neuron |
Cell transportation Microgel transportation |
| Hu et al. [122] | Bubble driven hydrogel disk | Mouse fibroblast | Cell transportation |
| Kwon et al. [123] | Magnetic microstructure attached with oscillating bubble | Fish egg | Cell transportation |
| Ye and Sitti [124] | Rotating magnetic bead | S. marcescens bacterium | Cell transportation |
| Schurle et al. [125] | Magnetic bead | Macrophage | Test engulfing |
| Hagiwara et al. [126], Feng et al. [127] | Magnetically driven microtool | Bovine oocyte | Cell transportation Cell cutting |
| Kawahara et al. [128] | Magnetically driven microtool | P. Laevis | Physical stimulation |
| Zhang et al. [129] | Ni nanowire | Epidermal cell Blood cell Microorganism with flagella |
Cell transportation Cargo delivery |
| Petit et al. [130] | Self-assembled superparamagnetic microsphere doublet | E. coli bacterium | Cell transportation |
While manipulation of immotile cell is relative easy, manipulation of flagellated bacteria is much more challenging, which is conventionally done by optical tweezers with the cell threaten by photo damage [131], [132]. To address this, Ye and Sitti used the rotational flows around the rotating magnetic microparticle to selectively trap S. marcescens bacterium [124]. The authors showed that a uniform magnetic field smaller than 3.5 mT was enough to drive the microparticle and translate it with at a speed up to 100 μm/s.
Besides microtransportation, several other cell manipulations could be achieved by untethered microrobots. For example, a microrobot with force sensor was designed by Kawahara et al. to mechanically stimulate and investigate the P. Laevis response [128]. In the future, such sensor could be used to distinguish abnormal cell by its mechanical properties [133]. Furthermore, Hagiwara et al. proposed a magnetically driven microtools that was able to orient, position and cut single cell [126]. These functions were used to enucleate the oocyte [127]. The authors argued that this method was significantly faster than the conventional mechanical micromanipulators and also caused less damage to the oocyte.
As the next approach, the cellular level manipulation capabilities of the untethered microrobot could be further strengthened to realize more applications as envisioned in Fig. 10. Control method, either on-board or off-board, could be introduced to render the microrobot to be a complete autonomous agent. In this case, a large number of microrobots could be released into the biomedical sample to finish predefined applications such as detecting circulating tumor cells [134] and systematically probing the cellular communication [135].
Fig. 10.
Conceptual figure/illustration showing all potential applications of microrobotic cell manipulation.
III. CHALLENGES AND EMERGING CONCEPTS IN MINIATURIZED BIOMEDICAL ROBOTS
To enable high-impact biomedical applications of miniaturized mobile robots, many fundamental challenges need to be addressed. As the functional robot size goes down to the millimeter scale and below, design, fabrication, and control of these systems require design principles which greatly differ from that of macro scale robotics. Moreover, medical activities inside the human body will require additional tasks such as feedback from the environment and communication with the operator. In this section, we discuss the challenges associated with miniaturization of untethered biomedical robots from their initial design to the preclinical testing steps. We also provide a future outlook toward a solution in light of the recent advances addressing some of these challenges.
A. Design and Modeling
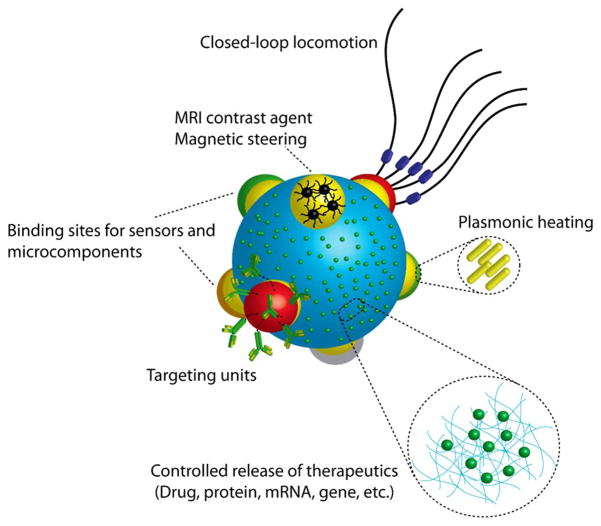
How can we design a mobile milli/microrobot for a specific biomedical task to achieve optimal operational performance, such as the shortest operation duration, minimum power consumption, and largest area coverage, while constrained by software, hardware, manufacturing, motion, control, lifetime, and safety? Given that biological environments are remarkably crowded, the design of physically and chemically, robust, and flexible milli/microrobots is of paramount importance. Such a design requires an integrated strategy where components, locomotion principles, materials, and power sources are considered altogether for functioning via a real-time closed-loop control system (Fig. 11).
Fig. 11.
Visionary design of a soft, modular microrobot with spatio-selective functionalization. Each functional component is assembled on a main board. The main board further serves as a large depot for therapeutics to launch controlled release at the site of action. A closed-loop autonomous locomotion (e.g., a biohybrid design) couples environmental signals to motility. Targeting units enable reaching and localization at the intended body site. MRI contrast agents loaded on the microrobot enables visualization as well as manual steering on demand. Gold nanorods enable plasmonic heating to decompose a tumor tissue.
These design problems can be addressed in many different perspectives. One primary design variable is the number of milli/microrobots: a single multitasking robot versus a team [48], [59], [122], [136] or a swarm [137] of robots with parallel and distributed functions. Considering the potential size of a human tissue or organ, a single microrobot would be insufficient for enough theranostic effect in a given operation, while a microrobot team functioning in a concerted manner could significantly amplify the expected throughput. In the multi-robot perspective, each individual robot could be either identical (i.e., homogeneous) with the same functions or different (i.e., heterogeneous) with varying functionality [48]. The team could move deterministically or stochastically using on-board or off-board (remote) actuation methods [48], [136], [138]–[140]. As locomotion, they could swim, crawl, roll, spin, or hop [30], [33], [50], [61], [136], [138], [141]. They could have integrated micro/nano-sensors, microactuators, and other components such as microcontrollers, power source, wireless communication, etc. [39], [49], [142]–[145].
Programming individual components to spontaneously assemble into fully equipped multifunctional microrobots is a promising design strategy (Fig. 11) [146]. Reprogrammable self-assembly of small components into larger, complex structures is a universal route of material fabrication by biological organisms [147]. Individual components, called building blocks, carry the necessary information for structural integration/disintegration as well as specific biological functions. Despite the complexity of the final ensemble, reprogrammable assembly is a simple and robust strategy for rapid adaptation of the organism to dynamic changes in the environment. Such level of intelligence in biological systems provides a powerful source of inspiration for making similarly complex, synthetic designs, which should be functionally capable of multitasking and autonomously responding to changes in the environmental conditions. Modular assembly of individual micro and nanocomponents could therefore enable flexible customization of optimally working milli/microrobots, which could be manufactured in large quantities in a feasible and reliable way. However, the reprogrammable material concept is still at its infancy, and there is a need for thoroughly understanding and controlling assembly and related processes using simple and robust strategies. A major challenge in macroscopic self-assembly is coding information in individual building blocks. Recent work has addressed this issue by designing self-assembling soft building blocks in various size and shapes [49], [114], [148]–[151]. For example, colloidal patchy particles, which can form directional and programmable interactions in 3D, are among the state-of-the-art examples [152]. By spatio-selective surface modification of individual building blocks, which range in 0.1 to a few micrometers, anisotropic and heterogeneous configuration could inspire similar robot designs based on the self-assembly concept (Fig. 11). In this regard, a similar approach would be useful for larger, i.e., 10 μm–1 mm, building blocks for manufacturing a microrobot. On the other hand, increased particle size creates many non-specific interaction sites, leading to the loss of the directionality and destabilized structural coherence. To this end, high fidelity directional bonding among the building blocks with high overall assembly yield remain as the major challenges to solve. One alternative to this would be remotely picking and then placing individual building blocks to assemble into 2D and 3D structures by the aid of a human operator [49], [114]. However, with this way, interactions between the individual building blocks usually remain weak, which does not support the overall structural integrity and the ensemble tends to fall apart. To surmount this, a secondary covalent cross-linking step is needed [49]. On the other hand, covalent cross-linking is an irreversible process that completely eliminates the intrinsic reprogrammable nature of the final ensemble. Therefore, another future task is to provide bonding stability while maintaining the dynamic nature of the self-assembly and ensure bonding directionality for building prescribed manufacturing of microrobots.
At the system level, real-time interactions and feedback among individual components of a milli/microrobot are essential for proper functioning. For an ideally autonomous microrobot, continuous sensing of the surrounding environment needs to be functionally coupled to mobility, cargo release, powering, and other operational components. Therefore, novel sensing mechanisms that modulate robot behavior would conditionally be able to activate operations. For example, sensing the location of a tumor site and subsequent taxis of microrobots to that location is crucial for carrying out a noninvasive medical operation. However, the major challenge of continuous sensing in the living environment is the unreliable biological signals that might cause false positive or false negatives, thereby leading to unintended microrobot activations. To surmount this problem, molecular logic gates sensing for multiple markers on a conditional basis would enable more accurate operational evaluations by milli/microrobots [153], [154]. In overall, there are alternative design approaches and variables one needs to select correctly for a given application. After developing approximate models of such milli/microrobot systems, rigorous numerical design optimization methods using evolutionary algorithms need to be developed as a significant future challenge.
