Abstract
Disruption of circadian rhythms, which frequently occurs during night shift work, may be associated with cancer progression. The effect of chronotype (preference for behaviors such as sleep, work, or exercise to occur at particular times of day, with an associated difference in circadian physiology) and alignment of bedtime (preferred vs. habitual), however, have not yet been studied in the context of cancer progression in women with breast cancer. Chronotype and alignment of actual bedtime with preferred chronotype were examined using the Morningness–Eveningness Scale (MEQ) and sleep-wake log among 85 women with metastatic breast cancer. Their association with disease-free interval (DFI) was retrospectively examined using the Cox proportional hazards model. Median DFI was 81.9 months for women with aligned bedtimes (“going to bed at preferred bedtime”) (n=72), and 46.9 months for women with misaligned bedtimes (“going to bed later or earlier than the preferred bedtime”) (n=13) (log rank p=0.001). In a multivariate Cox proportional hazard model, after controlling for other significant predictors of DFI, including chronotype (morning type/longer DFI; HR=0.539, 95% CI=0.320–0.906, p=0.021), estrogen receptor (ER) status at initial diagnosis (negative/shorter DFI; HR=2.169, 95% CI=1.124–4.187, p=0.028) and level of natural-killer cell count (lower levels/shorter DFI; HR=1.641, 95% CI=1.000–2.695, p=0.050), misaligned bedtimes was associated with shorter DFI, compared to aligned bedtimes (HR=3.180, 95% CI=1.327–7.616, p=0.018). Our data indicate that a misalignment of bedtime on a daily basis, an indication of circadian disruption, is associated with more rapid breast cancer progression as measured by DFI. Considering the limitations of small sample size and study design, a prospective study with a larger sample is necessary to explore their causal relationship and underlying mechanisms.
Keywords: Chronobiology phenomenon, circadian dysregulation, circadian rhythm, disease progression
INTRODUCTION
Individual differences in the relative timing of events influenced by the central circadian clock are described as chronotypes along a continuum of “morningness/ eveningness” (Horne & Östberg, 1976). For example, morning types prefer earlier wake times and earlier bedtimes, while evening types prefer later wake times and later bedtimes. Chronotypes also differ in the circadian timing of various physiologic events, such as melatonin rise and core body temperature minimum, relative to the timing of sleep (Duffy et al., 1999). As compared to the morning chronotype, the evening chronotype has been associated with poor general health (Paine et al., 2006), fatigue (Kantermann et al., 2012), fibromyalgia (Kantermann et al., 2012), intensified physiological arousal (Roeser et al., 2012), more perceived stress (Kantermann et al., 2012), more depression (Chelminski et al., 1999; Gaspar-Barba et al., 2009; Hidalgo et al., 2009; Kantermann et al., 2012; Levandovski et al., 2011; Pabst et al., 2009; Selvi et al., 2007), lower early morning salivary cortisol level (Bailey & Heitkemper, 1991; Griefahn & Robens, 2008), delay in the fitted peaks of cortisol and melatonin (Bailey & Heitkemper, 2001; Burgess & Fogg, 2008; Duffy et al., 1999), and lower amplitude of cortisol rhythm (Bailey & Heitkemper, 2001; Randler & Schaal, 2010). In theory, either directly through differences in circadian clock function, or indirectly through the effects of the circadian clock on behavior, endocrine, or immune function, there may be an association between chronotype and progression of cancer (Sephton et al., 2000). In one study of a large group of women in the Danish military, those with a morning chronotype exhibited an especially strong interaction between night shift work and breast cancer risk (Hansen & Lassen, 2012). The association of chronotype with progression of breast cancer independent of night shift work, however, has not yet to be examined.
Disruption of circadian rhythms is closely related to cancer progression (Greene, 2012). Nighttime shift work is a significant risk for the development of breast cancer in women (Davis et al., 2001; Hansen, 2001; Hansen & Lassen, 2012; Hansen & Stevens, 2012; Lie et al., 2006, 2011; Megdal et al., 2005; O’Leary et al., 2006; Schernhammer et al., 2001, 2006; Spiegel & Sephton, 2002; Straif et al., 2007). Indeed, the International Agency for Research on Cancer of the World Health Organization concluded, “Shift work that involves circadian disruption” is a probable human carcinogen (Stevens et al., 2011; Straif et al., 2007). While not as severe a disruption as shift work, the mismatch of biological time and social time may lead to chronic circadian desynchrony. This mismatch has been referred to as “social jetlag” (Wittmann et al., 2006) and has been associated with increased obesity, among other physiologic effects normally associated with disruption of circadian timing (Roenneberg et al., 2012). However, associations of a misalignment between preferred and habitual bedtime with health and disease, including cancer, and its clinical and biological implications, have not yet been studied. We were also interested in the relationships among sleep and disease progression variables with the number of natural killer (NK) cells in peripheral blood. NK (CD56þ) cells kill carcinogenically transformed cells and secrete the pro-inflammatory cytokines interferon gamma and interleukin-6. NK cell numbers in blood and their activity are reduced by stress and such reductions are associated with more rapid breast cancer progression (Levy et al., 1985, 1987).
