SUMMARY
Background
ALK-rearranged non-small-cell lung cancer (NSCLC) is sensitive to ALK tyrosine kinase inhibitors (ALKi) such as crizotinib, but resistance invariably develops, often with progression in the brain. Ceritinib is a more potent ALKi than crizotinib in vitro, crosses the blood-brain barrier in vivo and shows clinical responses in crizotinib-resistant disease. Here, we assessed whole-body and intracranial activity of ceritinib in both ALK-pretreated and ALKi-naïve patients with ALK-rearranged NSCLC.
Methods
The primary objective (to determine the maximum tolerated dose of ceritinib) of this first-in-human, phase I, open-label ASCEND-1 trial has been reported previously. In the analysis reported here, antitumour efficacy of ceritinib was evaluated in all patients with ALK-rearranged NSCLC (n=246) treated with ceritinib at the recommended dose of 750 mg/day. Additionally, as patients with untreated or locally treated neurologically stable brain metastases at baseline were permitted in this study, intracranial efficacy was retrospectively confirmed by independent neuroradiologists for 94 patients with baseline brain metastases and at least one post-baseline MRI/CT tumour assessment. This study is no longer recruiting patients; however, treatment and follow-up are ongoing. This study is registered with ClinicalTrials.gov, number NCT01283516.
Findings
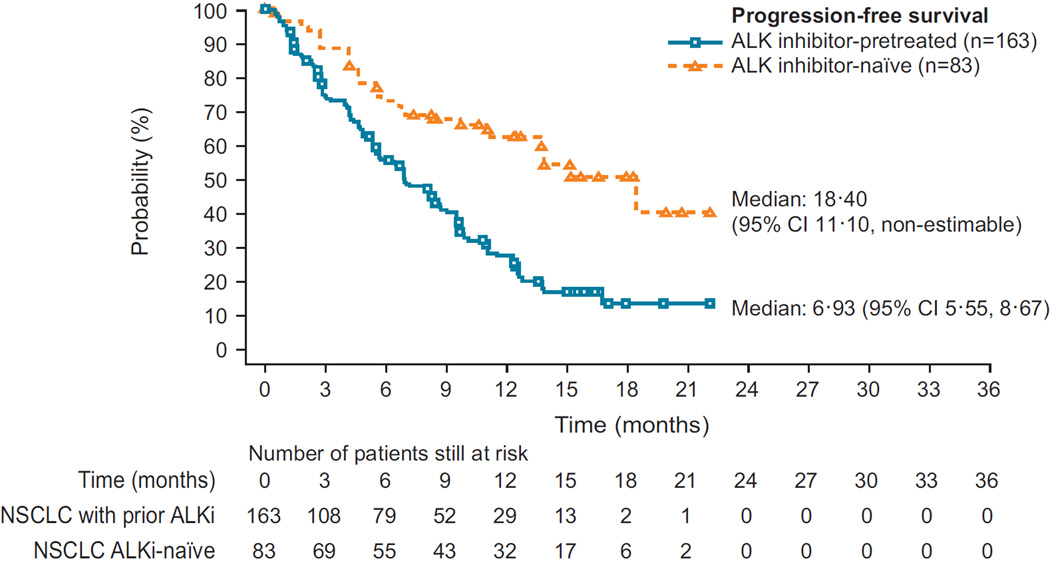
Median follow-up at the time of this report was 11 1 months (interquartile range 6·7–15·2). Patients were mainly heavily pretreated (105/246 [42·7%] at least three prior regimens). The overall response rate was 72·3% (60/83; 95% confidence interval [CI] 61·4–81·6) for ALKi-naïve (n=83) and 56·4% (92/163; 95% CI 48·5–64·2) for ALKi-pretreated (n=163) patients. Median progression-free survival in ALKi-naïve and ALKi-pretreated patients was 18·4 (95% CI 11·1-non-estimable) and 6·9 (95% CI 5·6–8·7) months, respectively. Brain metastases by investigator assessment were reported at study entry in 124 patients. Of these, 94 (n=19 ALKi-naïve and n=75 ALKi-pretreated) were included in the retrospective analysis; intracranial disease control rate was 78·9% (15/19; 95% CI 54·4– 93·9) in ALKi-naïve patients and 65·3% (49/75; 95% CI 53·5–76·0) in ALKi-pretreated patients. Of the 94 patients included in the retrospective analysis, 11 had measurable brain lesions and no prior radiotherapy to the brain: 6 of these achieved a partial intracranial response.
Safety was evaluated for all 246 patients with ALK-rearranged NSCLC. Serious adverse events were recorded for 117 (47·6%) patients. The most common grade 3/4 laboratory abnormalities were increased alanine aminotransferase and increased aspartate aminotransferase, occurring in 73 (29·7%) and 25 (10·2%) patients, respectively. The most common grade 3/4 non-laboratory adverse events were diarrhoea and nausea, both of which occurred in 15 (6.1%) patients. Two on-treatment deaths in the study were considered to be related to study drug by the investigators, one due to interstitial lung disease and one as a result of multi-organ failure that occurred in the context of infection and ischaemic hepatitis.
Interpretation
This study demonstrated clinically meaningful and durable responses in mainly heavily pretreated patients with ALK-rearranged NSCLC (ALKi-naïve and ALKi-pretreated) receiving ceritinib 750 mg/day. Treatment with ceritinib also achieved both whole-body and intracranial efficacy in patients with brain metastases at baseline, a common site of disease progression in patients with NSCLC. The durable whole-body responses reported, together with the intracranial efficacy, support a clinical benefit for treatment with ceritinib in patients post-crizotinib, or as an alternative to crizotinib in patients with ALK-rearranged NSCLC.
Funding
Sponsored by Novartis Pharmaceuticals Corporation.
Keywords: Ceritinib, ALK inhibitor, NSCLC, Brain metastases
INTRODUCTION
Anaplastic lymphoma kinase- (ALK-) rearrangement is a therapeutically tractable oncogenic driver that occurs in 2–7% of patients with non-small-cell lung cancer (NSCLC).1 To date, three ALK-targeted small-molecule tyrosine kinase inhibitors have been approved by several health authorities.2–5 The first ALK-targeted therapeutic was crizotinib, which targets cMET, ALK, and ROS1.6,7 Two phase 3 studies comparing crizotinib with chemotherapy in patients with advanced ALK-rearranged NSCLC demonstrated progression-free survival (PFS) and response rate benefits with crizotinib therapy in both second- and first-line settings.8,9 However, most responders acquire resistance within 1 year, with recurrence commonly occurring in the brain or liver.10–12 In a recent retrospective analysis of patients with ALK-rearranged NSCLC treated with crizotinib, the site of disease progression was brain in 41% of patients, and liver in 25% of patients.11 The high incidence of recurrence in the brain may partly be a result of limited blood-brain barrier penetration of crizotinib, which has been described in the clinical setting.13,14 Crizotinib resistance can result from both ALK-dependent and ALK-independent mechanisms.10,15,16 Therefore, treatment options for patients who progress on crizotinib are needed.17
Ceritinib (LDK378, Novartis Pharmaceuticals) is a potent and selective oral tyrosine kinase inhibitor of ALK (ALKi).18 In vitro, ceritinib inhibits ALK with a 20-fold greater potency than crizotinib, and has nanomolar potency against patient-derived crizotinib-resistant tumour cell lines.19 In preclinical xenograft studies, immediate tumour regrowth was observed following completion of crizotinib treatment, whereas regrowth upon stopping ceritinib treatment was notably delayed.19 Further, in a tissue distribution study utilising a rat model, ceritinib crossed the blood-brain barrier with a brain-to-blood exposure (AUCinf) ratio of approximately 15%.20 Consistent with these preclinical observations, ceritinib has demonstrated efficacy against crizotinib-resistant tumours in patients with ALK-rearranged NSCLC, including brain metastases.18
Preliminary efficacy and safety data from the dose-escalation phase of the ASCEND-1 study have been reported previously.18 The objective of the analyses reported here was to present updated results evaluating antitumour efficacy for a larger cohort of 246 patients with ALK-rearranged NSCLC, including both ALKi-naïve and ALKi-pretreated patients, treated at the recommended dose (RD) of 750 mg/day, with a longer median duration of follow-up of 11·1 (interquartile range [IQR] 6·7–15·2) months. The majority of patients were heavily pretreated (chemotherapy and/or ALKi). In addition, results from a retrospective review of brain MRI and CT scans conducted to assess intracranial activity are reported for patients with treated and untreated neurologically stable brain metastases at study entry.