B. Materials and Fabrication
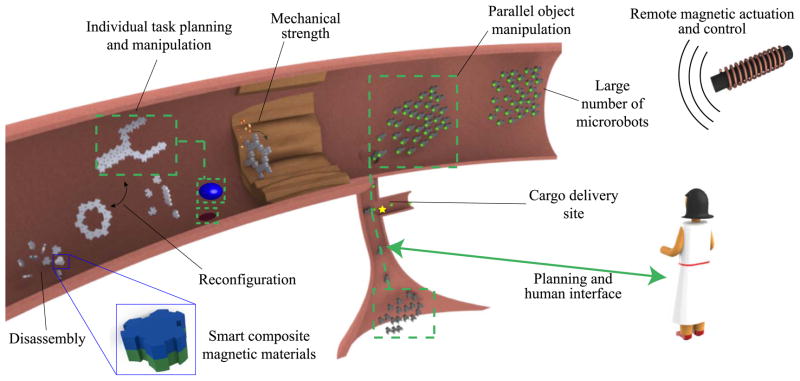
Robots designed to be operating at the small scale is essentially a materials science problem because intelligence of such robots would mainly come from their physical material, structure, mechanism, and design properties. For any material coming into contact with biological fluids need to be resistant to corrosion, as highly saline aqueous environment could easily cause leaching hazardous products from robots as well as causing irreversible robotic malfunctions. Mechanical resilience and durability of milli/microrobots are also highly critical, particularly in large vessels and load-bearing tissues. Inside arteries, for example, high blood flow rate and shear forces can easily disintegrate tiny robots or prevent their motion control [2]. One bioinspired solution toward overcoming that issue might be recapitulation of erythrocyte deformability in milli/microrobots. Erythrocytes can change shape under applied stress without undergoing plastic deformation. There has already been an ongoing effort for developing injectable, shape memory polymers for tissue engineering applications [155], [156]. These materials can be compressed under large mechanical force and then completely recover repeatedly. Such a design could greatly help robust locomotion in blood vessels with changing diameter. For multicomponent systems, surface bonds should also be stable as these interconnections sites are the weakest points under mechanical stress. On top of all of these, robots interacting with biological tissues or working inside the human body must be biocompatible while most of the existing microrobots are made of materials that are not biocompatible. In most biomedical applications, it is crucial to also have novel materials that are soft, biodegradable, multi-functional, smart, and compatible to existing micro and nanofabrication processes. On the other hand, current robot materials are typically rigid, non-biodegradable, and have single function. Creating milli/microrobots from such novel materials require many custom and novel micro/nanoscale fabrication and prototyping tools in 2D and 3D that could be based on optical lithography, two-photon stereo-lithography, self-folding thin-films, micro/nanomachining, micro/nanoimprinting, and micro/nanomolding [49], [138], [151], [157], [158]. Finally, it is crucial to manufacture these robots in large numbers for their potential medical use (Fig. 12). Robot mass-production at the micro/nanoscale is integral for their future commercial applications using roll-to-roll, directed self-assembly, and programmable self-folding methods.
Fig. 12.
Conceptual sketch of a large number of microrobots made of smart materials that can be remotely actuated and controlled inside the human body with a user interface to achieve different biomedical functions.
C. Functionality
In the millimeter scale, although active imaging is possible with current capsule millirobots, this function is primarily used for post-procedure diagnosis. In the future, it is imperative to go beyond this to advanced image processing for diagnosis of visually undetectable disease [159], to map the 3D environment of the given organ using visual simultaneous localization and mapping (SLAM) [160] or optical flow based advanced motion detection algorithms to predict the capsule motion precisely [161], and to propose new active focusing and 3D illumination methods to improve the imaging quality and diagnosis precision [162].
On the micron scale, the only practically available site for microrobot functionalization is its surface. Porous soft materials can also allow cargo encapsulation inside their 3D body. This would be a very useful strategy as it allows higher amount of cargo loading compared to 2D surface. There has been extensive experience over drug encapsulation and release for targeted therapy and controlled-release applications, which might be directly transferred to microrobotic applications [163]–[165]. For this purpose, a whole microrobot can be fabricated as a big cargo depot, which will significantly prolong the impact of single dose administration. In accordance with the special medical requirement, microrobot surface can be modified with operational microtools enabling the sensing of disease diagnosis, therapeutic functions, e.g., targeted drug or gene delivery, and surgical functions, e.g., cauterization and clearing clogged blood vessels. In this sense, mechanical microgrippers could be promising microtools for ablation and biopsy as well as drug/gene delivery [48], [117]. Similar microtools for drilling and heating local tissue sites could profoundly improve noninvasive surgical operations, particularly for removing tumor in deep tissue sites. High throughput or organized operations could find pervasive use in biomedicine. A typical example of microrobot swarms piece-by-piece building tissue scaffolds could revolutionize tissue engineering.
D. Mobility
For the capsule robots, there have been many 2D and 3D locomotion methods proposed. However, there are still many open challenges such as increasing the locomotion precision and speed for accurate and shorter operations, minimizing the power consumption during locomotion, increased safety for not damaging any tissue or not creating any negative reaction from the body, and robust operation against the relative organ motion such as respiration, heart beating, and peristalsis. Also, every person with different age, gender, and race has a different scale and property of biological tissues. Therefore, the given locomotion method could be adapted to such variations robustly.
Possible locomotion modes of untethered biomedical microrobots are swimming in 3D liquid environment and walking, crawling, sliding, spinning, hopping, and rolling on 2D surfaces. Using such locomotion modes, microrobots should be able to navigate in hard-to-reach regions of the human body with high degree of mobility i.e., 6-DOF actuation and high steering capability [166], speed (achieving the tasks in reasonable durations for realistic clinical use), range of motion, penetration depth (i.e., reaching to the deep regions of the body), precision, and autonomy in teams or large numbers. Depending on its given task, a microrobot can have either or both 3D and 2D mobility to reach a specific site inside body. In body sites with low velocity or stagnant fluid flows, swimming and remote directing would be more efficient and faster, whereas in solid tissues or organs, 2D mobility might be the best option to penetrate into deep regions. To this end, autonomously switchable locomotion modes by sensing the body environment are significant challenges. Even so, minimum interaction with solid tissue surface would be desirable to avoid potential irritation and injury-related side effects. Speed control of a mobile microrobot is another critical factor for timely achievement of a given medical task. Synthetic micropropellers harvesting energy from a local source are far from providing a useful locomotion speed even in the unrestricted liquid environment, i.e., without the limitation of a solid tissue barrier. To the best of our knowledge, no micropropeller system has been demonstrated that can move against the blood stream in large vessels due to high-speed blood flow. Despite the fact that biological microorganisms can reach faster speed than synthetic and biomimetic micropropellers, none of the available sources (either natural or synthetic) has inspired for a practically useful speed for biomedical applications. For example, average swimming speed of a flagellum-carrying E. coli is 30 μm s−1 inside water [167]. A biohybrid design involving remote mobilization of magnetotactic bacteria was demonstrated to reach a maximum swimming speed of 200 μm s−1 [137].
Speed control is important for reaching to target site and completing the medical operation. In order to speed up in low Reynolds number, forces acting on the microrobot should be higher. Therefore, there is room for novel micromotor designs that will elevate the efficiency of harvesting local energy source by increasing the micromotor speed. For remotely controlled microrobots, the remote actuation torque or field gradient can simply be tuned to adjust speed [34] while there is a maximum speed limit in magnetic microrobots due to the roll-off behavior depending on the rotational drag properties of the robot.
E. Powering
One of the most significant bottlenecks of untethered mobile milli/microrobots is powering their mobility, sensing, communication, tools, and computation for long enough durations required for a given medical task. Capsule millirobots are powered by silver-oxide coin batteries inside the capsule shell that provide for approximately from 1 min to 8 hours of operation; for example, on-board actuated capsule can last for 1 min when they are actuated all the time, and just on-board imaging and data transfer can last up to 8 hours or so. There is always need for high power density power sources for longer operation durations. On the other hand, minimizing the energy consumption for sensing, locomotion, data transfer, and computation would help such grand challenge. As an alternative solution, wireless power transmission techniques such as inductive powering and radio frequency, microwave radiation, and piezoelectric ultrasound systems are promising options because they are off-board providing space for other modules on the capsule and increasing the operation duration [101]. However, when you scale down the capsule robot size significantly or increase the distance of the device from the power transmitter, such wireless power transfer efficiency goes down exponentially, reducing the provided average power numbers to approximately 1–20 mW.
On the micron scale, especially mobility requires significant amount of power as the motion at low Reynolds numbers could be significantly affected by the viscous drag on the robot body. Moreover, high mechanical power is needed for stable mobility control inside pulsating blood flow. At the sub-millimeter scale, storing, harvesting, and transmitting power is not feasible in the conventional sense we are used to in our macroscopic world. Therefore, a significant effort has been concentrated on various power sources, including remote magnetic, electrical, acoustic, and optical actuation and self-powering, including self-electrophoresis, self-diffusiophoresis, and self-thermophoresis, for microrobot locomotion [24], [168]–[173].
Biological systems have adapted to living in this size domain by storing energy in the form of chemical energy, which is then converted to mechanical motion, sensing, communication and reproduction. Similarly, autonomous microrobots should be powered by available local chemical energy inside the human body. To this end, a proof-of-concept gold-platinum bimetallic nanorod was demonstrated to autonomously move via self-electrophoresis in the presence of 2–3 vol.% H2O2 as the fuel [172]. Translating this technology to the micrometer scale, platinum nanoparticle catalyzed generation of oxygen gas drove motion of polymer stomatocytes at as low as 0.3 vol.% H2O2. Similar conceptual designs were shown to be operational in other liquids containing N,N-dimethyl hydrazine or methanol, though, none of which is close to a biologically relevant environment [174], [175]. To overcome this, a strategy that harnesses locally available sources is crucial. Mano and Heller’s strategy of reacting glucose and oxygen was promising to drive locomotion, though it is only operational at water-oxygen interface and requires high oxygen pressures. Recently, enzyme-powered micropumps have been shown to be viable source of motion in biologically relevant conditions [176].
Energy conversion efficiency is another concept that has so far received little attention. Energy conversion efficiency of microrobots can be described as the ratio of the mechanical power output to the overall work done to drive the motion. The efficiency of the synthetic micro and nano-propellers remain around 1%, significantly lower than macroscopic motors [167]. This might be a limiting step for the overall success of robotic operations.
