Abstract
Avian-origin influenza represents a global public health concern. In 2013, the H10N8 virus caused documented human infections for the first time. Currently, there is no approved vaccine against H10 influenza. Recombinant virus-like particles (VLPs) represent a promising vaccine approach. In this study, we evaluated H10 VLPs containing hemagglutinin from H10N8 virus as an experimental vaccine in a ferret challenge model. In addition, we evaluated quadri-subtype VLPs co-localizing H5, H7, H9 and H10 subtypes. Both vaccines elicited serum antibody that reacted with the homologous H10 derived from H10N8 virus and cross-reacted with the heterologous H10N1 virus. Quadri-subtype vaccine also elicited serum antibody to the homologous H5, H7, and H9 antigens and cross-reacted with multiple clades of H5N1 virus. After heterologous challenge with the H10N1 virus, all vaccinated ferrets showed significantly reduced titers of replicating virus in the respiratory tract indicating protective effect of vaccination with either H10 VLPs or with quadri-subtype VLPs.
1. Introduction
Seasonal and pandemic influenza viruses represent a global health problem [1]. In the U.S. alone, over 200,000 people are hospitalized and over 20,000 people die annually from seasonal influenza epidemics as a result of influenza virus infections or secondary complications [2]. Influenza pandemics represent even a greater threat. Predicting pandemics is a difficult task [3]. Unlike seasonal influenza caused by H1N1, H3N2 and type B viruses, potentially pandemic viruses include multiple subtypes for which humans lack specific immunity. Human infections with avian-origin H5N1, H7N9, H9N2 subtypes have underscored the potential for avian influenza viruses to adapt to humans and start the next pandemic with high mortality rates [4, 5]. In addition to H5, H7, and H9 subtypes, other potentially pandemic avian viruses continue to circulate in nature [5-7]. In 2013, H10N8 virus caused human infections [5, 8, 9]. Currently, there is no approved vaccine against H10N8 virus.
Recombinant virus-like particles (VLPs) comprised of hemagglutinin (HA), neuraminidase (NA) and matrix (M1) proteins have been developed as a novel vaccine approach for prevention of influenza [4, 7, 10-14]. In some cases, retrovirus gag was used in place of M1 [15]. The HA antigen is the major vaccine component, which induces neutralizing antibodies preventing infectious virus from entering cells [4, 16, 17]. Expression within VLPs increases immunogenicity of the HA antigen [12, 18]. In previous studies, VLPs protected from pandemic influenza including the reconstructed 1918 virus, the 2009 swine-origin pandemic virus, and avian-origin influenza viruses [4, 11, 19]. Recombinant VLPs are produced by using cell culture methods and do not require traditional egg-based technology for production. Furthermore, VLPs are capable of co-localizing the HA proteins derived from several subtypes [20, 21]. Such multi-subtype VLP approach was designed to simultaneously elicit specific immunity to multiple influenza subtypes with no requirement for blending individual vaccines. Recombinant VLPs co-localizing three subtypes of HA protected ferrets, a highly sensitive to influenza infection animal model, from potentially pandemic viruses of H5, H7 and H9 subtypes [20, 21]. Recently, we have described a novel, quadri-subtype VLP design, which co-localized within a VLP the H5, H7, H9, and H10 hemagglutinins from influenza subtypes that have caused human infections in the past [15]. For preparation of the mono-subtype H10 and the quadri-subtype H5/H7/H9/H10 VLPs, we used the bovine immunodeficiency virus (BIV) gag protein (Bgag) as the inner core of the VLP in place of M1. Bgag has the advantage of a larger diameter providing more surface area to accommodate HA molecules [15]. The mono-subtype H10 VLPs that included H10 subtype have been also prepared. However, prior to this study, immunogenicity and protective effects of mono- or quadri-subtype VLPs containing H10 subtype against challenge with H10 virus have not yet been tested. Furthermore, limited data are available regarding cross-reaction of VLP-induced antisera with heterologous viruses.
Here, we used the ferret model to evaluate the H10- and quadri-subtype H5/H7/H9/H10 VLPs containing H10 protein for their safety and the induction of immune response against homologous and heterologous influenza subtypes. Cross-protective potential of the immune sera against heterologous influenza antigens was investigated. Finally, protective efficacy of mono- and quadri-subtype VLPs was evaluated in ferrets after heterologous H10 virus challenge.