METHODS
Study design and participants
The study methods for the phase 1, multicentre, open-label ASCEND-1 study have been published previously (see appendix page 1 for a list of study centres).18 Briefly, eligible patients had ALK-rearranged NSCLC, were ≥18 years old, had locally advanced or metastatic NSCLC that had progressed (by physician assessment) despite standard therapy (including chemotherapy or ALKi) or for which no effective standard therapy existed, an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≤2, adequate organ function and laboratory results (required laboratory tests: neutrophil count, haemoglobin, platelets, serum total bilirubin, aspartate aminotransferase, alanine aminotransferase, calculated creatinine clearance, serum amylase, serum lipase, and fasting plasma glucose) and a life expectancy of ≥12 weeks. Patients were required to have at least one measurable lesion at baseline according to the Response Evaluation Criteria in Solid Tumours (RECIST) 1.0. Patients with untreated or locally treated asymptomatic and stable (>4 weeks) central nervous system (CNS) disease were eligible. Patients with tumours other than NSCLC were permitted to be enrolled on the study; data from these patients were reported previously18 and were not included in the analysis described here.
Patients were not permitted to have received any chemotherapy, biologic therapy, or other investigational agent for 1–4 weeks (depending upon half-life) prior to starting ceritinib, or during the study. Patients with unresolved nausea, vomiting or diarrhoea (>Common Terminology Criteria for Adverse Events [CTCAE] grade 1), impairment of gastrointestinal function, a history of pancreatitis, liver disease, known HIV, clinically significant cardiac disease, prior or current second malignancy (other than adequately treated in situ carcinoma of the cervix, non-melanoma carcinoma of the skin, or any other curatively treated malignancy that has not recurred in the prior 3 years), and patients with symptomatic, neurologically unstable CNS disease were not eligible for inclusion.
Conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice, the study was approved by the local human investigations committee at each study centre; all patients provided written informed consent prior to screening.
Procedures
Patients with ALK-rearranged NSCLC were treated with ceritinib, administered orally in fasted patients at the RD of 750 mg/day in continuous 21-day treatment cycles. Treatment was continued until objective evidence of disease progression (treatment beyond progression was permitted in patients who continued to experience a clinical benefit), development of intolerable side effects, or withdrawal of consent. Dose adjustments were permitted for patients who experienced dose-limiting toxicity. In patients with a dose delay of more than 21 days due to ceritinib-related toxicity, treatment was discontinued unless the patient demonstrated evidence of a clinical benefit. Patients were permitted a maximum of three dose-reductions, after which they were required to discontinue treatment.
At baseline, imaging scans of the brain, chest, and abdomen were performed in all patients. During treatment, tumour response was assessed every 6 weeks (RECIST 1.0) to determine overall response rate (ORR), duration of response (DOR), and PFS. Responses were assessed only for patients with measurable disease at baseline and at least one post-baseline assessment. Routine follow-up brain MRI/CT scans were conducted only in patients with brain metastases at study entry. Efficacy was also assessed by a blinded independent review committee (BIRC).
Outcomes
After determination of maximum tolerated dose (MTD)/RD, which was the primary objective of the ASCEND-1 trial and has been reported previously,18 the study objectives were to evaluate antitumour efficacy (by investigator and by BIRC) and safety of ceritinib at the RD of 750 mg/day in patients with ALK-rearranged NSCLC.
The primary endpoint of the ASCEND-1 study was determination of MTD/RD, which has been reported previously.18 Secondary endpoints reported here include ORR, DOR, and PFS (by investigator and by BIRC). ORR was defined as the proportion of patients with complete response (CR) or partial response (PR), as assessed by whole-body (all sites of disease, including brain) responses (RECIST 1.0 criteria). Responses had to be confirmed by repeat assessments ≥4 weeks after response criteria were first met. DOR was defined as the time from first documented PR or CR to the date of first disease progression or death from any cause. PFS was defined as the time from start of treatment with ceritinib to the date of radiologically documented disease progression or death from any cause.
A retrospective central review was conducted by two independent neuroradiologists (blinded to investigator and BIRC assessment) according to RECIST 1.1; 94 patients had brain metastases at baseline confirmed by retrospective reading of MRI/CT scans and at least one post-baseline MRI/CT tumour assessment (n=20 by CT and n=74 by MRI). A further 30 patients with brain metastases at baseline, as assessed by the investigator, were excluded from the retrospective analysis due to no available baseline (n=8) or post-baseline (n=7) image for review, no consent for central review (n=2), no post-baseline assessment (either in brain or elsewhere; n=6), or because the image was deemed non-assessable by the neuroradiologists (n=7). Brain lesions with longest diameter ≥10 mm were defined as measurable. Prior radiotherapy information, including time from the last dose to start of ceritinib treatment, was collected.
All adverse events (AEs) reported during the study were recorded and graded according to the CTCAE 4.03. Assessments of laboratory parameters, ECOG PS, and overall physical condition were performed at baseline, days 1, 2, 8, and 15 of cycle 1, days 1, 2, and 15 of cycle 2, days 1 and 15 of cycles 3–6, and day 1 of each cycle thereafter, until end of treatment. Assessments were also performed at the end of study/time of withdrawal.
Statistical analysis
For determination of the MTD/RD (primary objective, dose-escalation phase, reported previously),18 no formal statistical power calculations to determine sample size were performed. For the dose-escalation phase of this study, it was estimated that 40 subjects would be enrolled including at least 6 subjects treated at the MTD level. For the expansion phase of this study, up to 310 patients were planned to be enrolled (including all patients treated at the MTD/RD during the dose-escalation phase who were eligible for the safety set) with at least 25 and up to 100 patients in each of the following patient groups: ALKi-naïve patients, ALKi-pretreated patients who progressed during prior ALKi treatment and ALKi-pretreated patients who did not progress during prior ALKi treatment. Approximately 10 patients were planned to be enrolled with tumours other than NSCLC. In general, preliminary evidence of antitumour activity of ceritinib would be demonstrated if the lower bound of the 95% credible interval is greater than 10% at the MTD/RD within that patient group. Given a sample size of 25 patients per arm, assuming observed ORR of 28%, the 95% credible interval would be (12.6%, 45.7%). Given a sample size of 100 patients per arm, assuming observed ORR of 25% the 95% credible interval would be (17.0%, 33.7%).
Investigator-assessed efficacy and safety assessments included all patients with ALK-rearranged NSCLC enrolled on the study who received at least one 750 mg dose of ceritinib (n=246). Data were summarised using descriptive statistics (continuous data) and/or contingency tables (categorical data) for demographic and baseline characteristics, efficacy measurements, and safety measurements. The data cut-off date was 14 April 2014. This study is registered with ClinicalTrials.gov, number NCT01283516.
In addition to assessment of ORR, DOR, and PFS, overall survival (OS) was evaluated, defined as time from start of treatment with ceritinib to date of death from any cause. Time to response was defined as time from start of treatment with ceritinib to first objective tumour response (CR or PR) that was subsequently confirmed. The median DOR, PFS, and OS times, and their associated 95% confidence intervals (CIs), were estimated using Kaplan– Meier methodology.
Intracranial responses were assessed as CR, PR, or stable disease (SD; defined as non-CR/non-progressive disease [PD] for patients with non-measurable lesions) according to RECIST 1.1. Endpoints included overall intracranial response rate (OIRR; CR+PR), intracranial disease control rate (IDCR; CR+PR+SD), and time to intracranial response (time from start of treatment with ceritinib to first objective intracranial response that was subsequently confirmed). Intracranial DOR was defined as time from first complete or partial intracranial response to intracranial disease progression (not considering extracranial disease progression) or death.