Our a priori hypothesis was that evening chronotype and misalignment of preferred and habitual bedtime would both be associated with faster cancer progression. In order to test our hypotheses, we examined the association of chronotype and alignment of preferred and habitual bedtime with disease-free interval (DFI) in women with metastatic breast cancer. DFI, the time from initial diagnosis of breast cancer to the date of identified metastasis, is a well-known prognostic indicator of overall survival (shorter DFI, shorter survival; Levy et al., 1988).
Materials and Methods
Sample
Participants were recruited for a study of stress and circadian rhythms in women with metastatic breast cancer over a 5-year period from July 2006 through June 2011. The breast cancer had to be documented as metastatic or recurrent and the women had to be postmenopausal and have Karnofsky performance ratings (Karnofsky & Burchenal, 1949) of at least 70%. Women were excluded if they had bilateral lymph nodes removed, had other active cancers within the past 10 years (except for basal cell or squamous cell carcinomas of the skin or in situ cancer of the cervix), had a history of major psychiatric illness that required hospitalization in the preceding year, exhibited substance abuse or dependence, or engaged in travel involving two or more time zones or shift work during the 2 weeks prior to study. One hundred twenty women were enrolled, 18 of whom did not provide data regarding their chronotype. Seventeen women with stage IV disease at initial diagnosis were excluded in this analysis because DFI was used as a dependent variable. Thus, 85 women with metastatic breast cancer and stage 0–III at their initial diagnosis were analyzed in this study (Table 1).
Table 1.
Characteristics of 85 subjects with metastatic breast cancer.
| Variables | Total (N=85) |
Aligned bedtime (N=72) |
Misaligned bedtime (N=13) |
|
|---|---|---|---|---|
| Age at initial diagnosis | ||||
| All subjects [Mean (SD, range)] years | 46.1 (8.0, 28– 67) |
45.3 (7.3, 32–65) | 50.5 (10.3, 28–67) | t = −2.210, df = 83, p = 0.030 |
| 28–39 years | 18 (21.2) | 16 (22.2) | 2 (15.4) | p = 0.025g |
| 40–49 years | 42 (49.4) | 39 (54.2) | 3 (23.1) | |
| 50–67 years | 25 (29.4) | 17 (23.6) | 8 (61.5) | |
| Ethnicity | ||||
| Asian | 3 (3.5) | 3 (4.2) | 0 | p=0.166g |
| Black | 6 (7.1) | 3 (4.2) | 3 (23.1) | |
| Hispanic | 1 (1.2) | 1 (1.4) | 0 (0.0) | |
| White | 75 (88.2) | 65 (90.3) | 10 (76.9) | |
| Educational level | ||||
| High school | 7 (8.2) | 5 (6.9) | 2 (15.4) | p=0.450g |
| College - Bachelor’s degree | 43 (50.6) | 35 (48.6) | 8 (61.5) | |
| Graduate school – Master’s degree | 25 (29.4) | 23 (31.9) | 2 (15.4) | |
| Ph.D., M.D., J.D. | 10 (11.8) | 9 (12.5) | 1 (7.7) | |
| Marital status | ||||
| Married | 6 (7.1) | 4 (5.6) | 2 (15.4) | p=0.14g |
| Never married | 47 (55.3) | 44 (61.1) | 3 (23.1) | |
| Separated | 4 (4.7) | 3 (4.2) | 1 (7.7) | |
| Divorced | 22 (25.9) | 18 (25.0) | 4 (30.8) | |
| Widowed | 5 (5.9) | 2 (2.8) | 3 (23.1) | |
| Others | 1 (1.2) | 1 (1.4) | 0 (0.0) | |
| Total gross household income | ||||
| < $20,000 | 11 (13.3) | 7 (9.9) | 4 (33.3) | p=0.026g |
| $20,000 – $39,999 | 13 (15.7) | 13 (18.3) | 0 (0.0) | |
| $40,000 – $59,999 | 10 (12.0) | 7 (9.9) | 3 (25.0) | |
| $60,000 – $79,999 | 7 (8.4) | 7 (9.9) | 0 (0.0) | |
| $80,000 – $99,999 | 13 (15.7) | 10 (14.1) | 3 (25.0) | |
| $100,000 or above | 29 (34.9) | 27 (38.0) | 2 (16.7) | |
| Employment status | ||||
| Full time | 23 (27.1) | 20 (27.8) | 3 (23.1) | p=0.408g |
| Part time | 17 (20.0) | 16 (22.2) | 1 (7.7) | |
| None | 45 (52.9) | 36 (50.0) | 9 (69.2) | |
| Amount of caffeine consumption, mg | ||||
| Morning [Mean (SD)] | 163.2 (268.4) | 176.3 (279.1) | 90.7 (191.4) | p=0.027h |
| Afternoon [Mean (SD)] | 36.0 (75.4) | 31.0 (45.5) | 63.3 (163.3) | p=0.830h |