2. Materials and Methods
2.1. Genes and recombinant baculovirus vectors (rBV)
Preparation of mono-subtype H10 and quadri-subtype H5/7/9/10 VLPs was described in detail elsewhere [15]. Briefly, mono-subtype H10 VLPs were expressed in Spodoptera frugiperda (Sf9) cells using a baculovirus expression vector system (BEVS). The rBV vector co-expressing H10, NA, and Bgag genes contained H10 gene derived from A/Jiangxi/IPB13a/2013 (H10N8) virus. Influenza HA, NA, and Bgag genes were cloned in tandem fashion, each gene within its own transcriptional cassette that included a polyhedrin promoter upstream from each gene, as described elsewhere [7, 15].
Quadri-subtype VLPs were expressed by using rBV co-expressing four HA genes (H5, H7, H9, and H10), as well as NA and Bgag genes. Four distinct full-length HA genes, as well as NA and Bgag genes were introduced in tandem fashion into the rBV resulting in the vector containing six VLP-relevant genes. Influenza HA gene sequences were derived from A/VietNam/1203/2004 (H5N1 clade 1), A/Shanghai/2/2013(H7N9), A/Hong Kong/33982/2009 (H9N2 clade G1), and A/Jiangxi/IPB13a/2013 (H10N8) viruses. The H5N1 clade 1 is a highly pathogenic avian influenza virus (HPAI). Therefore, H5 protein sequence contained polybasic cleavage site recognized by furin protease, while H7, H9 and H10 genes naturally did not contain furin cleavage site. Influenza NA and Bgag sequences were identical to these genes within the mono-subtype H10 VLPs. Based on the indicated sequences, genes were codonoptimized for high-level expression in Sf9 cells and synthesized (Genscript, Piscataway, NJ). All preparations of rBV were plaque-purified and titrated using standard plaque assay in Sf9 cells.
2.2. Expression of H10- and quadri-subtype VLP vaccines
To prepare H10- and quadri-HA VLP vaccines, Sf9 cells were maintained as suspension cultures in SF900II-SFM insect serum free medium at 27°C. For production of each VLP vaccine, Sf9 cells (2×106 cells/ml) were infected at a MOI of 3.0 for 72 h with rBV expressing indicated genes. VLPs were harvested from the growth medium supernatant, clarified using centrifugation and 0.2 μm filtration, concentrated by tangential flow filtration, and purified by anion exchange chromatography [22]. Purified VLPs were further concentrated and purified by ultracentrifugation at 100 000 × g and resuspended in the phosphate buffered saline (PBS) for vaccinations. VLPs were characterized, including particle morphology and size, antigenicity, hemagglutination and NA enzyme activity. Quantitation of HA within VLPs was done by semi-quantitative western blot using highly purified, full-length rH5, rH7, rH9, and rH10 reference antigens [15]. Amino acid sequences of the indicated reference HA antigens used for antigen quantitations were identical to the HA subtypes within the VLPs.
For transmission electron microscopy, purified VLP samples were adsorbed onto a freshly discharged 400 mesh carbon parlodion-coated copper grids, negatively stained with 1% phosphotungstic acid, and visualized on a Hitachi H-7600 transmission electron microscope (Hitachi High Technologies America, Schaumburg, IL).
2.3. Indirect immunofluorescence assay (IFA)
For fluorescent staining, 0.3 ml aliquots of rBV-infected Sf9 cells were seeded into eight-well Nunc LabTek chamber slides. After 72 h incubation at 28°C, Sf9 cells were fixed with cold acetone, and IFA was carried out using antisera specific for H5, H7, H9, and H10 influenza subtypes. The following antibodies were used anti-H5 (H5N1) (A/Indonesia/5/2005) IT-003-005M6 mouse IgG2a MAb, clone 268D8; anti-HA (H7N9) (A/Shanghai/1/2013) IT-003-0073M1 mouse IgG1 MAb, clone 9B12; anti-H9 (A/HongKong/33982/2009)(H9N2) IT-003-0094M5 mouse IgG1 MAb, clone17D8; and anti-H10 (A/blue-winged teal/Louisiana/Sg-00073/07(H10N7)) IT-003-034 rabbit polyclonal antibody (Immune Technology, New York, NY). Antigen-expressing cells were visualized using FITC-conjugated goat anti-mouse IgG (H+L) (KPL, Gaithersburg, MD). Mounting medium containing propidium iodide nuclear counterstain was used in IFA to visualize cell nuclei.