Role of the funding source
This study was funded by Novartis Pharmaceuticals Corporation. It was designed by Novartis Pharmaceuticals Corporation, study investigators, and an independent steering committee. Data were collected by investigators and analysed by Novartis Pharmaceuticals Corporation. All authors (DWK, RM, DSWT, EF, LQMC, DRC, JV, SS, TDP, GJR, BJS, JW, MT, MS, GL, AS, SS, SL, TS, AY, ATS) had full access to the data, and contributed to the development of and approved the manuscript for submission. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
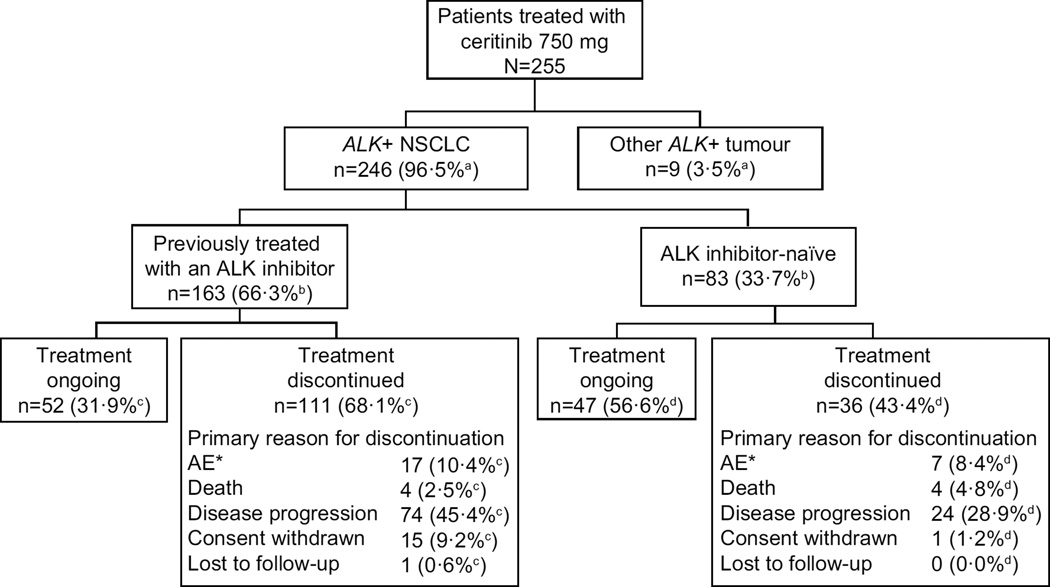
This global phase 1 trial included 255 patients enrolled between Jan 24, 2011 and Jul 31, 2013 across 20 centres who received at least one dose of ceritinib at the 750 mg/day RD. Of these, 246 (96·5%) had ALK-rearranged NSCLC; 83 (33·7%) of 246 were ALKi-naïve patients and 163 (66·3%) of 246 were ALKi-pretreated patients (Figure 1). Baseline demographics (Table 1) were consistent with those reported in other studies for patients with ALK-rearranged NSCLC, 6,21 and were mostly similar across both patient groups, apart from a numerically larger proportion of Asian patients, lower incidence of baseline brain metastases, and fewer prior treatment regimens in the ALKi-naïve group compared with the ALKi-pretreated group. Overall, the majority of patients were heavily pretreated, having received multiple antineoplastic therapies (chemotherapy and/or ALKi; Table 1).
Figure 1. Patient disposition.
Denominator used for a = 255, b = 246, c = 163, d = 83.
AE=adverse event. ALK=anaplastic lymphoma kinase. NSCLC=non-small-cell lung cancer.
* The total number of ALKi-pretreated and ALKi-naïve patients who discontinued treatment due to AEs were 18 and 8 patients, respectively. However, for one patient from each group, an AE was not considered the primary reason for discontinuation, and as such the number of patients listed in the patient disposition are 17 and 7, respectively.
Table 1.
Patient baseline characteristics in all patients with ALK-rearranged NSCLC (N=246)
| Characteristics | ALKi-naïve (n=83) |
ALKi-pretreated* (n=163) |
|---|---|---|
| Age, median (range), years | 55 (22–80) | 52 (24–80) |
| Sex, n (%) | ||
| Female | 44 (53·0) | 88 (54·0) |
| WHO/ECOG performance status, n (%) | ||
| 0 | 25 (30·1) | 38 (23·3) |
| 1 | 51 (61·4) | 104 (63·8) |
| 2 | 7 (8·4) | 20 (12·3) |
| ≥3 | 0 | 1 (0·6) |
| Smoking history | ||
| Never/ex-smoker | 82 (98·8%) | 158 (97·0%) |
| Current smoker | 1 (1·2%) | 5 (3·1%) |
| Race, n (%) | ||
| Caucasian | 48 (57·8) | 108 (66·3) |
| Black | 0 | 4 (2·5) |
| Asian | 35 (42·2) | 47 (28·8) |
| Other | 0 | 4 (2·5) |
| Tumour histology/cytology, n (%) | ||
| Adenocarcinoma | 76 (91·6) | 152 (93·3) |
| Other | 7 (8·4) | 11 (6·7) |
| Site of metastasis, n (%) | ||
| Brain | 26 (31·3) | 98 (60·1) |
| Lung | 62 (74·7) | 111 (68·1) |
| Liver | 30 (36·1) | 68 (41·7) |
| Bone | 26 (31·3) | 69 (42·3) |
| Prior treatment regimens, n (%) | ||
| 0 | 16 (19·3) | 0 |
| 1 | 38 (45·8) | 26 (16·0) |
| 2 | 16 (19·3) | 45 (27·6) |
| 3 | 7 (8·4) | 35 (21·5) |
| ≥4 | 6 (7·2) | 57 (35·0) |
| Median time from initial diagnosis to initiation of ceritinib, months (range) | 8·1 (1·0–109·3) | 21·2 (2·4–174·2) |
ALK=anaplastic lymphoma kinase. NSCLC=non-small-cell lung cancer.
All received crizotinib; five patients received the investigational ALK inhibitor CH5424802 after crizotinib.
At study entry, 124 patients with ALK-rearranged NSCLC had asymptomatic or controlled brain metastases (see appendix page 2), of whom 83 (66·9%) had received prior brain radiotherapy. Baseline patient characteristics for patients with and without brain metastases were mostly similar (see appendix pages 3 and 4), although the latter group appeared to have better ECOG PS and fewer prior lines of antineoplastic therapy.
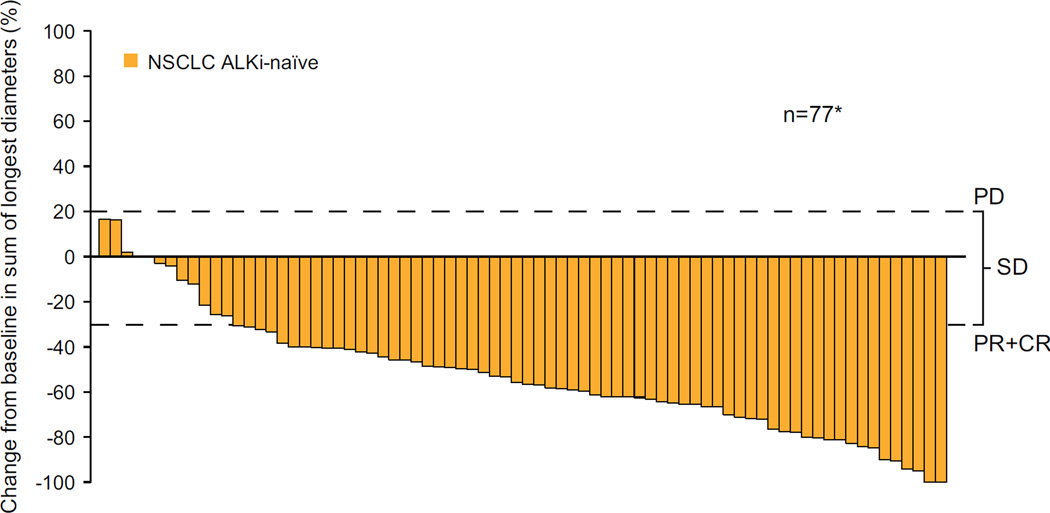
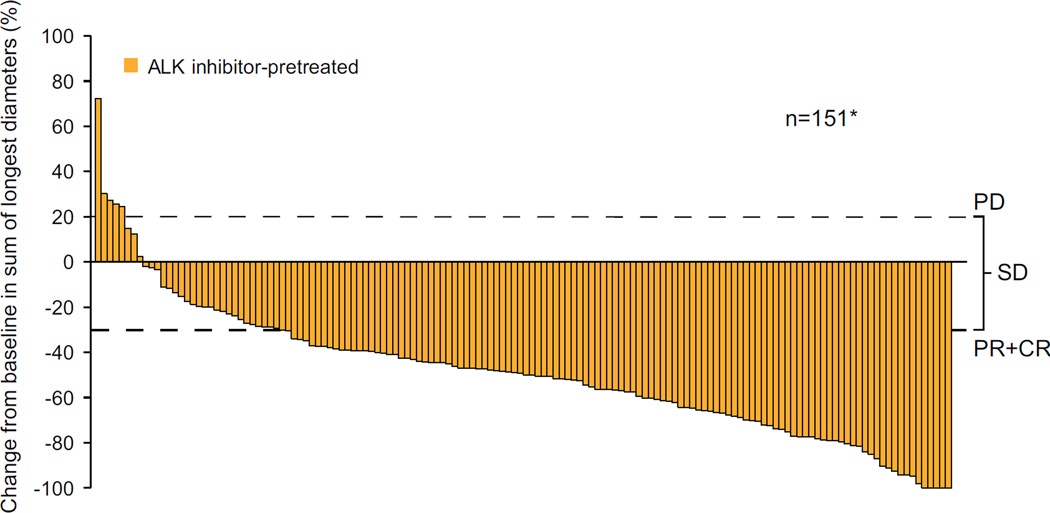
Based on investigator assessment of whole-body efficacy (all sites of disease, including brain; Table 2), the ORR for ALKi-naïve patients was 72·3% (60/83; 95% CI 61·4–81·6), and for ALKi-pretreated patients, 56·4% (92/163; 95% CI 48·5–64·2). Of the 83 ALKi-naïve patients, 16 (19.3%) had received no prior systemic anti-neoplastic therapy; the ORR for these treatment-naïve patients was 68.8% (11/16; 95% CI 41.3–89.0). Median time to response was 6·1 weeks for both ALKi-naïve (range 3·0–42·1 weeks) and ALKi-pretreated (range 4·6–24·1 weeks) patients. Among patients with measurable disease at baseline and at least one post-baseline assessment, a decrease from baseline in tumour burden was observed for the majority of ALKi-naïve and ALKi-pretreated patients (Figure 2A and 2B).