| Evening [Mean (SD)] | 11.9 (21.8) | 11.3 (22.0) | 14.8 (21.0) | p=0.560h |
| CES-D [Mean (SD)] | 8.9 (7.0) | 8.5 (6.4) | 11.2 (9.6) | P=0.423h |
| STAI, trait [Mean (SD)] | 34.1 (8.8) | 34.4 (8.6) | 32.5 (10.2) | p=0.343h |
| STAI, state [Mean (SD)] | 28.5 (7.6) | 28.6 (7.5) | 28.6 (8.5) | p=0.864h |
| PCL-C, total [Mean (SD)] | 28.6 (9.2) | 29.3 (9.4) | 24.8 (7.3) | p=0.060h |
| PCL-C, reexperience/intrusion [Mean (SD)] | 7.9 (3.5) | 8.1 (3.7) | 6.9 (1.8) | p=0.366h |
| PCL-C, avoidance/numbing [Mean (SD)] | 11.6 (4.2) | 12.0 (4.2) | 9.5 (4.0) | p=0.012h |
| PCL-C, arousal [Mean (SD)] | 9.1 (3.1) | 9.2 (3.2) | 8.5 (2.8) | p=0.514h |
| BPI, severity [Mean (SD)] | 2.0 (1.9) | 1.8 (1.7) | 3.2 (2.5) | p=0.052h |
| BPI, interference [Mean (SD)] | 1.7 (2.0) | 1.5 (1.7) | 2.9 (2.7) | p=0.086h |
| Stage at initial diagnosis | ||||
| 0 | 5 (5.9) | 4 (5.6) | 1 (7.7) | p=0.172g |
| I | 28 (32.9) | 24 (33.3) | 4 (30.8) | |
| II | 36 (42.4) | 33 (45.8) | 3 (23.1) | |
| III | 16 (18.8) | 11 (15.3) | 5 (38.5) | |
| Receptor status at initial diagnosis | ||||
| Estrogen receptor + | 70 (82.4) | 60 (83.3) | 10 (76.9) | p=0.692g |
| Progesterone receptor + a | 58 (71.6) | 51 (72.9) | 7 (63.6) | p=0.498g |
| HER2 receptor + b | 17 (22.7) | 17 (26.6) | 0 (0.0) | p=0.060g |
| Treatment before metastasis or recurrence | ||||
| Surgery + | 80 (94.1) | 68 (94.4) | 12 (92.3) | p=0.573g |
| Chemotherapy + | 54 (63.5) | 44 (61.1) | 10 (76.9) | p=0.358g |
| Radiation therapy + | 41 (48.2) | 36 (50.0) | 5 (38.5) | χ2=0.587, p=0.444 |
| Hormonal therapy + | 43 (50.6) | 37 (51.4) | 6 (46.2) | χ2=0.121, p=0.728 |
| Herceptin + | 2 (2.4) | 2 (2.8) | 0 (0.0) | p=1.000g |
| Natural killer cell count/mm3 [Mean (SD, range)]c |
136 (77, 24– 431) |
140 (77, 24–431) | 112 (73, 29–265) | p=0.353h |
| Higher natural killer cell counte | 36 (50.0) | 31 (50.0) | 5 (50.0) | χ2=0.000, p=1.000 |
| Karnofsky performance rating [Mean (SD)]d | 92.4 (10.2) | 92.3 (10.2) | 93.0 (10.6) | p=0.730h |
| Chronotype score [Mean (SD, range)] | 22.3 (4.4, 13– 32) |
22.6 (3.9, 14–32) | 20.7 (6.8, 13–32) | t=0.968, df=13.42, p=0.350 |
| Chronotypef | p=0.008g | |||
| Morning type | 43 (50.6) | 38 (52.8) | 5 (38.5) | |
| Neither type | 36 (42.4) | 32 (44.4) | 4 (30.8) | |
| Evening type | 6 (7.1) | 2 (2.8) | 4 (30.8) |
SD, standard deviation; CES-D, Center for Epidemiologic Studies-Depression; STAI, State and Trait Anxiety Index; PCL-C, Post-Traumatic Stress Disorder Checklist-Civilian version; BPI, Brief Pain Inventory; HER2, Human Epidermal Growth Factor Receptor 2.
: n=81,
: n=75,
: n=72,
n=71
NK-cell count was classified into higher and lower one by median value of 116.97/mm3
Chronotype was classified into three types according to MEQ score (morning type 32 – 23, neither type 22 – 16, evening type 15 – 6)
Fisher’s exact test
Mann-Whitney U-test
Data are shown as number of subjects (percentage of subjects) unless otherwise noted.
The Horne–Östberg Morningness–Eveningness Scale (MEQ) (Horne & Östberg, 1976), Center for Epidemiologic Studies-Depression (CES-D) (Radloff, 1977), State and Trait Anxiety Index (STAI) (Spielberger, 1983), Post-Traumatic Stress Disorder Checklist-Civilian version (PCL-C) (Weathers et al., 1993), and Brief Pain Inventory (BPI) (Daut et al., 1983) were administered at baseline. NK cell count for 72 participants was obtained 8 h after habitual wake time via an indwelling forearm venous catheter at the Stanford Hospital Clinical Translational Research Unit. All study procedures adhered to the ethical standards and methods for the conduct of human biological rhythm research (Portaluppi et al., 2010) and the intent and principles outlined in the Declaration of Helsinki (2008) and were approved by the Stanford University Institutional Review Board. Written informed consent was obtained from all participants.