2.4. Vaccinations of ferrets
All animal studies were approved by the CDC Institutional Animal Care and Use Committee and were consistent with applicable local, state, and federal regulations. For vaccinations, adult male Fitch ferrets (4 to 5 months of age, Triple F Farms, Sayre, PA) were used. One group of four animals was inoculated three times (weeks 0, 4 and 22) intramuscularly (i.m.) with H10 VLPs (30 μg of total HA). Two groups of five ferrets were vaccinated with quadri-subtype VLPs either i.m. or intranasally (i.n.) (120 μg of total HA containing 30 μg of each HA subtype). Finally, a control group of four ferrets received PBS as placebo. Prior to initial vaccination, ferrets were confirmed to be serologically negative by HI assay for currently circulating influenza viruses. All ferrets were bled on weeks 4, 8, 12, 16, and 26 for collection of serum to assess specific antibody titers by hemagglutination inhibition (HI). Animals were also bled after challenge with H10 virus as described below.
2.5. Challenge with H10 virus
Ferrets were anesthetized with an i.m. injection of a ketamine-xylazine-atropine cocktail and challenged on week 27 i. n. with 106 EID50 of influenza A/teal/Egypt/12908-NAMRU3/2005 (H10N1) virus in a total volume of 1 ml (500 μl per nostril) diluted in PBS. Viral challenge took place at week 27, five weeks after the final vaccination. Following challenge, ferrets were monitored daily for four days to observe changes in body weight and temperature, as well as clinical signs of illness. Nasal wash samples were collected at 2 and 4 days post-challenge (p.c.). At day 4 p.c., animals were euthanized and nasal turbinates, trachea, and lungs were collected. All the samples were tittered in eggs to determine virus titers in lower and upper respiratory tract.
2.6. Statistical analysis
The statistical significance of viral titer differences between vaccinated and PBS control animals were determined by two-way ANOVA statistical analysis.
2.7. Hemagglutination inhibition (HI) assays
All sera were treated with receptor-destroying enzyme from Vibrio cholerae (Denka Seiken, Tokyo, Japan). HI assay was performed using freshly prepared 0.5% turkey or 1% horse red blood cells (RBCs) with 4 HA units of each indicated antigen.
Homologous influenza antigens that were used to determine HI titers included the full-length homologous recombinant antigens, rH5 derived from A/Viet Nam/1203/2004 (H5N1); rH9 from A/Hong Kong/33982/2009 (H9N2); rH7 from A/Anhui/1/2013 (H7N9) (amino acid sequence identical to A/Shanghai/2/2013(H7N9)); and rH10 from A/Jiangxi-DongHu/346/2013 (H10N8). These reference antigens were expressed using BEVS and isolated and purified from Sf9 cells according to the method described elsewhere [16]. Additionally, heterologous influenza antigens were used for determination of HI titers, which included BPL-inactivated viruses A/teal/Egypt/12908-NAMRU3/2005 (H10N1), A/California/07/2009 NYMC X-197A (H1N1), A/Switzerland/9715293/2013 (H3N2) , as well as several BPL-inactivated H5N1 viruses, A/Hong Kong/156/1997 (clade 0), A/Cambodia/X012331/2013 (clade 1.1.2), A/Indonesia/05/2005 (clade 2.1.3.2), A/bar-headed goose/Qinghai/1A/2005:PR8, (clade 2.2), and A/Bangladesh/3233/2011 (clade 2.2.2). Geometric mean HI titers and standard deviations were calculated using Excel software.