Table 2.
Investigator-assessed whole-body responses for all patients with ALK-rearranged NSCLC receiving ceritinib 750 mg/day (N=246)
| Efficacy parameter | ALKi-naïve (n=83) | ALKi-pretreated (n=163) |
|---|---|---|
| Whole-body responses | ||
| Complete response, n (%) | 1 (1·2) | 3 (1·8) |
| Partial response, n (%) | 59 (71·1) | 89 (54·6) |
| Stable disease, n (%) | 14 (16·9) | 29 (17·8) |
| Progressive disease, n (%) | 0 | 16 (9·8) |
| Unknown, n (%) | 9 (10·8) | 26 (16·0) |
| Overall response rate, n (%) [95% CI] | 60 (72·3) [61·4–81·6] | 92 (56·4) [48·5–64·2] |
| DOR, median [95% CI] (months) | 17·0 [11·3–NE] | 8·3 [6·8–9·7] |
| 12-month DOR, % | 64·3 [48·7–76·3] | 25·6 [ 16·0–36·3] |
| PFS, median [95% CI] (months) | 18·4 [11·1–NE] | 6·9 [5·6–8·7] |
| 12-month PFS, % | 62·3 [50·0–72·4] | 27·2 [19·8–35·1] |
| OS, median [95% CI] (months) | NE [19·61-NE] | 16·7 [14·8–NE] |
| 12-month OS, % | 83·0 [72·4–89·8] | 67·2 [58·9–74·1] |
ALK=anaplastic lymphoma kinase. DOR=duration of response. NSCLC=non-small-cell lung cancer. OS=overall survival. PFS=progression-free survival.
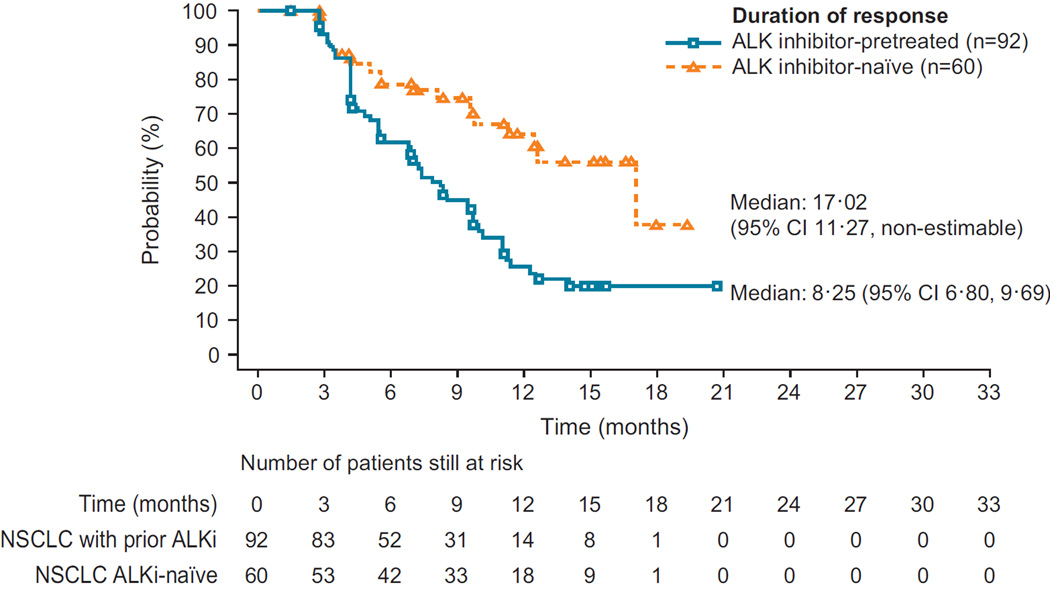
Figure 2. Best percentage change from baseline in tumour volume.
(A) ALKi-naïve patients (n=77) and (B) ALKi-pretreated patients (n=151); upper dotted line indicates threshold for disease progression (PD), lower dotted line represents the threshold of a 30% decrease from baseline (patients with a PR or CR will meet or exceed this threshold) and where the changes fall between the two dotted lines, SD is defined; *n=number of patients with measurable disease at baseline and at least one valid post baseline tumour assessment. (C) Duration of response in all patients with ALK-rearranged NSCLC who responded to ceritinib treatment (N=152) (D) Progression-free survival in all patients with ALK-rearranged NSCLC (N=246)
ALK=anaplastic lymphoma kinase. CR=complete response. PR=partial response. SD=stable disease.
For ALKi-naïve patients, median DOR and PFS were 17·0 (95% CI 11·3–non-estimable [NE]) and 18·4 (95% CI 11·1–NE) months, respectively (Figure 2C, Table 2); median OS had not yet been reached (95% CI 19·6–NE), but 12-month OS rate was 83·0% (95% CI 72·4– 89·8). ALKi-pretreated patients had median DOR and PFS of 8·3 (95% CI 6·8–9·7) and 6·9 (95% CI 5·6–8·7) months, respectively (Figure 2D, Table 2), and median OS of 16·7 months (95% CI 14·8–NE); the 12-month OS rate was 67·2% (95% CI 58·9–74·1). The median duration of follow-up for overall survival at data cut-off (14 April 2014) was 11·1 (IQR 6·7– 15·2) months.
In patients who progressed while on ceritinib, treatment was continued beyond disease progression (defined as ceritinib treatment for more than 3 weeks following documentation of PD) in 12 (14·5%) of 83 ALKi-naïve and 48 (29·4%) of 163 ALKi-pretreated patients. At time of data cut-off, treatment was ongoing in 5 (41·7%) of these 12 ALKi-naïve patients, with the remaining 7 (58·3%) patients having discontinued due to PD. Of the 48 ALKi-pretreated patients, treatment was ongoing in 18 (37·5%) patients, with the remaining patients having discontinued due to PD (n=25; 52·1%), AEs (n=1; 2·1%), lost to follow-up (n=1; 2·1%), and withdrawn consent (n=3; 6·3%). At time of data cut-off, death was reported for two of seven ALKi-naïve and 14 of 30 ALKi-pretreated patients who had discontinued following treatment beyond disease progression.
Whole-body responses for ALKi-naïve and ALKi-pretreated patients with brain metastases at study entry (n=124) were similar to those of the full patient population, with the same pattern of PFS and DOR times (see appendix page 5).
The results of BIRC assessment for efficacy were consistent with the results of the investigator-assessed efficacy analyses (see appendix page 6).
Given the evidence for whole-body response to ceritinib in patients with brain metastases at study entry, retrospective analyses were conducted to specifically evaluate intracranial responses in this subgroup. Of the 124 patients with baseline brain metastases by investigator assessment, brain metastases were retrospectively confirmed by independent neuroradiologists for 94 patients with baseline and at least one post-baseline MRI/CT tumour assessment.