Chronotype and preferred bedtime
Chronotype was classified as morning, neither, and evening type based on the score on the MEQ (Horne & Östberg, 1976). Aside from determining chronotype, we used question 1 of the MEQ to determine preferred bedtime, which was coded as 2000 h–2100 h (5), 2100 h– 2215 h (4), 2215 h–0030 h (3), 0030 h–0145 h (2), and 0145 h–0300 h (1).
Habitual bedtime
Participants were asked to complete a sleep-wake log within 30 min of arising each morning during a 2-week at-home study of ad libitum sleep. This post-sleep questionnaire queried recollection of bedtime, latency to sleep onset, number of nighttime awakenings, sleep hours, and wake time, as well as a subjective sleep quality scale and a subjective sleepiness scale (Stanford Sleepiness Scale) (Hoddes et al., 1973). Habitual bedtime was determined by averaging the data collected during the two weeks and transformed to time as coded by the MEQ (range=1–5).
Alignment of preferred and habitual bedtime
The difference between the code of preferred bedtime and that of habitual bedtime was used to determine alignment of (preferred and habitual) bedtime; aligned bedtime (“going to bed at preferred bedtime”), earlier misaligned bedtime (“going to bed earlier than preferred bedtime”), and later misaligned bedtime (“going to bed later than preferred bedtime”).
Disease-free interval
DFI was calculated by subtracting the date of surgical removal of the primary breast cancer from the date of metastasis or recurrence. It was based on the reports of physician and enrollee and confirmed by medical records.
Analysis
The association of chronotype and alignment of bedtime with DFI was retrospectively examined using the Cox proportional hazards model. In addition, analyses were performed to control for the effects of other predictors of DFI, including age, cancer stage, and receptor status (estrogen, progesterone, and HER2) at initial diagnosis, treatment (surgery, chemotherapy, radiation, hormonal therapy, herceptin treatment) before metastasis or recurrence, circulating NK cell count, and other physical and psychosocial variables (Karnofsky performance rating, severity and interference index of pain, depression, trait and state anxiety, posttraumatic stress disorder rating (re-experience/ intrusion, avoidance/numbing, arousal symptoms, total score), marital status, educational level, income, and employment status). The final model was formulated based on a series of univariate Cox proportional hazard analyses (variables with p value 50.100 were included) and our a priori hypotheses (association of chronotype and mismatch of preferred with actual bedtime with DFI). Kaplan–Meier curves and the log rank test were also used to evaluate the median DFI across conditions. Chi-square tests, Fisher’s exact test, independent-sample Student’s t-tests, and Mann– Whitney U tests were used to examine the characteristics of chronotype and alignment of bedtime. SPSS Statistics 20.0 was used and two-tailed tests were applied in all analyses.
Results
Table 1 shows characteristics of patients with different alignment types. Age at initial diagnosis (p=0.030), marital status (p=0.014), income (p=0.026), amount of caffeine consumption at morning (p=0.027), PCL-C avoidance/numbing score (p=0.012), and chronotype (p=0.008) were different between aligned/misaligned bedtimes (Table 1). Otherwise, ethnicity, employment status, educational level, depression score, anxiety score, pain rating, Karnofsky rating, stage, receptor status, treatment, and NK cell count were not different across alignment of bedtime. When misaligned bedtime was divided into earlier and later misalignment, later misalignment was common in morning types and earlier misalignment was common in evening types or neither types (Fisher’s exact test, p=0.005).