Altogether, despite some solid progress in self-powering methods for microrobots harvesting the environmental liquids and flows and for sub-millimeter scale robots are still primitive and not directly applicable inside biological environment. It is therefore a great challenge to achieve remote or autonomous microrobot actuation for long durations in a wide range of mobility and inside deep regions of the human body. Maximizing the power efficiency and minimizing the power consumption of microrobotic systems are crucial for long-term medical operations, which could be enabled by optimal design of microrobot’s mobility, sensing, and control methods.
F. Robot Localization
Determining the location and orientation of a medical robot in 3D is crucial for precise and safe motion control inside the human body. Many successful localization methods are available for millirobots [77], [78], [81]–[85], while localization of micron scale medical robots is a great challenge due to their much smaller size [94]. Thus, it is better to design such microrobot systems as swarms and facilitate stronger collective imaging signal [137]. Medical imaging systems such as MRI [71], [137], fluoroscopy [177], PET [178], NIR [179], and ultrasound [178] are possible candidates for microrobot localization. Under these systems, the localization could be registered with the medical images to plan and achieve medical tasks safely. At last, having multi-modal localization methods could enable more precise and safer medical operations [179], [180]. Even very early attempts towards precise localization of inside body will have profound impact in the field.
G. Communication
While many commercial transceivers are available for capsule millirobots, no one has tackled yet the challenge of wireless communication with microrobots inside the human body or communication among large number of microrobots, which could be crucial for data or information transfer from the robots to the doctor and vice versa and microrobot control and coordination. Magnetic actuation was proposed as a promising wireless strategy for cooperative [59], [70], [136] and distributed [48], [136] microrobotic tasks. However, effectiveness of distributed operations via magnetic actuation drastically diminishes with increase in the number of microrobots in the team. Further, magnetic actuation is an open-loop controller, lacking of autonomous decision-making based on real-time sensing of changes in the environment and state of individual microrobots. In this regard, principles that govern the social behaviors of biological microorganisms could be a valuable source of inspiration to address control and coordination of microrobot swarms. Microscopic species exhibit collective behaviors in response to environmental stimuli, which are sensed and transmitted among individual species by physical interactions and/or chemical secretions [181], [182]. Dictyostelium discoideum is a well-known example of such microorganisms, which, upon self-organization into a hierarchical colony with up to 105 residents, can reconfigure itself and migrate as a single unit [183]. Quorum sensing is another cell-to-cell communication process used in bacteria for sharing information among the population and eliciting a collective reaction [184]. An intriguing property of quorum sensing is that the population density is monitored in real-time by the whole colony and a communal response is elicited as a result [184]. This strategy is particularly inspirational for developing a population density-driven switch for microrobot operation inside body. microrobots gathering inside a specific body site and operating only after their population reaches a particular size would be a highly effective strategy.
H. Safety
It is mandatory to guarantee the safety of biomedical milli/microrobots while they are deployed, operated, extracted inside, and removed out of the human body. Such safety is only possible by designing and selecting proper materials and methods for fabrication, actuation, and powering from the very beginning of the system design and integration. Therefore, any robotic component, remote magnetic or other autonomous actuation or sensing methods should be within the FDA limits so that they don’t cause any discomfort, damage, or pain to the patients; synthetic microrobots should be made of biocompatible and biodegradable soft materials; biohybrid (e.g., muscle-cell- or bacteria- actuated) microrobots should not be pathogenic or not create any immunological negative response. Immunogenicity concerns of muscle-cell-actuated microrobots could be successfully evaded by producing functional cells from patient-derived induced pluripotent stem cells (iPSC) [185]. On the other hand, during microrobot fabrication, biohybrid constructs are highly prone to microbial contamination, which should be given a special emphasis [186], [187]. Bacteria-propelled micro robots must be sterilized from any sort of pathogenicity. One safest way is genetically engineering these organisms, so that their proliferation and hazardous by-products are eliminated [188], [189].
While the magnetic strength of the microrobot itself will not present an issue, the magnetic fields used to actuate the microrobot need to be considered [190]. The FDA currently classifies devices with static fields less than 8 T to be of nonsignificant risk. Current medical trials have shown fields upwards of 9.4 T to be safe, not affecting vital signs or cognitive ability [191]. A DC field of 16 T was shown to levitate a frog and other objects due to the weak diamagnetic properties of living tissue, with no observable negative effects [192]. Blood, which is electrically conductive, moving through a static field will generate a back electromotive force (EMF). A field of 10 T is calculated to reduce blood volume flow by 5% due to the effects of the induced voltage, possibly hazardous to susceptible patients [193]. The resulting current is expected to generate the upper limit of safe static fields [190]. However, the fringing fields at the end of an MRI device or solenoid can lead to large spatial magnetic gradients. A time-varying magnetic field or moving conductor will generate an induced current. The spatial magnetic gradient is used to push or pull magnetic microrobots. These spatial gradients will not harm the patient, however any movement will turn these gradients into time-varying magnetic gradients. On the other hand, if the magnetic microrobot is being precisely controlled, the spatial gradients will change with the control of the microrobot, causing time-varying gradients. Spatial gradients are reported for the patient accessible volume of MRI machines to reach several Tesla per meter, but are typically found outside the central bore and the procedures to measure the maximum possible spatial gradient are not well defined [194].
As discussed in Section III-F, there are several possible localization techniques, some of which may pose a risk to the patient. Using MRI to localize the medical microrobot has the same considerations as those above for actuating the microrobot. Imaging techniques based on ionizing radiation, such as fluoroscopy and positron emission tomography are only used when necessary. Fluoroscopy is limited by a patient dose to 88 mGy per minute by the FDA, and can even pose hazards to the operators [191]. Limitations on PET are already set for staff preparing the tracer nucleotide as well as during patient care. Exposure of 10–30 mSv have been reported for patients, and patients which underwent the procedure showed a higher incidence of cancer [195]. Ultrasonic radiation, while generally considered safe, is able to heat tissue and induce cavitation of gas bubbles. The FDA has set limits for beam intensity dependent on the frequency, pulse length, and number of pulses, ranging upwards to 2 W/cm2 for pulsed-averaged intensities [196].
I. Preclinical Assessment Models
For gaining mechanistic insight into behaviors of miniaturized robots in a complex living environment, realistic in vitro medical models/phantoms or freshly acquired organs or tissues are essential. For cargo delivery and controlled release applications, existing tissue engineering models could be adapted for proof-of-concept investigations. In this regard, organ-on-chip technologies could be a valuable platform as the clinical and physiological mimetic of human body environment [197]. In addition to such in vitro testing, it would be crucial to have in vivo small animal proof-of-concept tests to show the preclinical feasibility of the proposed novel concepts.
IV. CONCLUSION
Small-scale untethered mobile robots have a promising future in healthcare and bioengineering applications [198]. They are unrivalled for accessing into small, highly confined and delicate body sites, where conventional medical devices fall short without an invasive intervention. Reconfigurable and modular designs of these robots could also allow for carrying out multiple tasks such as theranostic, i.e., both diagnostic and therapeutic, strategies. Notwithstanding, mobility, powering, and localization are the cardinal challenges that significantly limit the transition of viable robotic designs from in vitro to preclinical stage. An ideal self-powered microrobot that can be actuated autonomously, targeting a specific location to carry out a programmed function by real-time reporting to an outside operator would truly trigger a paradigm shift in clinical practice. Besides, individual robots that can form swarm-like assemblies for parallel and distributed operations would dramatically amplify their expected clinical outcome. Design and fabrication of miniaturized robots, particularly at the submillimeter scale, require a fundamentally different strategy than the existing macroscale manufacturing. Because surface-surface interactions predominate inertial forces, design and manufacturing at this size domain requires an interdisciplinary effort, particularly the involvement of robotic researchers, chemists, biomedical engineers, and materials scientists. Overall, even the currently presented primitive examples of untethered mobile milli/microrobots have opened new avenues in biomedical applications paving the way for minimally invasive and cost-effective strategies, thereby leading to fast recovery and increased quality of life of patients.
Acknowledgments
This work was supported by the NIH R01-NR014083 grant, the NSF Cyber Physical Systems Program (CNS-1135850), and the NSF National Robotics Initiative Program (NRI-1317477).
Biographies

Metin Sitti (Fellow, IEEE) received the B.Sc. and M.Sc. degrees in electrical and electronics engineering from Bogazici University, Istanbul, Turkey, in 1992 and 1994, respectively, and the Ph.D. degree in electrical engineering from the University of Tokyo, Tokyo, Japan, in 1999.
He was a research scientist at the University of California at Berkeley, Berkeley, CA, USA, during 1999–2002. Since 2002, he has been a professor in Department of Mechanical Engineering and Robotics Institute at Carnegie Mellon University, Pittsburgh, PA, USA. In 2014, he became a director in Max-Planck Institute for Intelligent Systems in Stuttgart, Germany. His research interests include physical intelligence, mobile micro-robots, bioinspired materials and miniature robots, medical milli/micro-robotics, and micro/nanomanipulation.
Dr. Sitti received the SPIE Nanoengineering Pioneer Award in 2011 and the National Science Foundation CAREER Award in 2005. He received the IEEE/ASME Best Mechatronics Paper Award in 2014, the Best Poster Award in the Adhesion Conference in 2014, the Best Paper Award in the IEEE/RSJ International Conference on Intelligent Robots and Systems in 2009 and 1998, respectively, the first prize in the World RoboCup micro-robotics Competition in 2012 and 2013, the Best Biomimetics Paper Award in the IEEE Robotics and Biomimetics Conference in 2004, and the Best Video Award in the IEEE Robotics and Automation Conference in 2002. He is the editor-in-chief of Journal of Micro-Bio Robotics.

Hakan Ceylan received the B.S. degree in molecular biology from Bilkent University, Ankara, Turkey, in 2010 and the Ph.D. degree in materials science and nanotechnology from National Nanotechnology Research Center affiliated to Bilkent University in August 2014.