Median time to intracranial response was 6·1 weeks (range 5·1–30·1), consistent with that reported for whole-body responses in the full patient population (n=246). For ALKi-naïve (n=19) and ALKi-pretreated (n=75) patients, median time to intracranial response was 9·9 (range 5·4–30·1) and 6·1 (range 5·1–19·1) weeks, respectively (see appendix page 7). Based on central review by two independent neuroradiologists, ceritinib treatment resulted in an IDCR of 78·9% (15/19; 95% CI 54·4–93·9) in ALKi-naïve patients and 65·3% (49/75; 95% CI 53·5–76·0) in ALKi-pretreated patients with baseline brain metastases (Table 3).
Table 3.
Retrospective analyses of intracranial responses in patients with ALK-rearranged NSCLC with baseline brain metastases and evaluable MRI/CT scans (in all patients and as a function of prior radiotherapy to the brain)
| Efficacy parameter | ALKi-naïve | ALKi-pretreated | ||||
|---|---|---|---|---|---|---|
| Intracranial response in all patients with baseline brain metastases (measurable and non-measurable) by MRI/CT (N=94) | ||||||
| All NSCLC (n=19) |
No prior RT (n=8) |
Prior RT (n=11) |
All NSCLC (n=75) |
No prior RT (n=23) |
Prior RT (n=52) |
|
| Complete response, n | 3 (15·8%) | 1 | 2 | 4 (5·3%) | 2 | 2 |
| Partial response, n | 5 (26·3%) | 3 | 2 | 10 (13·3%) | 3 | 7 |
| Stable disease*, n | 7 (36·8%) | 3 | 4 | 35 (46·7%) | 10 | 25 |
| Progressive disease, n | 0 (0·0%) | 0 | 0 | 12 (16·0%) | 5 | 7 |
| Unknown#, n | 4 (21·1%) | 1 | 3 | 14 (18·7%) | 3 | 11 |
| IDCR, n (%) [95% CI] |
15 (78·9) [54·4–93·9] |
N/A | N/A | 49 (65·3) [53·5–76·0] |
N/A | N/A |
| Intracranial DOR, median [95% CI] (months) |
NE [5·6–NE] |
N/A | N/A | 6·9 [2·9–NE] |
N/A | N/A |
| Intracranial response in patients with measurable baseline brain metastases by MRI/CT (N=36) | ||||||
| All NSCLC (n=8) |
No prior RT (n=4) |
Prior RT (n=4) |
All NSCLC (n=28) |
No prior RT (n=7) |
Prior RT (n=21) |
|
| Complete response (CR), n | 0 (0·0%) | 0 | 0 | 0 (0·0%) | 0 | 0 |
| Partial response (PR), n | 5 (62·5%) | 3 | 2 | 10 (35·7%) | 3 | 7 |
| Stable disease (SD), n | 0 (0·0%) | 0 | 0 | 7 (25·0%) | 1 | 6 |
| Progressive disease (PD), n | 0 (0·0%) | 0 | 0 | 6 (21·4%) | 2 | 4 |
| Unknown, n | 3 (37·5%) | 1 | 2 | 5 (17·9%) | 1 | 4 |
| OIRR (CR+PR), n (%) [95% CI] |
5 (62·5) [24·5–91·5] |
N/A | N/A | 10 (35·7) [18·6–55·9] |
N/A | N/A |
| IDCR (CR+PR+SD), n (%) [95% CI] |
5 (62·5) [24·5–91·5] |
N/A | N/A | 17 (60·7) [40·6–78·5] |
N/A | N/A |
| Intracranial DOR, median [95% CI] (months) |
8·2 [5·6–NE] |
N/A | N/A | 11·1 [2·8–NE] |
N/A | N/A |
ALK=anaplastic lymphoma kinase. CI=confidence interval. CT=computed tomography. DOR=duration of response. IDCR=intracranial disease control rate. MRI=magnetic resonance imaging. N/A=non-applicable. NSCLC=non-small-cell lung cancer. OIRR=overall intracranial response rate. RT=radiotherapy.
Non-CR/non-PD in patients with non-measurable brain lesions at baseline.
A response was listed as unknown if all post-baseline assessments had overall response as unknown (n=5), there was no valid post-baseline assessment (n=1), assessment of PD was considered too late (e.g. no valid assessment before week 12; n=3), or assessment of SD was too early (e.g. assessed before day 42 with no further valid assessment; n=9).
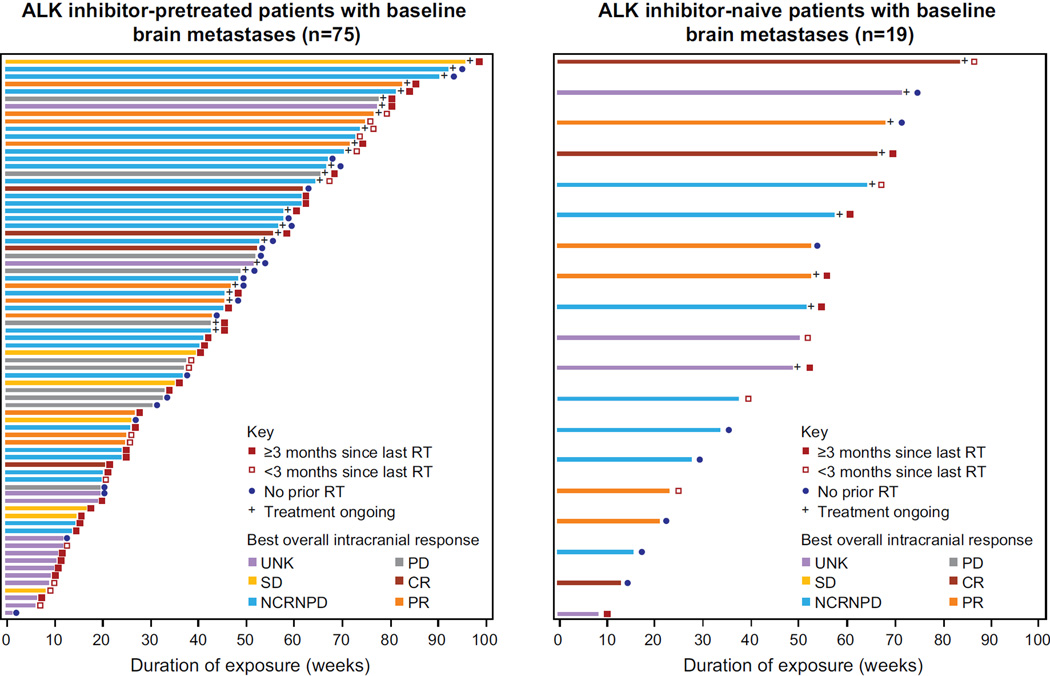
Of the 94 patients included in the retrospective analysis, 63 (67·0%) had previously received radiotherapy to the brain (see appendix page 8). In the 31 (33·0%) of 94 patients who had not previously received radiotherapy to the brain, IDCR (CR+PR+SD) was observed in 7 of 8 ALKi-naïve patients and 15 of 23 ALKi-pretreated patients. This IDCR was comparable to those patients previously treated with radiotherapy.
Based on RECIST 1.1, 36 of 94 patients had measurable intracranial lesions at baseline; for these, the IDCR was 62·5% (5/8; 95% CI 24·5–91·5) in ALKi-naïve patients and 60·7% (17/28; 95% CI 40·6–78·5) in ALKi-pretreated patients (Table 3). The median intracranial DOR in patients with measurable disease was 8·2 months (95% CI 5·6–NE) in ALKi-naïve and 11 1 months (95% CI 2·8–NE) in ALKi-pretreated patients. The majority of patients with measurable brain lesions at baseline had received prior radiotherapy to the brain (25/36; 69·4%); 17 of these had received the last radiotherapy treatment more than 3 months prior to starting ceritinib treatment (see appendix page 9). Among 11 patients (4 ALKi-naïve, 7 ALKi-pretreated) with measurable brain lesions at baseline who had not received prior brain radiotherapy, six achieved a PR (Table 3, see appendix page 9). Intracranial responses were similar in those patients who had received prior brain radiotherapy, regardless of timing of radiotherapy (≥3 months vs <3 months prior to starting ceritinib).
Median duration of exposure to ceritinib 750 mg/day for all 246 patients was 38·6 (range 0·4–105·9) weeks, with a median average daily dose of 664.2 mg (range 357·9–750·0 mg) and median relative dose intensity of 82·8% (range 30·2–100%). Overall, 181 (73·6%) of 246 patients had at least one dose interruption, and 152 (61·8%) of 246 patients had at least one dose reduction. Dose reductions occurred throughout the dosing period (see appendix page 10) with 88 (35·8%) of 246 patients having one dose reduction, 48 (19·5%) two dose reductions, and 16 (6·5%) three or more dose reductions. In patients who discontinued ceritinib treatment, regardless of primary reason, 19 (22·9%) of 83 ALKi-naïve and 43 (26·4%) of 163 ALKi-pretreated patients received further anti-neoplastic therapy following discontinuation (see appendix page 11). However, data on anti-neoplastic therapy following discontinuation were collected for only 28 days following discontinuation, limiting the clinical interpretation of these data.