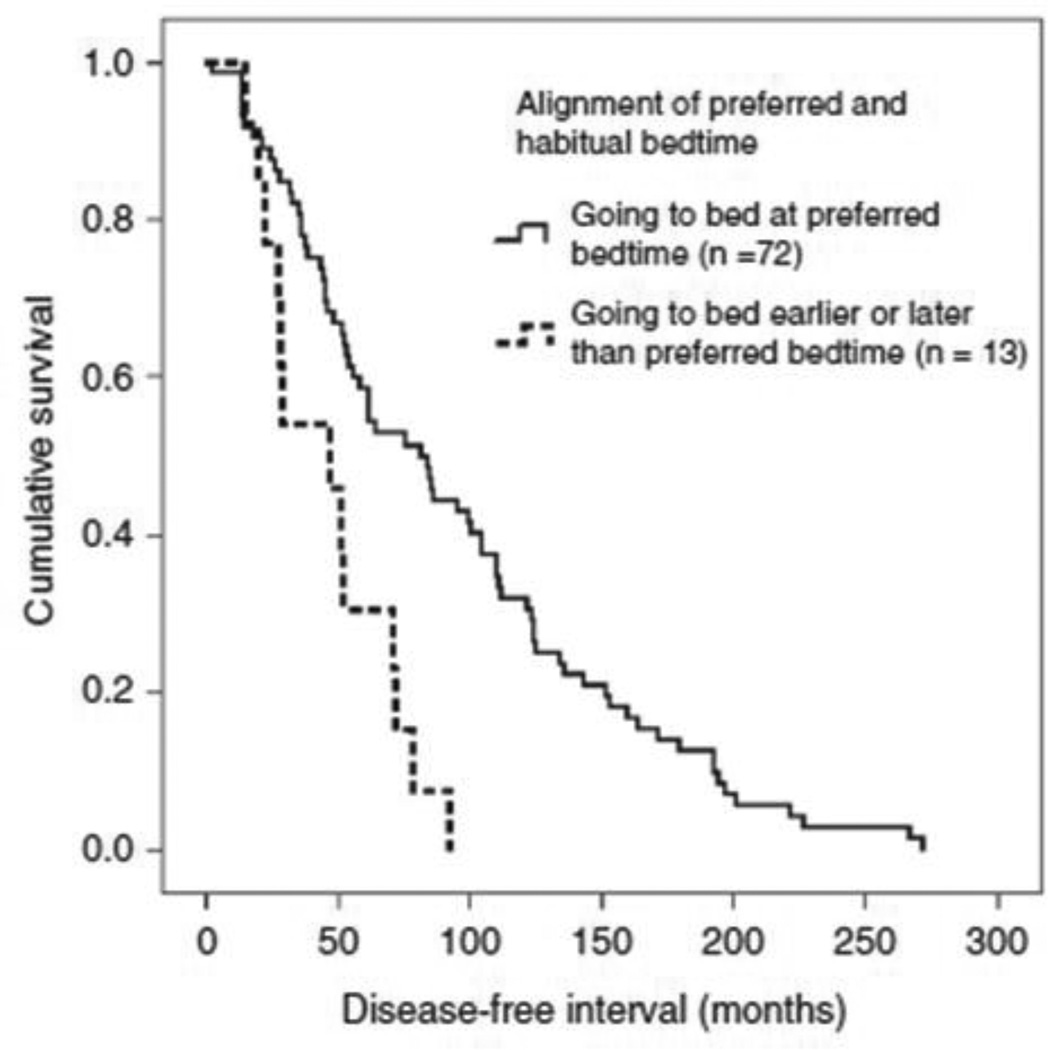
Median DFI was 81.9 months for women with aligned bedtimes (“going to bed at the preferred bedtime”) (n=72), and 46.9 months for women with misaligned bedtimes (“going to bed later or earlier than the preferred bedtime”) (n=13) (log rank χ2=11.279, df=1, p=0.001) (Figure 1). When misaligned bedtime was divided into earlier and later misalignment, median DFIs were 81.9 months for women “going to bed at the preferred bedtime” (n=72), 51.4 months for those “going to bed later than the preferred bedtime” (n=7), and 29.0 months for those “going to bed earlier than the preferred bedtime” (n=6) (log rank χ2 for difference=12.863, df=2, p=0.002).
Figure 1.
Kaplan–Meier curve on disease-free interval for “goingto bed at preferred bedtime” (n=72, solid line) and “going to bedlater or earlier than preferred bedtime” (n=13, dashed line) in 85subjects with metastatic breast cancer.
In a univariate Cox proportional model, misaligned bedtimes were associated with shorter DFI, compared to aligned bedtimes. While age at initial diagnosis, estrogen receptor status, and NK cell count were associated with DFI, stage, progesterone receptor status, HER2 status, surgery before metastasis, chemotherapy before metastasis, radiation therapy before metastasis, hormonal therapy before metastasis, and chronotype were not (Table 2).
Table 2.
Univariate Cox regression on disease-free interval for alignment of bedtime among 85 subjects with metastatic breast cancer.
| B | SE | χ2f | df | p | Hazard Ratio |
95% Confidence Interval |
|
|---|---|---|---|---|---|---|---|
| Age at initial diagnosisa | 0.040 | 0.016 | 5.762 | 1 | 0.016 | 1.040 | 1.008–1.074 |
| Stage at initial diagnosis (reference: III) | 1.514 | 3 | 0.679 | ||||
| 0 | −0.413 | 0.522 | 0.665 | 1 | 0.415 | 0.661 | 0.238–1.841 |
| I | −0.376 | 0.322 | 1.305 | 1 | 0.253 | 0.687 | 0.365–1.291 |
| II | −0.354 | 0.313 | 1.212 | 1 | 0.271 | 0.702 | 0.380–1.298 |
| Estrogen receptor, negative | 0.854 | 0.294 | 7.119 | 1 | 0.008 | 2.349 | 1.319–4.183 |
| Progesterone receptor, negativec | 0.380 | 0.250 | 2.185 | 1 | 0.139 | 1.462 | 0.896–2.388 |
| HER2, negatived | 0.105 | 0.277 | 0.145 | 1 | 0.704 | 1.110 | 0.644–1.912 |
| Surgery before metastasis | −0.569 | 0.466 | 1.273 | 1 | 0.259 | 0.566 | 0.227–1.412 |
| Chemotherapy before metastasis | 0.072 | 0.227 | 0.103 | 1 | 0.749 | 1.075 | 0.689–1.677 |
| Radiation therapy before metastasis | −0.039 | 0.222 | 0.032 | 1 | 0.859 | 0.961 | 0.623–1.485 |
| Hormonal therapy before metastasis | −0.049 | 0.221 | 0.049 | 1 | 0.824 | 0.952 | 0.618–1.467 |
| Natural-killer cell count,b lowere | 0.0517 | 0.244 | 4.467 | 1 | 0.035 | 1.676 | 1.039–2.704 |
| Chronotype score (reference: neither type) | 3.281 | 2 | 0.194 | ||||
| Morning type | −0.267 | 0.230 | 1.338 | 1 | 0.247 | 0.766 | 0.488–1.201 |
| Evening type | 0.510 | 0.449 | 1.150 | 1 | 0.284 | 1.665 | 0.691–4.012 |
| Misalignment of bedtime (“Going to bed earlier or later than preferred bedtime’) |
1.045 | 0.325 | 8.611 | 1 | 0.003 | 2.844 | 1.504–5.378 |
Continuous variables
Natural-killer cell count was classified into higher and lower one by median value of 116.97/mm3 (n = 72)
n=81;
n=75;
n=72.