Since September 2014, he has been a postdoctoral researcher in the Max Planck Institute of Intelligent Systems, Stuttgart, Germany. His research interests include self-organization, programmable matter, reconfigurable processes, and bioinspired adhesive interfaces. He is the author of one book chapter and seven research articles.
Dr. Ceylan’s awards and fellowships include Lindau Nobel Laureate Meeting Fellowship (2011), undergraduate and graduate scholarships from the Scientific and Technological Council of Turkey (TUBITAK) in the periods of 2005–2010 and 2010–2014, respectively, and summer research fellowship from Marie Curie Research Institute, Oxted, UK, in 2009. He was a recipient of Ultratech/Cambridge NanoTech 2013 Best Paper Award.

Wenqi Hu (Student Member, IEEE) received the B.S. degree in electrical engineering from the University of Electronic Science and Technology of China, Chengdu, China, in 2005, and the Ph.D. degree in electrical engineering from the University of Hawai’i at Manoã, Honolulu, HI, USA, in 2014.
He is currently working as a postdoctoral researcher at Max Planck Institute for Intelligent Systems, Stuttgart, Germany. His research interest is biomedical applications of micro-robots.
Dr. Hu was the recipient of the University of Hawai’i College of Engineering’s 2014 Outstanding Ph.D. Research Award.

Joshua Giltinan (Student Member, IEEE) is currently pursuing the Ph.D. degree in Department of Mechanical Engineering at Carnegie Mellon University, Pittsburgh, USA.
In 2014, he joined the Physical Intelligence department at the Max-Planck Institute for Intelligent Systems, Stuttgart, Germany.

Mehmet Turan received the Diploma degree in electronics and telecommunication engineering from RWTH Aachen University, Germany in 2011. Since June 2014, he has been a Ph.D. student in the Max Planck Institute for Intelligent Systems, Stuttgart, Germany.
His research interests include computer vision in medical robotics, SLAM, and robot control.

Sehyuk Yim (Student Member, IEEE) received the B.Eng. degree in mechanical engineering, the B.Eng. degree in electronic engineering in 2007, and the M.S. degree in mechanical engineering in 2009, all from Sogang University, Seoul, Korea, and the Ph.D. degree in mechanical engineering from Carnegie Mellon University, Pittsburgh, PA, USA, in December 2012.
He is currently working as a postdoctoral researcher at Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA. His research interests include the design, modeling, and control of bioinspired robots, soft robots for medical applications, modular robots, and programmable matter.

Eric Diller (Member, IEEE) received the B.S. and M.S. degrees in mechanical engineering from the Case Western Reserve University, Cleveland, OH, USA, in 2009. He received the Ph.D. degree in mechanical engineering from Carnegie Mellon University, Pittsburgh, PA, USA, in 2013, where he continued as a postdoctoral researcher. Since 2014, he has been an Assistant Professor in the Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, ON, Canada.
Dr. Diller’s research interests are in microscale robotics and bioinspired novel locomotion systems, fabrication and control relating to remote actuation of microscale devices using magnetic fields, microscale robotic manipulation, smart materials, and swimming at low Reynolds number.
Contributor Information
Metin Sitti, Email: sitti@is.mpg.de, Max-Planck Institute for Intelligent Systems, 70569 Stuttgart, Germany, and also are with Department of Mechanical Engineering, Carnegie Mellon University, Pittsburgh, PA 15238 USA.
Hakan Ceylan, Email: ceylan@is.mpg.de, Max-Planck Institute for Intelligent Systems, 70569 Stuttgart, Germany.
Wenqi Hu, Email: wenqi@is.mpg.de, Max-Planck Institute for Intelligent Systems, 70569 Stuttgart, Germany.
Joshua Giltinan, Email: giltinan@is.mpg.de, Max-Planck Institute for Intelligent Systems, 70569 Stuttgart, Germany, and also are with Department of Mechanical Engineering, Carnegie Mellon University, Pittsburgh, PA 15238 USA.
Mehmet Turan, Max-Planck Institute for Intelligent Systems, 70569 Stuttgart, Germany.
Sehyuk Yim, Email: sam.sehyuk@gmail.com, Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 02139 USA.
Eric Diller, Email: ediller@mie.utoronto.ca, Department of Mechanical and Industrial Engineering, University of Toronto, Toronto, ON M5S3G8, Canada.
References
- 1.Sitti M. Miniature devices: Voyage of the micro-robots. Nature. 2009 Apr;458:1121–1122. doi: 10.1038/4581121a. [DOI] [PubMed] [Google Scholar]
- 2.Nelson BJ, Kaliakatsos IK, Abbott JJ. Micro-robots for minimally invasive medicine. Annu Rev Biomed Eng. 2010 Aug;12:55–85. doi: 10.1146/annurev-bioeng-010510-103409. [DOI] [PubMed] [Google Scholar]
- 3.Hale MF, Sidhu R, McAlindon ME. Capsule endoscopy: Current practice and future directions. World J Gastroenterol. 2014 Jun;20:7752–7759. doi: 10.3748/wjg.v20.i24.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, retention rates of small-bowel capsule endoscopy: A systematic review. Gastrointest Endosc. 2010 Feb;71:280–286. doi: 10.1016/j.gie.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Liao Z, McAlindon M. Handbook of Capsule Endoscopy. Dordrecht, The Netherland: Springer Netherlands; 2014. [Google Scholar]
- 6.Goenka MK, Majumder S, Goenka U. Capsule endoscopy: Present status and future expectation. World J Gastroenterol. 2014 Aug;20:10024–10037. doi: 10.3748/wjg.v20.i29.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura T, Terano A. Capsule endoscopy: Past, present, future. J Gastroenterol. 2008;43:93–99. doi: 10.1007/s00535-007-2153-6. [DOI] [PubMed] [Google Scholar]
- 8.Munoz F, Alici G, Li W. A review of drug delivery systems for capsule endoscopy. Adv Drug Deliv Rev. 2014 May;71:77–85. doi: 10.1016/j.addr.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Carpi F, Kastelein N, Talcott M, Pappone C. Magnetically controllable gastrointestinal steering of video capsules. IEEE Trans Biomed Eng. 2011 Feb;58:231–234. doi: 10.1109/TBME.2010.2087332. [DOI] [PubMed] [Google Scholar]
- 10.Keller H, et al. Method for navigation and control of a magnetically guided capsule endoscope in the human stomach. Proc. 2012 4th IEEE RAS EMBS Int. Conf. Biomed. Robot. Biomechatron. (BioRob); Rome, Italy. 2012. pp. 859–865. [Google Scholar]
- 11.Mahoney AW, Wright SE, Abbott JJ. Managing the attractive magnetic force between an untethered magnetically actuated tool and a rotating permanent magnet. Proc. 2013 IEEE Int. Conf. Robot. Automation (ICRA); Karlsruhe. 2013. pp. 5366–5371. [Google Scholar]
- 12.Yim S, Gultepe E, Gracias DH, Sitti M. Biopsy using a magnetic capsule endoscope carrying, releasing, retrieving untethered microgrippers. IEEE Trans Biomed Eng. 2014 Feb;61:513–521. doi: 10.1109/TBME.2013.2283369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petruska AJ, Abbott JJ. An omnidirectional electromagnet for remote manipulation. Proc. 2013 IEEE Int. Conf. Robot. Automation (ICRA); Karlsruhe. 2013. pp. 822–827. [Google Scholar]
- 14.Carlsen RW, Sitti M. Biohybrid cell-based actuators for microsystems. Small. 2014 Oct;10:3831–3851. doi: 10.1002/smll.201400384. [DOI] [PubMed] [Google Scholar]
- 15.Sitti M. Microscale and nanoscale robotics systems [Grand Challenges of Robotics] IEEE Robot Autom Mag. 2007;14:53–60. [Google Scholar]
- 16.Meeker DC, Maslen EH, Ritter RC, Creighton FM. Optimal realization of arbitrary forces in a magnetic stereotaxis system. IEEE Trans Magn. 1996;32:320–328. [Google Scholar]
- 17.Caprari G, Balmer P, Piguet R, Siegwart R. The autonomous micro robot ‘alice’: A platform for scientific and commercial applications. Proc. 1998 Int. Symp. Micromechatron. Human Sci. (MHS’98); 1998. pp. 231–235. [Google Scholar]
- 18.Kawahara N, Shibata T, Sasaya T. In-pipe wireless micro-robot. Proc. Photonics East’99; Boston, MA, USA. 1999. pp. 166–171. [Google Scholar]
- 19.Fearing RS, et al. Wing transmission for a micromechanical flying insect. Proc. 2000 IEEE Int. Conf. Robot. Automation (ICRA); San Francisco, CA, USA. 2000. pp. 1509–1516. [Google Scholar]
- 20.Ishiyama K, Sendoh M, Yamazaki A, Arai KI. Swimming micro-machine driven by magnetic torque. Sensor Actuat A-Phys. 2001;91:141–144. [Google Scholar]
- 21.Hollar S, Flynn A, Bellew C, Pister K. Solar powered 10 mg silicon robot. Proc. 2003 IEEE Sixteenth Annu. Int. Conf. Micro Electro Mechanical Systems (MEMS); Kyoto, Japan. 2003. pp. 706–711. [Google Scholar]
- 22.Patronik NA, Zenati MA, Riviere CN. Crawling on the heart: A mobile robotic device for minimally invasive cardiac interventions. Medical Image Computing and Computer-Assisted Intervention-MICCAI 2004; New York, NY, USA: Springer; 2004. pp. 9–16. [Google Scholar]