In all patients with brain metastases at baseline (n=124) and in those included in the retrospective analysis (n=94; Figure 3), duration of exposure to ceritinib 750 mg/day was similar to that reported for all 246 patients (see appendix page 12).
Figure 3. Exposure and response to ceritinib.
Duration of exposure and response to ceritinib in patients with ALK-rearranged NSCLC with brain metastases at baseline by MRI/CT (retrospective, independent readings; n=94). Whether or not the patient received prior radiotherapy to the brain is indicated on the plots; in patients who did received prior radiotherapy, the duration between last radiotherapy treatment and start of ceritinib treatment (≥ or < than 3 months) is also indicated.
ALK=anaplastic lymphoma kinase. BIOR=best intracranial overall response. CR=complete response. NCRNPD=non-complete response non-progressive disease. NSCLC=non-small cell lung cancer. PD=progressive disease. PR=partial response. RT=radiotherapy. SD=stable disease. UNK=unknown.
In the 60 patients who continued ceritinib 750 mg/day beyond disease progression, the median post-progression exposure was 10·1 (range 3·3–71·7) weeks.
All patients with ALK-rearranged NSCLC treated at 750 mg/day (n=246) experienced at least one AE; 96.7% (238/246) were suspected to be drug-related. The most frequent AEs are presented in Table 4; gastrointestinal (GI) toxicity was most common (>60% of patients, mostly grade 1/2), including diarrhoea, nausea, and vomiting, generally occurring early in treatment (median time to onset, days [quartiles 1, 3]: 4 [1, 13], 8 [1, 22], and 8 [2, 32], respectively). These common GI toxicities were manageable through administration of concomitant medication and, where required, dose modification. One (0·4%) of the 246 patients discontinued ceritinib because of a GI AE (grade 1 nausea).
Table 4.
Adverse events occurring at grades 1–2 in ≥10% or at grade 3 or grade4a in ≥2% of patients with ALK-rearranged NSCLC
| Adverse event | Patients with ALK-rearranged NSCLC treated with 750 mg/day (N=246) |
||
|---|---|---|---|
| Grade 1–2 n (%) |
Grade 3 n (%) |
Grade 4 n (%) |
|
| Diarrhoea | 198 (80.5) | 15 (6.1) | 0 (0.0) |
| Nausea | 190 (77.2) | 15 (6.1) | 0 (0.0) |
| Vomiting | 139 (56.5) | 11 (4.5) | 0 (0.0) |
| Fatigue | 94 (38.2) | 12 (4.9) | 0 (0.0) |
| Abdominal pain | 91 (37.0) | 3 (1.2) | 0 (0.0) |
| Decreased appetite | 89 (36.2) | 4 (1.6) | 0 (0.0) |
| Constipation | 75 (30.5) | 0 (0.0) | 0 (0.0) |
| Cough | 71 (28.9) | 0 (0.0) | 0 (0.0) |
| Abdominal pain, upper | 57 (23.2) | 2 (0.8) | 0 (0.0) |
| Dyspnoea | 52 (21.1) | 9 (3.7) | 1 (0.4) |
| Back pain | 49 (19.9) | 1 (0.4) | 0 (0.0) |
| Headache | 47 (19.1) | 4 (1.6) | 0 (0.0) |
| Asthenia | 45 (18.3) | 2 (0.8) | 0 (0.0) |
| Weight decreased | 41 (16.7) | 4 (1.6) | 0 (0.0) |
| Insomnia | 37 (15.0) | 0 (0.0) | 0 (0.0) |
| Pyrexia | 37 (15.0) | 0 (0.0) | 0 (0.0) |
| Musculoskeletal pain | 36 (14.6) | 0 (0.0) | 0 (0.0) |
| Rash | 33 (13.4) | 0 (0.0) | 0 (0.0) |
| Dizziness | 31 (12.6) | 0 (0.0) | 0 (0.0) |
| Dyspepsia | 30 (12.2) | 1 (0.4) | 0 (0.0) |
| Arthralgia | 26 (10.6) | 0 (0.0) | 0 (0.0) |
| Musculoskeletal chest pain | 26 (10.6) | 0 (0.0) | 0 (0.0) |
| Anaemia | 18 (7.3) | 12 (4.9) | 0 (0.0) |
| Pneumonia | 13 (5.3) | 12 (4.9) | 0 (0.0) |
| Convulsion | 7 (2.8) | 7 (2.8) | 1 (0.4) |
| Pneumonitis | 1 (0.4) | 6 (2.4) | 1 (0.4) |
| Respiratory failure | 0 (0.0) | 1 (0.4) | 5 (2.0) |
| Laboratory Abnormalities | |||
| Aspartate aminotransferase increased | 56 (22.8) | 20 (8.1) | 5 (2.0) |
| Blood creatinine increased | 42 (17.1) | 0 (0.0) | 0 (0.0) |
| Alanine aminotransferase increased | 36 (14.6) | 66 (26.8) | 7 (2.8) |
| Blood alkaline phosphatase increased | 31 (12.6) | 13 (5.3) | 0 (0.0) |
| Hypokalaemia | 17 (6.9) | 10 (4.1) | 1 (0.4) |
| Amylase increased | 10 (4.1) | 7 (2.8) | 1 (0.4) |
| Hyponatraemia | 8 (3.3) | 11 (4.5) | 0 (0.0) |
| Hypophosphataemia | 8 (3.3) | 8 (3.3) | 0 (0.0) |
| Lipase increased | 8 (3.3) | 13 (5.3) | 3 (1.2) |
| Gamma-glutamyl transferase increased | 7 (2.8) | 6 (2.4) | 1 (0.4) |
| Hyperglycaemia | 6 (2.4) | 12 (4.9) | 3 (1.2) |
Grade 5 adverse events were not specifically recorded, per the protocol. However, there were two deaths during the study that were considered to be related to study drug: one from interstitial lung disease and the other from multi-organ failure.
ALK=anaplastic lymphoma kinase. NSCLC=non-small-cell lung cancer.
Grade 3/4 AEs (regardless of study drug relationship; Table 4; see appendix pages 13–15) were reported for 200 (81·3%) of 246 patients and serious AEs (SAEs) for 117 (47·6%) of 246 patients. Grade 3/4 AEs and SAEs (of any grade) suspected to be drug-related were experienced by 125 (50·8%) and 29 (11·8%) of 246 patients, respectively. Grade 3/4 increases (regardless of study drug relationship) in alanine aminotransferases and aspartate aminotransferases were reported for 73 (29·7%) and 25 (10·2%) of 246 patients, respectively, but were manageable through dose interruption until resolution; there were no cases of Hy’s law in this study. Although grade 3/4 lipase increase was reported in 16 (6·5%) of 246 patients, there were no cases of increased lipase as an SAE, nor did any patients discontinue treatment as a result of this AE.
Hyperglycaemia was reported at grade 3/4 for 15 (6·1%) of 246 patients, and as an SAE in 6 (2·4%) patients; no patients discontinued treatment due to AE/SAEs associated with hyperglycaemia. Diabetic ketoacidosis (not suspected to be study drug related) was reported at grade 3 in one patient; no action was taken with the study drug and the AE resolved, without recurrence of hyperglycaemia. Interstitial lung disease (ILD)/pneumonitis was reported for 9 (3·7%) of 246 patients, leading to treatment discontinuation in 3 (1·2%) patients; one case was fatal. There were no cases of grade 3/4 bradycardia. There were no cases of corrected QT interval >500 ms; changes from baseline of the corrected QT interval >60 ms occurred in 8 (3·3%) of 246 patients. Overall, 26 (10·6%) of 246 patients discontinued treatment due to AEs, of which nine were suspected to be related to study drug. Two on-treatment deaths were considered related to study drug, one due to ILD (noted above) and the other due to multi-organ failure that occurred in the context of infection and ischaemic hepatitis.
AEs in patients with brain metastases at baseline (n=124) were largely consistent with those reported for the full study population (see appendix page 16).