Likelihood ratio test was performed
Table 3 shows the results from our multivariate Cox proportional hazard model after controlling for other significant predictors of DFI, including chronotype, age and estrogen receptor (ER) status at initial diagnosis, and level of NK cell count. It is shown in Table 3 that in the presence of various competing predictors, misaligned bedtimes was still associated with shorter DFI, compared to aligned bedtimes. When misaligned bedtime was divided into earlier and later misalignment in this multivariate model, later misaligned betimes was associated with shorter DFI, compared to aligned bedtimes (HR=4.611, 95% CI=1.645–12.924, χ2=6.409, p=0.011), while earlier misaligned bedtimes was not associated with DFI. We examined the sensitivity of our findings by including additional predictors in the model (results not shown). Even after entering marital status, income, and PCL-C avoidance/numbing score in a multivariate model, alignment of bedtime was still significantly associated with DFI. Chronotype was not a significant predictor of DFI by itself, but showed a significant association with DFI when controlling for the misalignment (see Table 3). In this analysis, being a morning type was associated with longer DFI (β=−0.618, HR=0.539, 95% CI=0.320–0.906, p=0.021), while being an evening type was not associated with DFI.
Table 3.
Multivariate Cox regression on disease-free interval for alignment of bedtime among 72 subjects with metastatic breast cancer.
| B | SE | χ2c | df | P | Hazard Ratio |
95% Confidence Interval |
|
|---|---|---|---|---|---|---|---|
| Age at initial diagnosisa | 0.024 | 0.018 | 1.664 | 1 | 0.197 | 1.024 | 0.988–1.061 |
| Estrogen receptor, negative | 0.774 | 0.335 | 4.813 | 1 | 0.028 | 2.169 | 1.124–4.187 |
| Natural-killer cell countb, lower | 0.496 | 0.253 | 3.830 | 1 | 0.050 | 1.641 | 1.000–2.695 |
| Chronotype (ref: neither type) | 5.467 | 2 | 0.065 | ||||
| Morning type | −0.618 | 0.265 | 5.328 | 1 | 0.021 | 0.539 | 0.320–0.906 |
| Evening type | −0.213 | 0.593 | 0.132 | 1 | 0.716 | 0.808 | 0.253–2.582 |
| Misalignment of bedtime, “going to bed later or earlier than preferred bedtime” |
1.157 | 0.446 | 5.645 | 1 | 0.018 | 3.180 | 1.327–7.616 |
Continuous variables.
Natural-killer cell count was classified into higher and lower one by median value of 116.97/mm3 (n = 72).
Likelihood ratio test was performed.
Discussion
The misalignment of preferred and habitual bedtime was associated with shortened DFI in this cohort of women with metastatic breast cancer. Our results also show the possibility of differential impact of later and earlier misalignment bedtime on DFI. While the sample size was small, in our cohort, later misalignment of bedtime (i.e. going to bed later than the preferred bedtime) was associated with shorter DFI after controlling for other predictors including chronotype. This indicates that the later misalignment of bedtime may have more of an impact on DFI than an earlier misalignment, but the establishment of this more specific relationship will require more study. Due to our limited sample size, we could not reliably examine the interaction between chronotype and misalignment, but this will be critical to future studies. While the expected factors of estrogen receptor status at initial diagnosis as well as NK cell count were associated with DFI, other sleep-related variables including sleep hours and subjective sleep quality measured by sleep wake log were not, however, associated with DFI. These findings indicate that misalignment of bedtime may be more important to the progression of breast cancer than the absolute quantity or subjective quality of sleep.