- 23.Darnton N, Turner L, Breuer K, Berg HC. Moving fluid with bacterial carpets. Biophys J. 2004 Mar;86:1863–1870. doi: 10.1016/S0006-3495(04)74253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreyfus R, et al. Microscopic artificial swimmers. Nature. 2005 Oct;437:862–865. doi: 10.1038/nature04090. [DOI] [PubMed] [Google Scholar]
- 25.Song YS, Sitti M. Surface-tension-driven biologically inspired water strider robots: Theory and experiments. IEEE Trans Robot. 2007;23:578–589. [Google Scholar]
- 26.Hoover AM, Steltz E, Fearing RS. RoACH: An autonomous 2.4 g crawling hexapod robot. Proc. IEEE/RSJ 2008 Int. Conf. Intell. Robots Syst. (IROS); Nice, France. 2008. pp. 26–33. [Google Scholar]
- 27.Quirini M, Webster R, Menciassi A, Dario P. Design of a pill-sized 12-legged endoscopic capsule robot. Proc. 2007 IEEE Int. Conf. Robot. Automation (ICRA); Rome, Italy. 2007. pp. 1856–1862. [Google Scholar]
- 28.Degani A, Choset H, Wolf A, Zenati MA. Highly articulated robotic probe for minimally invasive surgery. Proc. 2006 IEEE Int. Conf. Robot. Automation (ICRA); Orlando, FL, USA. 2006. pp. 4167–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martel S, et al. Automatic navigation of an untethered device in the artery of a living animal using a conventional clinical magnetic resonance imaging system. Appl Phys Lett. 2007;90:114105. [Google Scholar]
- 30.Donald BR, Levey CG, McGray CD, Paprotny I, Rus D. An untethered, electrostatic, globally controllable MEMS micro-robot. J Micromech Microeng. 2006;15:1–15. [Google Scholar]
- 31.Sul OJ, Falvo MR, Taylor RM, Washburn S, Superfine R. Thermally actuated untethered impact-driven locomotive microdevices. Appl Phys Lett. 2006;89:203512. [Google Scholar]
- 32.Martel S. Magnetotactic bacteria as controlled functional carriers in microsystems, microelectronic circuits and interconnections. Proc. 16th Eur. Microelectron. Packaging Conf. (EMPC); Qulu. 2007. [Google Scholar]
- 33.Pawashe C, Floyd S, Sitti M. Modeling and experimental characterization of an untethered magnetic micro-robot. Int J Robot Res. 2009;28:1077–1094. [Google Scholar]
- 34.Zhang L, et al. Characterizing the swimming properties of artificial bacterial flagella. Nano Lett. 2009 Oct;9:3663–3667. doi: 10.1021/nl901869j. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh A, Fischer P. Controlled propulsion of artificial magnetic nanostructured propellers. Nano Lett. 2009 Jun;9:2243–2245. doi: 10.1021/nl900186w. [DOI] [PubMed] [Google Scholar]
- 36.Sehyuk Y, Sitti M. Shape-programmable soft capsule robots for semi-implantable drug delivery. IEEE Trans Robot. 2012;28:1198–1202. [Google Scholar]
- 37.Miyashita S, Diller E, Sitti M. Two-dimensional magnetic micro-module reconfigurations based on inter-modular interactions. Int J Robot Res. 2013;32:591–613. [Google Scholar]
- 38.Pawashe C, Floyd S, Sitti M. Multiple magnetic micro-robot control using electrostatic anchoring. Appl Phys Lett. 2009;94:164108–164108-3. [Google Scholar]
- 39.Hu W, Ishii KS, Ohta AT. Micro-assembly using optically controlled bubble micro-robots. Appl Phys Lett. 2011;99:094103. [Google Scholar]
- 40.Kummer MP, et al. OctoMag: An electromagnetic system for 5-DOF wireless micromanipulation. IEEE Trans Robot. 2010;26:1006–1017. [Google Scholar]
- 41.Magdanz V, Sanchez S, Schmidt OG. Development of a sperm-flagella driven micro-bio-robot. Adv Mater. 2013 Dec;25:6581–6588. doi: 10.1002/adma.201302544. [DOI] [PubMed] [Google Scholar]
- 42.Solovev AA, Mei Y, Bermudez Urena E, Huang G, Schmidt OG. Catalytic microtubular jet engines self-propelled by accumulated gas bubbles. Small. 2009 Jul;5:1688–1692. doi: 10.1002/smll.200900021. [DOI] [PubMed] [Google Scholar]
- 43.Búzás A, et al. Light sailboats: Laser driven autonomous micro-robots. Appl Phys Lett. 2012;101:041111. [Google Scholar]
- 44.Martel S, Mohammadi M. Using a swarm of self-propelled natural micro-robots in the form of flagellated bacteria to perform complex micro-assembly tasks. Proc. 2014 IEEE Int. Conf. Robot. Automation (ICRA); Hong Kong. 2010. pp. 500–505. [Google Scholar]
- 45.Rubenstein M, Cornejo A, Nagpal R. Programmable self-assembly in a thousand-robot swarm. Science. 2014;345:795–799. doi: 10.1126/science.1254295. [DOI] [PubMed] [Google Scholar]
- 46.Ma KY, Chirarattananon P, Fuller SB, Wood RJ. Controlled flight of a biologically inspired, insect-scale robot. Science. 2013 May 3;340:603–607. doi: 10.1126/science.1231806. [DOI] [PubMed] [Google Scholar]
- 47.Diller E, Zhuang J, Lum GZ, Edwards MR, Sitti M. Continuously distributed magnetization profile for millimeter-scale elastomeric undulatory swimming. Appl Phys Lett. 2014;104:174101. [Google Scholar]
- 48.Diller E, Sitti M. Three-dimensional programmable assembly by untethered magnetic robotic micro-grippers. Adv Funct Mater. 2014;24:4397–4404. [Google Scholar]
- 49.Tasoglu S, Diller E, Guven S, Sitti M, Demirci U. Untethered micro-robotic coding of three-dimensional material composition. Nat Commun. 2014 Jan;5:3124. doi: 10.1038/ncomms4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou Y, Regnier S, Sitti M. Rotating magnetic miniature swimming robots with multiple flexible flagella. IEEE Trans Robot. 2014;30:3–13. [Google Scholar]
- 51.Caprari G, Estier T, Siegwart R. Fascination of down scaling-Alice the sugar cube robot. J Micromechatron. 2001;1:177–189. [Google Scholar]
- 52.Quirini M, Menciassi A, Scapellato S, Stefanini C, Dario P. Design and fabrication of a motor legged capsule for the active exploration of the gastrointestinal tract. IEEE/ASME Trans Mechatron. 2008;13:169–179. [Google Scholar]
- 53.Yim S, Sitti M. Design and rolling locomotion of a magnetically actuated soft capsule endoscope. IEEE Trans Robot. 2012;28:183–194. doi: 10.1109/TRO.2013.2266754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Edd J, Payen S, Rubinsky B, Stoller ML, Sitti M. Biomimetic propulsion for a swimming surgical micro-robot. Proc. 2003 IEEE/RSJ Int. Conf. Intelligent Robots Syst. (IROS); Las Vegas, NV, USA. 2003. pp. 2583–2588. [Google Scholar]
- 55.Behkam B, Sitti M. Bacterial flagella-based propulsion and on/off motion control of microscale objects. Appl Phys Lett. 2007;90:023902. [Google Scholar]
- 56.Yesin KB. Modeling and control of untethered biomicro-robots in a fluidic environment using electromagnetic fields. Int J Robot Res. 2006;25:527–536. [Google Scholar]
- 57.Peyer KE, Tottori S, Qiu F, Zhang L, Nelson BJ. Magnetic helical micromachines. Chemistry. 2013 Jan;19:28–38. doi: 10.1002/chem.201203364. [DOI] [PubMed] [Google Scholar]
- 58.Martel S, Tremblay CC, Ngakeng S, Langlois G. Controlled manipulation and actuation of micro-objects with magnetotactic bacteria. Appl Phys Lett. 2006;89:233904. [Google Scholar]
- 59.Frutiger DR, Vollmers K, Kratochvil BE, Nelson BJ. Small, fast, under control: Wireless resonant magnetic micro-agents. Int J Robot Res. 2009 Apr;29:613–636. [Google Scholar]
- 60.Sakar MS, Steager EB, Cowley A, Kumar V, Pappas GJ. Wireless manipulation of single cells using magnetic microtransporters. Proc. 2011 IEEE Int. Conf. Robot. Automation (ICRA); Shanghai, China. 2011. pp. 2668–2673. [Google Scholar]
- 61.Jiang GL, et al. Development of rolling magnetic micro-robots. J Micromech Microeng. 2010;20:085042. [Google Scholar]
- 62.Pelrine R, et al. Diamagnetically levitated robots: An approach to massively parallel robotic systems with unusual motion properties. Proc. 2012 IEEE Int. Conf. Robot. Automation (ICRA); St. Paul, MN, USA. 2012. pp. 739–744. [Google Scholar]
- 63.Snezhko A, Aranson IS. Magnetic manipulation of self-assembled colloidal asters. Nat Mater. 2011 Sep;10:698–703. doi: 10.1038/nmat3083. [DOI] [PubMed] [Google Scholar]
- 64.Jing W, et al. A magnetic thin film micro-robot with two operating modes. Proc. 2014 IEEE Int. Conf. Robot. Automation (ICRA); Hong Kong. 2011. pp. 96–101. [Google Scholar]
- 65.Donald BR, Levey CG, Paprotny I. Planar microassembly by parallel actuation of MEMS micro-robots. J Micromech Microeng. 2008;17:789–808. [Google Scholar]
- 66.Ivan IA, et al. First experiments on MagPieR: A planar wireless magnetic and piezoelectric micro-robot. Proc. 2014 IEEE Int. Conf. Robot. Automation (ICRA); Hong Kong. 2011. pp. 102–108. [Google Scholar]
- 67.Schaler E, Tellers M, Gerratt A, Penskiy I, Bergbreiter S. Toward fluidic micro-robots using electrowetting. Proc. 2012 IEEE Int. Conf. Robot. Automation (ICRA); St. Paul, MN, USA. 2012. pp. 3461–3466. [Google Scholar]
- 68.Hwang G, et al. Electro-osmotic propulsion of helical nanobelt swimmers. Int J Robot Res. 2011;30:806–819. [Google Scholar]