DISCUSSION
Ceritinib treatment resulted in clinically meaningful, rapid, and durable antitumour responses in both ALKi-naïve and ALKi-pretreated patients with ALK-rearranged NSCLC, most of whom had received multiple prior lines of antineoplastic therapy. In addition, ceritinib antitumour activity was demonstrated in patients with asymptomatic or controlled baseline brain metastases, with both extracranial and intracranial responses observed.
Despite initial efficacy, development of resistance to crizotinib (and other targeted therapeutics) remains an ongoing challenge that has limited the benefit in patients with NSCLC.10,15,22,23 The first-in-human phase 1 study of crizotinib evaluated 143 patients with advanced stage ALK-rearranged NSCLC who had not previously received treatment with an ALKi. In these ALKi-naïve patients, who had baseline characteristics consistent with those reported for other trials in patients with ALK-rearranged NSCLC, 8,21 the ORR was 60·8% and median PFS was 9·7 months.7 In the updated analysis of ceritinib activity in the ASCEND-1 study reported here, those patients who were ALKi-naïve achieved an ORR of 72·3% and median PFS of 18·4 months. Moreover, the median PFS in these patients was longer than that reported for patients treated with crizotinib post-chemotherapy (7·7 months),8 and for patients who received crizotinib as first-line therapy (10·9 months) in the recently published phase 3 PROFILE 1014 trial.9 Of note, patients in the crizotinib PROFILE 1014 study were systemic treatment naïve; in the ASCEND-1 study, 81% of ALKi-naïve patients had received at least one line of prior antineoplastic therapy.
Patients who were ALKi-pretreated also achieved responses following treatment with ceritinib; the ORR in these patients was 56·4% and median PFS was 6·9 months. In this patient subgroup, over half of the patients had received at least three prior lines of therapy. The observed response is consistent with that reported for another phase 1 study (conducted in the USA) investigating the second-generation ALKi alectinib in patients previously treated with crizotinib; the ORR in this study was 55%.21
The high ORR and median DOR reported with ceritinib in this study, particularly in ALKi-naïve patients (72·3% and 17.0 months, respectively), are indicative of durable responses. Overall, outcomes in patients with advanced NSCLC are poor, with first-line chemotherapy reporting ORRs in the range of 28–45% and DORs of 4·5–5·3 months.9,24 Responses with targeted therapies in patients with NSCLC with oncogenic driver mutations are consistently higher.25 Nonetheless, in the first-in-human phase 1 study of crizotinib, ORR was 60·8%, with a corresponding DOR of 49·1 weeks.7
Brain metastases have been reported on diagnosis in around 24% of patients with advanced ALK-rearranged NSCLC, making activity in the brain an important feature of ALK-targeted therapies.26 Despite evidence for potential clinical benefit of crizotinib in patients with baseline brain metastases,27 the brain is the most common site of disease progression following acquired resistance to crizotinib.11,12 Further, in a retrospective pooled analysis of two crizotinib trials (PROFILE 1005 and 1007), the OIRR with crizotinib was substantially lower (18%) than the extracranial response rate (53%).28 The limited activity reported for crizotinib in the brain may be related to lower concentrations of the drug in cerebrospinal fluid compared with the plasma concentration (0·616 ng/ml compared with 237 ng/ml, respectively, 5 hours after a 250 mg dose).13
Brain metastases are associated with a poor prognosis. In the general NSCLC population, survival is rarely extended beyond 12 months, and median PFS times fall in the range of 3–6 months.29–31 Local ablative therapy is an option for patients with ALK-rearranged NSCLC receiving crizotinib who have progression in the brain.12 However, recent data suggest that second-generation ALK inhibitors demonstrate both extracranial and intracranial antitumour activity,21,32 representing an alternative to local ablative therapy. Nonetheless, these data should be interpreted with caution, as the contribution of prior radiotherapy to efficacy outcomes in this patient population is unknown.
This retrospective central analysis of intracranial responses was conducted in patients with baseline brain metastases who were asymptomatic or had stable CNS disease. As it was carried out retrospectively, this analysis has some limitations, including that the pre-defined study assessments, data collection schedule, and sample size were not specifically designed to evaluate this endpoint. Nevertheless, there was promising evidence of intracranial activity, with a high IDCR being achieved, in both ALKi-naïve (78·9%) and ALKi-pretreated (65·3%) patients. Moreover, the median time to intracranial response was similar to that reported for whole-body responses. As radiotherapy is often used to treat brain lesions,31 67% of patients included in the central analysis had previously received radiotherapy to the brain (although, as a retrospective analysis, whether radiotherapy was whole-brain or stereotactic was not recorded). Nonetheless, 6/11 patients with measurable brain disease were radiotherapy-naïve and achieved a PR, indicative of blood-brain barrier penetration of this highly potent ALK inhibitor.20 A confirmatory phase II clinical trial, which is expected to enrol approximately 125 patients, is ongoing to evaluate ceritinib activity in patients with ALK-rearranged NSCLC and metastases to the brain or leptomeninges (NCT02336451). Patients will be stratified according to prior ALKi treatment (pretreated or naïve), whether or not they have received prior whole-brain radiotherapy and leptomeningeal disease.
Longer follow-up shows that the safety profile for ceritinib is similar to that reported previously, and is broadly consistent in patients with or without brain metastases.18 GI AEs, mostly grade 1/2, are the most frequent AEs following ceritinib treatment. These AEs were manageable (only one patient discontinued ceritinib due to a GI AE) and highlight the potential need for early, proactive management and patient education.33 It is not possible to directly compare AE frequency between different studies, limiting the direct comparison of AEs between ceritinib and crizotinib. Common side-effects listed for crizotinib include diarrhoea and nausea, reported in approximately 50–60% of patients, and visual disorders in approximately 60% of patients.7,8 Elevated liver aminotransferases have also been reported as a common side effect with crizotinib; however, this appears more variable.7,8 In the study reported here, grade 3/4 elevations in liver enzymes were common, but manageable through dose interruption or reduction (after which patients could resume ceritinib treatment). ILD/pneumonitis, also a known complication of crizotinib treatment,8 was reported for a small proportion of patients in this study. Overall, rates of discontinuation due to AEs with ceritinib were low. In addition, data on patient-reported outcomes from ongoing phase 2 studies (including both patients previously treated with crizotinib and ALKi-naïve patients) have shown that quality of life is maintained, with reductions in lung symptoms during the course of treatment with ceritinib.34,35
In this 1-year follow-up to the ASCEND-1 study, treatment with ceritinib 750 mg/day continued to result in a high rate of rapid, durable responses and prolonged PFS in ALKi-naïve and ALKi-pretreated patients, along with a manageable safety profile and low rates of study discontinuation. A high level of response was observed in patients with and without baseline brain metastases. Furthermore, intracranial responses were high, including in patients with measurable brain lesions that were radiotherapy-naïve. Taken together, these data expand our understanding of the efficacy and safety of ceritinib and its role in the management of patients with ALK-rearranged NSCLC.
Acknowledgments
The authors thank the participating patients, their families, all study co-investigators, and research coordinators. Thanks are also extended to the central laboratories that performed ALK testing during the trial: NeoGenomic Laboratories, Florida, USA; Institute of Pathology of the University of Heidelberg, Heidelberg, Germany; Institute of Pathology and Genetics (IPG), Gosselies, Belgium, and Molecular Profiling Laboratory of Novartis Institutes for Biomedical Research, Massachusetts, USA. Medical writing support was provided by Sarah Jane Rutherford, PhD, QXV Comms (an Ashfield business, part of UDG Healthcare plc), Macclesfield, UK, and was fully funded by Novartis Pharmaceuticals Corporation.
GRANT SUPPORT
This study was funded by Novartis Pharmaceuticals Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONTRIBUTORS
DWK contributed to data collection, analysis and interpretation, and manuscript preparation and review. RM contributed to study design, data collection, analysis and interpretation, and manuscript preparation and review. DSWT contributed to data collection, analysis, interpretation, manuscript drafting and revision. EF contributed to protocol assessment, data analysis and interpretation, and manuscript preparation and review. LQMC contributed as Principal Investigator at site (University of Washington/Seattle Cancer Care Alliance) for study, enrolled patients on study, helped in study design, data collection, and helped in manuscript writing and review. DRC contributed to data analysis and interpretation, and manuscript preparation and review. JV contributed to data collection, data analysis, data interpretation and editing and approval of the final version. SSh contributed to protocol assessment, data analysis and interpretation, and manuscript preparation and review. TDP contributed to data collection, analysis and interpretation, and manuscript preparation and review. GJR contributed to data collection, analysis and interpretation, and manuscript preparation and review. BJS contributed to accrual of patients to the trial, data collection, data analysis and interpretation and manuscript preparation and review. JW contributed to protocol assessment, data analysis and interpretation, and manuscript preparation and review. MT contributed to data analysis and interpretation, and manuscript preparation and review. MS contributed to conduct of the study, data analysis and interpretation, and manuscript preparation and review. GL contributed to data collection, analysis and interpretation, and manuscript preparation and review. AS contributed to study design, data interpretation and writing. SSu, as an employee of Novartis Pharmaceutical Corporation, has contributed to data analysis and interpretation and review. SL contributed to data analysis and interpretation, and manuscript preparation and review. TS contributed to the data analysis, data interpretation, and manuscript drafting and revision. AY contributed to study design and set up, data cleaning, analysis and interpretation, and manuscript preparation and review. ATS contributed to study design, data collection, analysis and interpretation, and manuscript preparation and review.