The connection between disruption of circadian rhythms and breast cancer has been reported mainly in the context of shift work (Davis et al., 2001; Hansen, 2001; Hansen & Lassen, 2012; Hansen & Stevens, 2012; Lie et al., 2006, 2011; Megdal et al., 2005; O’Leary et al., 2006; Schernhammer et al., 2001, 2006; Spiegel & Sephton, 2002; Straif et al., 2007). A more insidious and potentially widespread type of disruption of circadian rhythms is the mismatch between preferred and actual bedtime – a situation in which individuals are often sleeping at an altered time vis-a`-vis their circadian system. This type of mismatch has previously been described as the difference between mid-sleep times on free days and on workdays and been termed “social jetlag” (Wittmann et al., 2006). Social jetlag is associated with increased BMI and depression (Levandovski et al., 2011; Roenneberg et al., 2012), though to our knowledge, there is no report on its relation to cancer progression. Our study shows that misaligned bedtime is associated with shorter DFI after controlling for other predictors of DFI in women with metastatic breast cancer. Although misaligned bedtimes were more common in evening chronotypes, alignment of bedtime was not chronotype-specific as misaligned bedtime was still significantly associated with shorter DFI after controlling the effect of chronotype. The misalignment of bedtime was not explained by other conditions that could affect sleep behaviors, including depression, anxiety, pain, physical performance status, and employment status. Separated/divorced/widowed state and lower income were more common among those with misaligned bedtimes, but marital status and income did not influence the association between alignment of bedtime and DFI. The misalignment of bedtimes could impact the circadian system in a gradual, more subtle fashion then is observed in shift work, as the chronicity of the behavior is likely to be additive (Greene, 2012). While NK cell counts were marginally associated with DFI, as hypothesized, there was no relationship between NK cell levels and misalignment of bedtime.
Limitations of the study include the fact that it was performed using a cross-sectional design and the association of alignment of bedtime with DFI was retrospectively examined. Therefore, a causal relationship of alignment of bedtime and DFI cannot be assumed. It is possible that those with shorter DFI experienced mismatch between preferred and actual sleep times as a result of more rapid disease progression or anxiety related to it. The small number of the subsamples was a major limitation in statistical analyses. It is therefore possible that we might have missed significant associations among other sleep-related variables and DFI due to low statistical power. Finally, habitual bedtime was determined by self-report, not by objective observation; however, participants were encouraged to record bedtime within 30 min after waking to render correct and reliable information.
Despite these limitations, stability of chronotype and the fact that the association remains even after controlling for other salient variables suggest that misalignment of bedtime on a daily basis may be a significant disruption of circadian rhythms that could be associated with cancer progression. A prospective study with a larger sample is necessary to replicate these associations, explore causality, and identify underlying mechanisms. Sleep has crucial effects on quality of life among women with breast cancer (Liu et al., 2013; Spiegel, 2008). These findings suggest the possibility that improved sleep practices, especially matching preferred to actual sleep times, could have beneficial effects on quality and potentially quantity of life for women with metastatic breast cancer.
Acknowledgments
The authors thank the research participants for their time, wisdom, and willingness to participate, and the Dr. Susan Love Research Foundation’s Love/Avon Army of Women Program for their assistance in recruitment.
References
- Bailey SL, Heitkemper MM. Morningness–eveningness and early-morning salivary cortisol levels. Biol Psychol. 1991;32:181–192. doi: 10.1016/0301-0511(91)90009-6. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Heitkemper MM. Circadian rhythmicity of cortisol and body temperature: Morningness–eveningness effects. Chronobiol Int. 2001;18:249–261. doi: 10.1081/cbi-100103189. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008;3:e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelminski I, Ferraro FR, Petros TV, Plaud JJ. An analysis of the “eveningness–morningness” dimension in “depressive” college students. J Affect Disord. 1999;52:19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17:197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- Declaration of Helsinki. 59th World Medical Association General Assembly; October 2008; Seoul, Korea. [Google Scholar]
- Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- Gaspar-Barba E, Calati R, Cruz-Fuentes CS, et al. Depressive symptomatology is influenced by chronotypes. J Affect Disord. 2009;119:100–106. doi: 10.1016/j.jad.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Greene MW. Circadian rhythms and tumor growth. Cancer Lett. 2012;318:115–123. doi: 10.1016/j.canlet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Griefahn B, Robens S. The cortisol awakening response: A pilot study on the effects of shift work, morningness and sleep duration. Psychoneuroendocrinology. 2008;33:981–988. doi: 10.1016/j.psyneuen.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12:74–77. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- Hansen J, Lassen CF. Nested case-control study of night shift work and breast cancer risk among women in the Danish military. Occup Environ Med. 2012;69:551–556. doi: 10.1136/oemed-2011-100240. [DOI] [PubMed] [Google Scholar]