3. Results
3.1. Preparation of H10 VLP vaccine
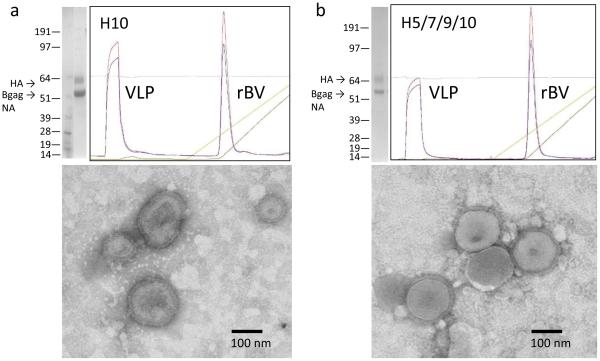
The H10 VLPs were prepared using H10 from avian-origin influenza H10N8 virus, which caused a fatal human infection in an elderly patient in China in 2013 and has been identified as a pathogen of pandemic concern [8, 9]. The rBV vector was configured to co-express three genes, H10, NA and Bgag [15]. The resulting rBV was used to infect Sf9 cells to prepare the mono-subtype H10 VLPs (Figure 1a). During chromatography, VLPs migrated in the flow-through fraction, while majority of the rBV vector particles were bound and separated. In agreement with the previous observations [15], HA was expressed as HA0 of approximately 60-64 kDa, with no detectable processing into HA1 and HA2 (Figure 1a). Prominent band of approximately 55 kDa corresponding to the expected sizes of NA and Bgag was also detected. Functional NA enzyme activity was confirmed in a previous study [15]. Three lots of H10 VLPs were prepared (Table 1). In all lots, the H10 VLPs exhibited hemagglutination activity (titer 8,192 to 65,536 per 50 μl of ~3.5 mg/ml of total protein). The observed lot-to-lot variations could be explained by differences in manufacturing or testing conditions. In general, agreement was consistently observed between the hemagglutination titers and the HA content (Table 1). By electron microscopy, enveloped pleomorphic influenza-like particles of approximately 100-180 nm in diameter were observed in the H10 VLP preparation (Figure 1a). Only a few rod-shaped particles consistent with rBV morphology were detected suggesting efficient purification of the VLPs (Table 1).
Fig. 1.
Preparation and characterization of mono-subtype H10 VLP (a) and quadri-subtype H5/H7/H9/H10 VLPs (b). Coomassie-stained is shown on the left upper panels, with molecular weight and locations of the HA, NA and Bgag are indicated. Anion exchange chromatogram is also shown in the upper panels. Bottom panels indicate the result of transmission electron microscopy of VLPs. Bar, 100nm.
Table 1.
Preparation of H10 VLPs.
| Production# | Lot # | Total Protein *, (mg/ml) |
HA Content**, mg/ml |
HA Titer*** | Spherical/Rod- shaped**** |
|---|---|---|---|---|---|
| 1 | 020215 | 3.48 | 0.4 | 8 192 | 82/3 |
| 2 | 081715 | 3.5 | 1.0 | 32 768 | ND |
| 3 | 082515 | 3.7 | 1.5 | 65 536 | ND |
Determined using Qubit 2.0 fluorometer (Thermo).
By SDS-PAGE and densitometry.
By HA assay using turkey RBC.
Total number of spherical and rod-shaped particles in three representative observation fields, by transmission electron microscopy. ND, not determined.
3.2. Preparation of quadri-subtype H5/7/9/10 VLP vaccine
To prepare quadri-subtype H5/7/9/10 VLPs for vaccinations, HA proteins were derived from the four avian-origin influenza viruses, H5N1, H7N9, H9N2, and H10N8. The H10N8 virus was described above. The clade 1 HPAI H5N1 virus was isolated from a fatal human case [23]. The H7N9 virus was isolated from a hospitalized patient with a fatal disease [24]. The G1 clade H9N2 virus was derived from a nasopharyngeal aspirate of an adult non-fatal patient [25]. In addition, we used NA and Bgag as structural components of VLP. For preparation of VLPs, Sf9 cells were infected with rBV to allow expression and secretion of VLPs containing H5, H7, H9, H10, NA, and Bgag genes.