- 69.Yamazaki A, et al. Wireless micro swimming machine with magnetic thin film. J Magn Magn Mater. 2004;272–276:E1741–E1742. [Google Scholar]
- 70.Tottori S, et al. Magnetic helical micromachines: Fabrication, controlled swimming, cargo transport. Adv Mater. 2012 Feb;24:811–816. doi: 10.1002/adma.201103818. [DOI] [PubMed] [Google Scholar]
- 71.Martel S, et al. MRI-based medical nanorobotic platform for the control of magnetic nanoparticles and flagellated bacteria for target interventions in human capillaries. Int J Rob Res. 2009 Sep;28:1169–1182. doi: 10.1177/0278364908104855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim DH, Kim PSS, Julius AA, Kim MJ. Three-dimensional control of Tetrahymena pyriformis using artificial magnetotaxis. Appl Phys Lett. 2012;100:053702. [Google Scholar]
- 73.Williams BJ, Anand SV, Rajagopalan J, Saif MT. A self-propelled biohybrid swimmer at low Reynolds number. Nat Commun. 2014;5:3081. doi: 10.1038/ncomms4081. [DOI] [PubMed] [Google Scholar]
- 74.Kim DH, Kim PSS, Julius AA, Kim MJ. Three-dimensional control of engineered motile cellular micro-robots. Proc. 2012 IEEE Int. Conf. Robot. Automation (ICRA); St. Paul, MN, USA. 2012. [Google Scholar]
- 75.Ciuti G, Menciassi A, Dario P. Capsule endoscopy: From current achievements to open challenges. IEEE Rev Biomed Eng. 2011;4:59–72. doi: 10.1109/RBME.2011.2171182. [DOI] [PubMed] [Google Scholar]
- 76.Pan G, Wang L. Swallowable wireless capsule endoscopy: Progress and technical challenges. Gastroenterol Res Pract. 2012;2012:841691. doi: 10.1155/2012/841691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Than TD, Alici G, Zhou H, Li W. A review of localization systems for robotic endoscopic capsules. IEEE Trans Biomed Eng. 2012 Sep;59:2387–2399. doi: 10.1109/TBME.2012.2201715. [DOI] [PubMed] [Google Scholar]
- 78.Fluckiger M, Nelson BJ. Ultrasound emitter localization in heterogeneous media. Proc. 29th Annu. Int. Conf. IEEE Eng. Medicine Biology Society; Lyon, France. 2007. pp. 2867–2870. [DOI] [PubMed] [Google Scholar]
- 79.Rubin JM, et al. Sonographic elasticity imaging of acute and chronic deep venous thrombosis in humans. J Ultrasound Med. 2006 Sep;25:1179–1186. doi: 10.7863/jum.2006.25.9.1179. [DOI] [PubMed] [Google Scholar]
- 80.Kim K, et al. Noninvasive ultrasound elasticity imaging (UEI) of Crohn’s disease: Animal model. Ultrasound Med Biol. 2008 Jun;34:902–912. doi: 10.1016/j.ultrasmedbio.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Than TD, et al. An effective localization method for robotic endoscopic capsules using multiple positron emission markers. IEEE Trans Robot. 2014;30:1174–1186. doi: 10.1118/1.4881316. [DOI] [PubMed] [Google Scholar]
- 82.Di Natali C, Beccani M, Valdastri P. Real-time pose detection for magnetic medical devices. IEEE Trans Magn. 2013;49:3524–3527. [Google Scholar]
- 83.Hu C, Meng MQH, Mandal M. Efficient magnetic localization and orientation technique for capsule endoscopy. Int J Inform Acquisition. 2005;2:23–36. [Google Scholar]
- 84.Soon D, Yim S, Sitti M. A 5-D localization method for a magnetically manipulated untethered robot using a 2-D array of Hall-effect sensors. doi: 10.1109/TMECH.2015.2488361. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yim S, Sitti M. 3-D localization method for a magnetically actuated soft capsule endoscope and its applications. IEEE Trans Robot. 2013 Jun;29:1139–1151. doi: 10.1109/TRO.2013.2266754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.AIWABA. [Online]. Available: http://www.awaiba.com/product/naneye/
- 87.Ergeneman O, Dogangil G, Kummer MP, Abbott JJ, Nazeeruddin MK, Nelson BJ. A magnetically controlled wireless optical oxygen sensor for intraocular measurements. IEEE Sensors J. 2008;8:29–37. [Google Scholar]
- 88.Timko BP, Dvir T, Kohane DS. Remotely triggerable drug delivery systems. Adv Mater. 2010 Nov;22:4925–4943. doi: 10.1002/adma.201002072. [DOI] [PubMed] [Google Scholar]
- 89.Woods SP, Constandinou TG. Wireless capsule endoscope for targeted drug delivery: Mechanics and design considerations. IEEE Trans Biomed Eng. 2013 Apr;60:945–953. doi: 10.1109/TBME.2012.2228647. [DOI] [PubMed] [Google Scholar]
- 90.Cui J, Zheng X, Hou W, Zhuang Y, Pi X, Yang J. The study of a remote-controlled gastrointestinal drug delivery and sampling system. Telemed J E Health. 2008 Sep;14:715–719. doi: 10.1089/tmj.2007.0118. [DOI] [PubMed] [Google Scholar]
- 91.Xitian P, Hongying L, Kang W, Yulin L, Xiaolin Z, Zhiyu W. A novel remote controlled capsule for site-specific drug delivery in human GI tract. Int J Pharm. 2009 Dec;382:160–164. doi: 10.1016/j.ijpharm.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 92.Yim S, Goyal K, Sitti M. Magnetically actuated soft capsule with the multimodal drug release function. IEEE ASME Trans Mechatron. 2013 Jan;18:1413–1418. doi: 10.1109/TMECH.2012.2235077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martel S. Bacterial microsystems and micro-robots. Biomed Microdevices. 2012 Dec;14:1033–1045. doi: 10.1007/s10544-012-9696-x. [DOI] [PubMed] [Google Scholar]
- 94.Martel S. micro-robotics in the vascular network: Present status and next challenges. J Micro-Bio Robot. 2013;8:41–52. [Google Scholar]
- 95.Pouponneau P, Leroux JC, Soulez G, Gaboury L, Martel S. Co-encapsulation of magnetic nanoparticles and doxorubicin into biodegradable microcarriers for deep tissue targeting by vascular MRI navigation. Biomaterials. 2011 May;32:3481–3486. doi: 10.1016/j.biomaterials.2010.12.059. [DOI] [PubMed] [Google Scholar]
- 96.Fusco S, Chatzipirpiridis G, Sivaraman KM, Ergeneman O, Nelson BJ, Pane S. Chitosan electrodeposition for micro-robotic drug delivery. Adv Healthc Mater. 2013 Jul;2:1037–1044. doi: 10.1002/adhm.201200409. [DOI] [PubMed] [Google Scholar]
- 97.Martel S, Taherkhani S, Tabrizian M, Mohammadi M, de Lanauze D, Felfoul O. Computer 3D controlled bacterial transports and aggregations of microbial adhered nano-components. J Micro-Bio Robot. 2014;9:23–28. [Google Scholar]
- 98.Carlsen RW, Edwards MR, Zhuang J, Pacoret C, Sitti M. Magnetic steering control of multi-cellular biohybrid microswimmers. Lab Chip. 2014 Oct;14:3850–3859. doi: 10.1039/c4lc00707g. [DOI] [PubMed] [Google Scholar]
- 99.Kong K, Cha J, Jeon D, Cho D. A rotational micro biopsy device for the capsule endoscope. Proc. 2005 IEEE/RSJ Int. Conf. Intell. Robots Syst., 2005 (IROS 2005); Edmonton, AB, Canada. 2005. pp. 1839–1843. [Google Scholar]
- 100.Park S, et al. A novel micro-biopsy actuator for capsular endoscope using LIGA process. Proc. 2007 Solid-State Sensors, Actuators and Microsystems Conf; Lyon. 2007. p. 209. [Google Scholar]
- 101.Park S, et al. A novel microactuator for microbiopsy in capsular endoscopes. J Micromech Microeng. 2008;18 [Google Scholar]
- 102.Simi M, Gerboni G, Menciassi A, Valdastri P. Magnetic torsion spring mechanism for a wireless biopsy capsule. J Med Devices. 2013;7:041009. [Google Scholar]
- 103.Ullrich F, et al. Mobility experiments with micro-robots for minimally invasive intraocular surgery. Invest Ophthalmol Vis Sci. 2013 Apr;54:2853–2863. doi: 10.1167/iovs.13-11825. [DOI] [PubMed] [Google Scholar]
- 104.Yu C, et al. Novel electromagnetic actuation system for three-dimensional locomotion and drilling of intravascular micro-robot. Sensor Actuat A-Phys. 2010;161:297–304. [Google Scholar]
- 105.Miloro P, Sinibaldi E, Menciassi A, Dario P. Removing vascular obstructions: A challenge, yet an opportunity for interventional microdevices. Biomed Microdevices. 2012 Jun;14:511–532. doi: 10.1007/s10544-011-9627-2. [DOI] [PubMed] [Google Scholar]
- 106.Fox IJ, Daley GQ, Goldman SA, Huard J, Kamp TJ, Trucco M. Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science. 2014 Aug;345:1247391. doi: 10.1126/science.1247391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim S, et al. Fabrication and characterization of magnetic micro-robots for three-dimensional cell culture and targeted transportation. Adv Mater. 2013 Nov;25:5863–5868. doi: 10.1002/adma.201301484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Nat Acad Sci USA. 2006 Feb;103:2480–2487. doi: 10.1073/pnas.0507681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Masuda S, Shimizu T, Yamato M, Okano T. Cell sheet engineering for heart tissue repair. Adv Drug Deliv Rev. 2008 Jan;60:277–285. doi: 10.1016/j.addr.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 110.Elbert DL. Bottom-up tissue engineering. Curr Opin Biotechnol. 2011 Oct;22:674–680. doi: 10.1016/j.copbio.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ling Y, et al. A cell-laden microfluidic hydrogel. Lab Chip. 2007 Jun;7:756–762. doi: 10.1039/b615486g. [DOI] [PubMed] [Google Scholar]