- Hansen J, Stevens RG. Case-control study of shift-work and breast cancer risk in Danish nurses: Impact of shift systems. Eur J Cancer. 2012;48:1722–1729. doi: 10.1016/j.ejca.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Hidalgo MP, Caumo W, Posser M, et al. Relationship between depressive mood and chronotype in healthy subjects. Psychiatry Clin Neurosci. 2009;63:283–290. doi: 10.1111/j.1440-1819.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, et al. Quantification of sleepiness: A new approach. Psychophysiology. 1973;10:431–436. doi: 10.1111/j.1469-8986.1973.tb00801.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Östberg O. A self-assessment questionnaire to determine morningness–eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Kantermann T, Theadom A, Roenneberg T, Cropley M. Fibromyalgia syndrome and chronotype: Late chronotypes are more affected. J Biol Rhythms. 2012;27:176–179. doi: 10.1177/0748730411435999. [DOI] [PubMed] [Google Scholar]
- Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer. In: Macleod CM, editor. Evaluation of chemotherapeutic agents. New York, NY: Columbia University Press; 1949. pp. 191–205. [Google Scholar]
- Levandovski R, Dantas G, Fernandes LC, et al. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int. 2011;28:771–778. doi: 10.3109/07420528.2011.602445. [DOI] [PubMed] [Google Scholar]
- Levy S, Herberman R, Lippman M, d’Angelo T. Correlation of stress factors with sustained depression of natural killer cell activity and predicted prognosis in patients with breast cancer. J Clin Oncol. 1987;5:348–353. doi: 10.1200/JCO.1987.5.3.348. [DOI] [PubMed] [Google Scholar]
- Levy SM, Herberman RB, Maluish AM, et al. Prognostic risk assessment in primary breast cancer by behavioral and immunological parameters. Health Psychol. 1985;4:99–113. doi: 10.1037//0278-6133.4.2.99. [DOI] [PubMed] [Google Scholar]
- Levy SM, Lee J, Bagley C, Lippman M. Survival hazards analysis in first recurrent breast cancer patients: Seven-year follow-up. Psychosom Med. 1988;50:520–528. doi: 10.1097/00006842-198809000-00008. [DOI] [PubMed] [Google Scholar]
- Lie JA, Kjuus H, Zienolddiny S, et al. Night work and breast cancer risk among Norwegian nurses: Assessment by different exposure metrics. Am J Epidemiol. 2011;173:1272–1279. doi: 10.1093/aje/kwr014. [DOI] [PubMed] [Google Scholar]
- Lie JA, Roessink J, Kjaerheim K. Breast cancer and night work among Norwegian nurses. Cancer Causes Control. 2006;17:39–44. doi: 10.1007/s10552-005-3639-2. [DOI] [PubMed] [Google Scholar]
- Liu L, Fiorentino L, Rissling M, et al. Decreased Health-Related Quality of Life in women with breast cancer is associated with poor sleep. Behav Sleep Med. 2013;11:189–206. doi: 10.1080/15402002.2012.660589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megdal SP, Kroenke CH, Laden F, et al. Night work and breast cancer risk: A systematic review and meta-analysis. Eur J Cancer. 2005;41:2023–2032. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- O’Leary ES, Schoenfeld ER, Stevens RG, et al. Shift work, light at night, and breast cancer on Long Island, New York. Am J Epidemiol. 2006;164:358–366. doi: 10.1093/aje/kwj211. [DOI] [PubMed] [Google Scholar]
- Pabst SR, Negriff S, Dorn LD, et al. Depression and anxiety in adolescent females: The impact of sleep preference and body mass index. J Adolesc Health. 2009;44:554–560. doi: 10.1016/j.jadohealth.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine SJ, Gander PH, Travier N. The epidemiology of morningness/eveningness: Influence of age, gender, ethnicity, and socioeconomic factors in adults (30–49 years) J Biol Rhythms. 2006;21:68–76. doi: 10.1177/0748730405283154. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;27:1911–1929. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measurement. 1977;1:385–401. [Google Scholar]
- Randler C, Schaal S. Morningness–eveningness, habitual sleep-wake variables and cortisol level. Biol Psychol. 2010;85:14–18. doi: 10.1016/j.biopsycho.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22:939–943. doi: 10.1016/j.cub.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Roeser K, Obergfell F, Meule A, et al. Of larks and hearts – Morningness/eveningness, heart rate variability and cardiovascular stress response at different times of day. Physiol Behav. 2012;106:151–157. doi: 10.1016/j.physbeh.2012.01.023. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health Study. J Natl Cancer Inst. 2001;93:1563–1568. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Kroenke CH, Laden F, Hankinson SE. Night work and risk of breast cancer. Epidemiology. 2006;17:108–111. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- Selvi Y, Gulec M, Agargun MY, Besiroglu L. Mood changes after sleep deprivation in morningness–eveningness chronotypes in healthy individuals. J Sleep Res. 2007;16:241–244. doi: 10.1111/j.1365-2869.2007.00596.x. [DOI] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Spiegel D. Losing sleep over cancer. J Clin Oncol. 2008;26:2431–2432. doi: 10.1200/JCO.2008.16.2008. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Sephton S. Re: Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2002;94:530. doi: 10.1093/jnci/94.7.530. author reply 532–3. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-trait anxiety inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stevens RG, Hansen J, Costa G, et al. Considerations of circadian impact for defining ‘shift work’ in cancer studies: IARC Working Group Report. Occup Environ Med. 2011;68:154–162. doi: 10.1136/oem.2009.053512. [DOI] [PubMed] [Google Scholar]
- Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8:1065–1066. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, et al. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at the 9th Annual Meeting of the International Society for Traumatic Stress Studies; San Antonio, TX. 1993. [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: Misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]