DECLARATION OF INTERESTS
Dr Kim reports personal fees from Novartis, personal fees from Pfizer, outside the submitted work. Dr. Mehra reports personal fees from Bristol Meyers Squibb, Novartis, non-financial support from Bristol Meyers Squibb, Novartis, Pfizer, outside the submitted work. Dr Tan reports research grants from Novartis, advisory role for Novartis, Boehringer Ingelheim, Pfizer. Dr. Felip reports personal fees from Lily, Roche, BI, BMS, Novartis, outside the submitted work. Dr. Chow reports non-financial support from Novartis, during the conduct of the study; grants from Novartis, non-financial support from Novartis, personal fees from Novartis, grants from Novartis, outside the submitted work. Dr. Camidge reports personal fees from Servier, Lilly, Roche, Astex, Ariad, ImmunoGen, Clarient, Excelixis, IndiPharm, Astellas, BI, Chugai, Clovis, Array Biopharma, AZ, Novartis, Synta. Honoraria: Ariad, BI, Synta, Array Biopharma, Pfizer, grants from Ariad, outside the submitted work. Dr. Vansteenkiste reports personal fees from Novartis, during the conduct of the study. Dr. Sharma reports grants and personal fees from Novartis, other from Salarius Pharmaceuticals, Beta Cat Pharmaceuticals, ConverGene, and personal fees from VBL Therapeutics IDMC, Arrien Pharma, outside the submitted work. Dr. De Pas has nothing to disclose. Dr. Riely reports grants from Novartis, during the conduct of the study; personal fees from Novartis, outside the submitted work. Dr Solomon reports personal fees from Novartis, Pfizer, Clovis, Clovis Oncology, AZ, Roche, outside the submitted work. Dr Wolf reports personal fees from University Hospital of Cologne; grants and personal fees from AZ, Novartis, Roche, Pfizer, BI, BMS, Clovis, non-financial support from Novartis, Roche BI, outside of the submitted work. Dr. Thomas reports personal fees from Lilly, BMS, Roche, Boehringer, personal fees from Lilly, BMS, Roche, Novartis, Pfizer, MSD, Boehringer, Celgene, outside the submitted work. Dr. Schuler reports personal fees from AZ, BI, Novartis, Pfizer, grants from BI, Novartis, personal fees from BI, Celgene, GSK, Lilly, Novartis, Pfizer, outside the submitted work. Dr. Liu reports personal fees from AZ, Pfizer, Novartis, outside the submitted work. Dr. Santoro has nothing to disclose. Dr. Sutradhar reports other from Novartis Pharmaceutical Corporation, outside the submitted work. Dr. Li reports other from Novartis Pharmaceutical Corporation, outside the submitted work. Dr. Szczudlo reports personal fees from Novartis, outside the submitted work. Dr. Yovine reports personal fees and other from Novartis, outside the submitted work. Dr. Shaw reports grants and personal fees from Novartis, during the conduct of the study; personal fees from Ignyta, Blueprint, personal fees from Pfizer, Ariad, Chugai, Genentech, Roche, outside the submitted work.
REFERENCES
- 1.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song Z, Wang M, Zhang A. Alectinib: a novel second generation anaplastic lymphoma kinase (ALK) inhibitor for overcoming clinically-acquired resistance. Acta Pharmaceutica Sinica B. 2015;5(1):34–37. doi: 10.1016/j.apsb.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chustecka Z. [accessed 20 May 2015];Ceritinib (Zykadia) Recommended for Approval in EU. 2015. 2015 http://www.medscape.com/viewarticle/840585.
- 4.Malik SM, Maher VE, Bijwaard KE, et al. U.S. Food and Drug Administration approval: Crizotinib for treatment of advanced or metastatic non-small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-13-3077. [DOI] [PubMed] [Google Scholar]
- 5. [accessed 16 July 2014];Administration UFaD. http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm395386.htm.
- 6.Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med. 2014;371(21):1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camidge DR, Bang YJ, Kwak EL, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–1019. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 9.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 10.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120) doi: 10.1126/scitranslmed.3003316. 120ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ou SH, Janne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol. 2014;25(2):415–422. doi: 10.1093/annonc/mdt572. [DOI] [PubMed] [Google Scholar]
- 12.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7(12):1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 14.Metro G, Lunardi G, Floridi P, et al. CSF concentration of crizotinib in two ALK-positive non-small-cell lung cancer patients with CNS metastases deriving clinical benefit from treatment. J Thorac Oncol. 2015;10(5):e26–e27. doi: 10.1097/JTO.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 15.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanizaki J, Okamoto I, Okabe T, et al. Activation of HER family signaling as a mechanism of acquired resistance to ALK inhibitors in EML4-ALK-positive non-small cell lung cancer. Clin Cancer Res. 2012;18(22):6219–6226. doi: 10.1158/1078-0432.CCR-12-0392. [DOI] [PubMed] [Google Scholar]
- 17. [accessed 14 August 2014];NCCN Guidelines. http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
- 18.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung Cancer. N Engl J Med. 2014;370(13):1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4(6):662–673. doi: 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novartis. Zykadia Prescribing Information. 2014 [Google Scholar]
- 21.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncology. 2014;15(10):1119–1128. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen KS, Kobayashi S, Costa DB. Acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancers dependent on the epidermal growth factor receptor pathway. Clin Lung Cancer. 2009;10(4):281–289. doi: 10.3816/CLC.2009.n.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 25.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer. 2015;88(1):108–111. doi: 10.1016/j.lungcan.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mok T, Kim D-W, Wu YL, et al. First-line crizotinib versus pemetrexed–cisplatin or pemetrexed–carboplatin in patients (pts) with advanced ALK-positive non-squamous non-small cell lung cancer (NSCLC): results of a phase III study (PROFILE 1014) J Clin Oncol. 2014 [Google Scholar]
- 28.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33(17):1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fokas E, Steinbach JP, Rodel C. Biology of brain metastases and novel targeted therapies: time to translate the research. Biochim Biophys Acta. 2013;1835(1):61–75. doi: 10.1016/j.bbcan.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Khan AJ, Dicker AP. On the merits and limitations of whole-brain radiation therapy. J Clin Oncol. 2013;31(1):11–13. doi: 10.1200/JCO.2012.46.0410. [DOI] [PubMed] [Google Scholar]
- 31.Lukas RV, Lesniak MS, Salgia R. Brain metastases in non-small-cell lung cancer: better outcomes through current therapies and utilization of molecularly targeted approaches. CNS Oncol. 2014;3(1):61–75. doi: 10.2217/cns.13.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerstein D, Gettinger S, Gold K, et al. Evaluation of anaplastic lymphoma kinase (ALK) inhibitor brigatinib [AP26113] in patients (Pts) with ALK+ non–small cell lung cancer (NSCLC) and brain metastases. Ann Oncol. 2015;(suppl 1):i60–i61. [Google Scholar]
- 33.Rothenstein JM, Letarte N. Managing treatment-related adverse events associated with Alk inhibitors. Curr Oncol. 2014;21(1):19–26. doi: 10.3747/co.21.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mok T, Spigel D, Felip E, DeMarinis F, et al. ASCEND-2: A single-arm, open-label, multicenter phase II study of ceritinib in adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with chemotherapy and crizotinib (CRZ); 2015 ASCO Annual Meeting: Meeting Library.2015. [Google Scholar]
- 35.Felip E, Orlov S, Park K, Yu CJ, et al. ASCEND-3: A single-arm, open-label, multicenter phase II study of ceritinib in ALKi-naïve adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC); 2015 ASCO Annual Meeting: Meeting Library.2015. [Google Scholar]