- 112.Zorlutuna P, et al. Microfabricated biomaterials for engineering 3D tissues. Adv Mater. 2012 Apr;24:1782–1804. doi: 10.1002/adma.201104631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu JS, Gartner ZJ. Directing the assembly of spatially organized multicomponent tissues from the bottom up. Trends Cell Biol. 2012 Dec;22:683–691. doi: 10.1016/j.tcb.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tasoglu S, et al. Guided and magnetic self-assembly of tunable magnetoceptive gels. Nat Commun. 2014;5:4702. doi: 10.1038/ncomms5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mirsaidov U, et al. Live cell lithography: Using optical tweezers to create synthetic tissue. Lab Chip. 2008 Dec;8:2174–2181. doi: 10.1039/b807987k. [DOI] [PubMed] [Google Scholar]
- 116.Albrecht DR, Underhill GH, Wassermann TB, Sah RL, Bhatia SN. Probing the role of multicellular organization in three-dimensional microenvironments. Nat Methods. 2006 May;3:369–375. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- 117.Giltinan J, Diller E, Mayda C, Sitti M. Three-dimensional robotic manipulation and transport of micro-scale objects by a magnetically driven capillary micro-gripper. Proc. IEEE Int. Conf. Robot. Automation (ICRA); Hong Kong. 2014. pp. 2077–2082. [Google Scholar]
- 118.Di Carlo D, Tse HTK, Gossett DR. Introduction: Why Analyze Single Cells? In: Lindström S, Andersson-Svahn H, editors. Single-Cell Analysis. Vol. 853. Upper Saddle River, NJ, USA: Humana Press; 2012. pp. 1–10. [Google Scholar]
- 119.Brehm-Stecher BF, Johnson EA. Single-cell microbiology: Tools, technologies, applications. Microbiol Mol Biol Rev. 2004 Sep;68:538–559. doi: 10.1128/MMBR.68.3.538-559.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu Z, Moraes C, Ye G, Simmons CA, Sun Y. Single cell deposition and patterning with a robotic system. PLoS One. 2010;5:e13542. doi: 10.1371/journal.pone.0013542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steager EB, et al. Automated biomanipulation of single cells using magnetic micro-robots. Int J Robot Res. 2013;32:346–359. [Google Scholar]
- 122.Hu W, Ishii KS, Fan Q, Ohta AT. Hydrogel micro-robots actuated by optically generated vapour bubbles. Lab Chip. 2012 Oct;12:3821–3826. doi: 10.1039/c2lc40483d. [DOI] [PubMed] [Google Scholar]
- 123.Kwon JO, Yang JS, Chae JB, Chung SK. Micro-object manipulation in a microfabricated channel using an electromagnetically driven micro-robot with an acoustically oscillating bubble. Sensor Actuat A-Phys. 2014;215:77–82. [Google Scholar]
- 124.Ye Z, Sitti M. Dynamic trapping and two-dimensional transport of swimming microorganisms using a rotating magnetic micro-robot. Lab Chip. 2014 Jul;14:2177–2182. doi: 10.1039/c4lc00004h. [DOI] [PubMed] [Google Scholar]
- 125.Schurle S, et al. Three-dimensional, automated magnetic biomanipulation with subcellular resolution. Proc. 2013 IEEE Int. Conf. Robot. Automation (ICRA); Karlsruhe, Germany. pp. 1452–1457. [Google Scholar]
- 126.Hagiwara M, et al. On-chip magnetically actuated robot with ultrasonic vibration for single cell manipulations. Lab Chip. 2011 Jun;11:2049–2054. doi: 10.1039/c1lc20164f. [DOI] [PubMed] [Google Scholar]
- 127.Feng L, Hagiwara M, Ichikawa A, Arai F. On-chip enucleation of bovine oocytes using micro-robot-assisted flow-speed control. Micromachines. 2013;4:272–285. [Google Scholar]
- 128.Kawahara T, et al. On-chip micro-robot for investigating the response of aquatic microorganisms to mechanical stimulation. Lab Chip. 2013 Mar;13:1070–1078. doi: 10.1039/c2lc41190c. [DOI] [PubMed] [Google Scholar]
- 129.Zhang L, Petit T, Peyer KE, Nelson BJ. Targeted cargo delivery using a rotating nickel nanowire. Nanomedicine. 2012 Oct;8:1074–1080. doi: 10.1016/j.nano.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 130.Petit T, Zhang L, Peyer KE, Kratochvil BE, Nelson BJ. Selective trapping and manipulation of microscale objects using mobile microvortices. Nano Lett. 2012 Jan;12:156–160. doi: 10.1021/nl2032487. [DOI] [PubMed] [Google Scholar]
- 131.Thalhammer G, Steiger R, Bernet S, Ritsch-Marte M. Optical macro-tweezers: Trapping of highly motile micro-organisms. J Optics. 2011;13:044024. [Google Scholar]
- 132.Zhang H, Liu KK. Optical tweezers for single cells. J R Soc Interface. 2008 Jul;5:671–690. doi: 10.1098/rsif.2008.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim DH, Wong PK, Park J, Levchenko A, Sun Y. Microengineered platforms for cell mechanobiology. Annu Rev Biomed Eng. 2009;11:203–233. doi: 10.1146/annurev-bioeng-061008-124915. [DOI] [PubMed] [Google Scholar]
- 134.Hajba L, Guttman A. Circulating tumor-cell detection and capture using microfluidic devices. TrAC Trend Anal Chem. 2014;59:9–16. [Google Scholar]
- 135.Collins CH. Cell-cell communication special issue. ACS Synth Biol. 2014 Apr;3:197–198. doi: 10.1021/sb500231j. [DOI] [PubMed] [Google Scholar]
- 136.Huang TY, et al. Cooperative manipulation and transport of microobjects using multiple helical microcarriers. RSC Adv. 2014;4:26771–26771. [Google Scholar]
- 137.Martel S, Mohammadi M, Felfoul O, Lu Z, Pouponneau P. Flagellated magnetotactic bacteria as controlled MRI-trackable propulsion and steering systems for medical nanorobots operating in the human microvasculature. Int J Rob Res. 2009 Apr;28:571–582. doi: 10.1177/0278364908100924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zeeshan MA, et al. Hybrid helical magnetic micro-robots obtained by 3D template-assisted electrodeposition. Small. 2014;10:1284–1288. doi: 10.1002/smll.201302856. [DOI] [PubMed] [Google Scholar]
- 139.Bergbreiter S, Pister KSJ, editors. Design of an Autonomous Jumping micro-robot. Proc. IEEE Int. Conf. Robot. Automation (ICRA); Rome, Italy. 2007. [Google Scholar]
- 140.Churaman WA, Currano LJ, Morris CJ, Rajkowski JE, Bergbreiter S. The first launch of an autonomous thrust-driven micro-robot using nanoporous energetic silicon. J Microelectromech Syst. 2012;21:198–205. [Google Scholar]
- 141.Jing W, Pagano N, Cappelleri DJ. A novel micro-scale magnetic tumbling micro-robot. J of Micro-Bio Robotics. 2013 Feb;8:1–12. [Google Scholar]
- 142.McNaughton BH, Anker JN, Kopelman R. Magnetic microdrill as a modulated fluorescent pH sensor. J Magn Magn Mater. 2005;293:696–701. [Google Scholar]
- 143.Ergeneman O, et al. Cobalt-nickel microcantilevers for biosensing. J Intell Mater Syst Struct. 2012 Oct;24:2215–2220. [Google Scholar]
- 144.Jing W, Cappelleri DJ. Incorporating in-situ force sensing capabilities in a magnetic micro-robot. Proc. 2014 IEEE/RSJ Int. Conf. Intell. Robots Syst. (IROS 2014); Chicago, IL, USA. 2014. pp. 4704–4709. [Google Scholar]
- 145.Ergeneman O, et al. In vitro oxygen sensing using intraocular micro-robots. IEEE Trans Biomed Eng. 2012 Nov;59:3104–3109. doi: 10.1109/TBME.2012.2216264. [DOI] [PubMed] [Google Scholar]
- 146.Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002 Mar;295:2418–2421. doi: 10.1126/science.1070821. [DOI] [PubMed] [Google Scholar]
- 147.Kushner DJ. Self-assembly of biological structures. Bacteriol Rev. 1969;33:302–345. doi: 10.1128/br.33.2.302-345.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tasoglu S, et al. Paramagnetic levitational assembly of hydrogels. Adv Mater. 2013;25:1137–1143. doi: 10.1002/adma.201200285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu F, et al. The assembly of cell-encapsulating microscale hydrogels using acoustic waves. Biomaterials. 2011 Jul;32:7847–7855. doi: 10.1016/j.biomaterials.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu F, et al. Three-dimensional magnetic assembly of microscale hydrogels. Adv Mater. 2011;23:4254–4260. doi: 10.1002/adma.201101962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qi H, et al. DNA-directed self-assembly of shape-controlled hydrogels. Nat Commun. 2013 Sep;4 doi: 10.1038/ncomms3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yi GR, Pine DJ, Sacanna S. Recent progress on patchy colloids and their self-assembly. J Phys: Condensed Matter. 2013;25:193101. doi: 10.1088/0953-8984/25/19/193101. [DOI] [PubMed] [Google Scholar]
- 153.de Silva PA, Gunaratne NHQ, McCoy CP. A molecular photoionic AND gate based on fluorescent signalling. Nature. 1993;364:42–44. [Google Scholar]
- 154.Ozlem S, Akkaya EU. Thinking outside the silicon box: Molecular and logic as an additional layer of selectivity in singlet oxygen generation for photodynamic therapy. J Amer Chem Soc. 2009 Jan;131:48–49. doi: 10.1021/ja808389t. [DOI] [PubMed] [Google Scholar]
- 155.Bencherif SA, et al. Injectable preformed scaffolds with shape-memory properties. Proc Nat Acad Sci USA. 2012 Nov;109:19 590–19 595. doi: 10.1073/pnas.1211516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Behl M, Lendlein A. Shape-memory polymers. Materials Today. 2007;10:20–28. [Google Scholar]
- 157.Rajurkar KP, et al. Micro and nano machining by electro-physical and chemical processes. CIRP Annals—Manufacturing Technology. 2006;55:643–666. [Google Scholar]
- 158.Shao J, et al. Generation of fully-covering hierarchical micro-/nano- structures by nanoimprinting and modified laser swelling. Small. 2014;10:2595–2601. doi: 10.1002/smll.201303656. [DOI] [PubMed] [Google Scholar]
- 159.Messmann H, Knuchel R, Baumler W, Holstege A, Scholmerich J. Endoscopic fluorescence detection of dysplasia in patients with Barrett’s esophagus, ulcerative colitis, or adenomatous polyps after 5-aminolevulinic acid-induced protoporphyrin IX sensitization. Gastrointest Endoscopy. 1999 Jan;49:97–101. doi: 10.1016/s0016-5107(99)70453-0. [DOI] [PubMed] [Google Scholar]
- 160.Mountney P, Yang GZ. Motion compensated SLAM for image guided surgery. Med Image Comput Computat Assist Interv. 2010;13:496–504. doi: 10.1007/978-3-642-15745-5_61. [DOI] [PubMed] [Google Scholar]
- 161.Lee HG, Choi MK, Lee SC. Motion analysis for duplicate frame removal in wireless capsule endoscope. Medical Imaging 2011: Image Processing. 2011;7962 [Google Scholar]
- 162.Yoshizaki S, Serb A, Yan L, Constandinou TG. Octagonal CMOs image sensor with strobed RGB LED illumination for wireless capsule endoscopy. Proc. 2014 IEEE Int. Symp. Circuits Syst. (ISCAS); Melbourne, Australia. 2014. pp. 1857–1860. [Google Scholar]
- 163.Lam PL, Gambari R. Advanced progress of microencapsulation technologies: In vivo and in vitro models for studying oral and transdermal drug deliveries. J Control Release. 2014 Mar;178:25–45. doi: 10.1016/j.jconrel.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 164.Kiryukhin MV. Active drug release systems: Current status, applications and perspectives. Curr Opin Pharmacol. 2014 Sep;18C:69–75. doi: 10.1016/j.coph.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 165.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013 Nov;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 166.Diller E, Giltinan J, Lum GZ, Ye Z, Sitti M. Six-degrees-of-freedom remote actuation of magnetic micro-robots. Proc Robot: Sci Syst. 2014 [Google Scholar]
- 167.Purcell EM. Life at low Reynolds number. Amer J Phys. 1977;45:3. [Google Scholar]
- 168.Loget G, Kuhn A. Electric field-induced chemical locomotion of conducting objects. Nat Commun. 2011 Nov;2:535. doi: 10.1038/ncomms1550. [DOI] [PubMed] [Google Scholar]
- 169.Wang W, Castro LA, Hoyos M, Mallouk TE. Autonomous motion of metallic microrods propelled by ultrasound. ACS Nano. 2012 Jul;6:6122–6132. doi: 10.1021/nn301312z. [DOI] [PubMed] [Google Scholar]
- 170.Sen A, Ibele M, Hong Y, Velegol D. Chemo and phototactic nano/microbots. Faraday Discuss. 2009;143:15–27. doi: 10.1039/b900971j. discussion 81–93. [DOI] [PubMed] [Google Scholar]
- 171.Jiang HR, Yoshinaga N, Sano M. Active motion of a Janus particle by self-thermophoresis in a defocused laser beam. Phys Rev Lett. 2010 Dec;105:268302. doi: 10.1103/PhysRevLett.105.268302. [DOI] [PubMed] [Google Scholar]
- 172.Paxton WF, et al. Catalytic nanomotors: Autonomous movement of striped nanorods. J Am Chem Soc. 2004 Oct;126:13 424–13 431. doi: 10.1021/ja047697z. [DOI] [PubMed] [Google Scholar]
- 173.Kagan D, Balasubramanian S, Wang J. Chemically triggered swarming of gold microparticles. Angew Chem Int Ed Engl. 2011 Jan;50:503–506. doi: 10.1002/anie.201005078. [DOI] [PubMed] [Google Scholar]
- 174.Yoshizumi Y, Date Y, Ohkubo K, Yokokawa M, Suzuki H. Bimetallic micromotor autonomously movable in biofuels. Proc. 2013 IEEE 26th Int. Conf. Micro Electro Mechanical Syst. (MEMS); Taipei, Taiwan. 2013. pp. 540–543. [Google Scholar]
- 175.Ibele ME, Wang Y, Kline TR, Mallouk TE, Sen A. Hydrazine fuels for bimetallic catalytic microfluidic pumping. J Amer Chem Soc. 2007 Jun;129:7762–7763. doi: 10.1021/ja0726512. [DOI] [PubMed] [Google Scholar]
- 176.Sengupta S, et al. Self-powered enzyme micropumps. Nat Chem. 2014 May;6:415–422. doi: 10.1038/nchem.1895. [DOI] [PubMed] [Google Scholar]
- 177.Choi H, et al. Electromagnetic actuation system for locomotive intravascular therapeutic micro-robot. Proc. 2014 5th IEEE RAS EMBS Int. Conf. Robot. Biomechatron; Sao Paulo, Brazil. 2014. pp. 831–834. [Google Scholar]
- 178.Chen F, et al. In vivo tumor vasculature targeted PET/NIRF imaging with TRC105(Fab)-conjugated, dual-labeled mesoporous silica nanoparticles. Mol Pharm. 2014 Nov 3;11:4007–4014. doi: 10.1021/mp500306k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wehrl HF, et al. Preclinical and translational PET/MR imaging. J Nucl Med. 2014 May 15;55:11S–18S. doi: 10.2967/jnumed.113.129221. [DOI] [PubMed] [Google Scholar]
- 180.Judenhofer MS, et al. Simultaneous PET-MRI: A new approach for functional and morphological imaging. Nat Med. 2008 Apr;14:459–465. [Google Scholar]
- 181.Siu CH, Harris TJC, Wang J, Wong E. Regulation of cell-cell adhesion during Dictyostelium development. Seminars in Cell Develop Biol. 2004 Dec;15:633–641. doi: 10.1016/j.semcdb.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 182.Loomis WF. Cell signaling during development of Dictyostelium. Dev Biol. 2014 Jul 1;391:1–16. doi: 10.1016/j.ydbio.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Shaulsky G, Kessin RH. The cold war of the social amoebae. Current Biology. 2007 Aug;17:R684–R692. doi: 10.1016/j.cub.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 184.Rutherford ST, Bassler BL. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harbor Perspectives in Medicine. 2012 Nov 1;2 doi: 10.1101/cshperspect.a012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Robinton DA, Daley GQ. The promise of induced pluripotent stem cells in research and therapy. Nature. 2012 Jan;481:295–305. doi: 10.1038/nature10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Patah PA, et al. Microbial contamination of hematopoietic progenitor cell products: Clinical outcome. Bone Marrow Transplant. 2007 Jun;40:365–368. doi: 10.1038/sj.bmt.1705731. [DOI] [PubMed] [Google Scholar]
- 187.Stormer M, et al. Bacterial safety of cell-based therapeutic preparations, focusing on haematopoietic progenitor cells. Vox Sang. 2014 May;106:285–296. doi: 10.1111/vox.12097. [DOI] [PubMed] [Google Scholar]
- 188.Patyar S, et al. Bacteria in cancer therapy: A novel experimental strategy. J Biomed Sci. 2010;17:21. doi: 10.1186/1423-0127-17-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Loeffler M, Le’Negrate G, Krajewska M, Reed J. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Nat Acad Sci. 2007;104:12 879–12 883. doi: 10.1073/pnas.0701959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Schenck JF. Safety of strong, static magnetic fields. J Magn Reson Imaging. 2000 Jul;12:2–19. doi: 10.1002/1522-2586(200007)12:1<2::aid-jmri2>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 191.Atkinson IC, Renteria L, Burd H, Pliskin NH, Thulborn KR. Safety of human MRI at static fields above the FDA 8 T guideline: Sodium imaging at 9.4 T does not affect vital signs or cognitive ability. J Magnet Resonance Imaging. 2007;26:1222–1227. doi: 10.1002/jmri.21150. [DOI] [PubMed] [Google Scholar]
- 192.Berry MV, Geim AK. Of flying frogs and levitrons. European Journal of Physics. 1997;18:307. [Google Scholar]
- 193.Kinouchi Y, Yamaguchi H, Tenforde TS. Theoretical analysis of magnetic field interactions with aortic blood flow. Bioelectromagnetics. 1996;17:21–32. doi: 10.1002/(SICI)1521-186X(1996)17:1<21::AID-BEM3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 194.Shellock FG, Kanal E, Gilk TB. Regarding the value reported for the term ‘spatial gradient magnetic field’ and how this information is applied to labeling of medical implants and devices. Amer J Roentgenol. 2011;196:142–145. doi: 10.2214/AJR.10.5004. [DOI] [PubMed] [Google Scholar]
- 195.Huang B, Law MWM, Khong PL. Whole-body PET/CT scanning: Estimation of radiation dose and cancer risk 1. Radiology. 2009;251:166–174. doi: 10.1148/radiol.2511081300. [DOI] [PubMed] [Google Scholar]
- 196.Guidance for Industry and FDA Staff-Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. U.S. Food Drug Administration; 2012. [Google Scholar]
- 197.Capulli AK, et al. Approaching the in vitro clinical trial: Engineering organs on chips. Lab Chip. 2014 Sep 7;14:3181–3186. doi: 10.1039/c4lc00276h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Diller E, Sitti M. Micro-scale mobile robotics. Foundations and Trends in Robotics. 2013;2(3):143–259. [Google Scholar]