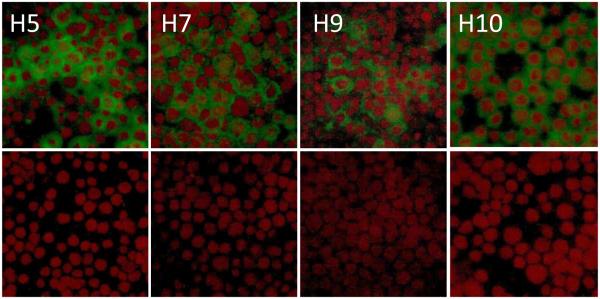
Initially, expression of H5, H7, H9, and H10 genes was evaluated by IFA, with expression of each subtype confirmed using subtype-specific antibodies (Figure 2). The VLPs were harvested from Sf9 culture supernatant and separated from rBV by anion exchange chromatography followed by ultracentrifugation. Quadri-subtype VLPs migrated on the flow-through fraction (Figure 1b). The HAs within VLPs represented uncleaved HA0 polypeptides of approximately 62-64 kDa, in agreement with expected sizes of the H5, H7, H9, and H10 genes of 64.5 kDa, 62.1 kDa, 62.9 kDa, and 62.3 kDa, respectively. Processing into HA1 and HA2 was not detected including H5 protein containing polybasic furin cleavage site. The prominent band of ~55 kDa corresponded to the expected molecular weight of the NA and Bgag (Figure 1b). By SDS-PAGE, the HA bands in the H5/H7/H9/H10 VLPs and H10 VLPs were comparable, while 55 kDa NA/Bgag band was more prominent in the H10 VLPs (Figure 1a,b; top left panels). This can be explained by the increased total HA content in the H5/H7/H9/H10 VLPs. The NA functional enzyme was confirmed by using fluorescence-based influenza NA assay as described elsewhere [15]. Three lots of quadri-subtype H5/7/9/10 VLPs were prepared and characterized (Table 2). Lot-to-lot variations were observed; however, the functional ability of H5/H7/H9/H10 VLPs to agglutinate RBCs was confirmed in all lots by the HA assay using turkey RBCs. Co-localization of subtypes and quantitation of each subtype within quadri-subtype H5/H7/H9/H10 VLPs have been reported in a previous study, with H5, H7, H9, and H10 represented at 24%, 23%, 34%, and 19% of the total HA content, respectively [15]. Finally, by negative-staining transmission electron microscopy, the H5/H7/H9/H10 VLPs were identified as largely spherical, pleomorphic, enveloped particles approximately 150-200 nm in diameter and containing typical influenza HA spikes protruding from the VLP envelope (Figure 1b).
Fig. 2.
Indirect immunofluorescence assays (IFA) for Sf9 cells infected with recombinant baculovirus (rBV) expressing H5, H7, H9, H10, NA, and Bgag genes. Aliquots of the infected cells (upper panel) and uninfected controls (bottom panel) were seeded into chamber slides and incubated for 48h. Cell monolayers were fixed with cold acetone and probed with primary antibodies specific for H5, H7, H9 or H10 antigens as indicated. After incubation, monolayers were probed with appropriate species-specific, FITC-labeled antibodies to visualize expressed HA antigens (in green color). Slides were covered with mounting medium containing propidium iodide counterstain to visualize nuclei (in red).
Table 2.
Preparation of H5/7/9/10 VLPs.
| Production# | Lot # | Total Protein *, (mg/ml) |
HA Content**, mg/ml |
HA Titer*** | Spherical/Rod- shaped**** |
|---|---|---|---|---|---|
| 1 | 031815 | 3.5 | 0.5 | 8 192 | 76/2 |
| 2 | 043015 | 3.4 | 0.5 | 32 768 | ND |
| 3 | 072715 | 2.9 | 0.4 | 16 384 | ND |
Determined using Qubit 2.0 fluorometer (Thermo).
By SDS-PAGE and densitometry.
By HA assay using turkey RBC.
Total number of spherical and rod-shaped particles in three representative observation fields, by transmission electron microscopy. ND, not determined.
3.3. Immunogenicity of mono- and quadri-subtype VLPs
Mono-subtype H10 VLPs and quadri-subtype H5/H7/H9/H10 VLPs were formulated in PBS for vaccinations. As there are only limited studies available regarding vaccination against H10N8, we took into account data available for H5N1 vaccines. Vaccination of healthy adults with 2 intramuscular injections of an A/Vietnam/1203/04 (H5N1) vaccine resulted in the potentially protective neutralizing antibody responses mostly at high doses (2 doses of 45 μg and 90 μg) [26]. Therefore, for vaccination of ferrets with H10 VLP we used 30 μg dose of HA. For quadri-subtype VLP experimental vaccine we used a dose of 120 μg. Because H5, H7, H9, and H10 antigens were each present at approximately 20-30% [15], this dose corresponded to approximately 30 μg of each subtype. We also used two vaccination routes, i.m. and i.n., for the quadri-subtype VLP vaccine. Vaccination regimen included three vaccinations (primary vaccination and two boost vaccinations on weeks 0, 4 and 22). Initially, we measured HI titers against the homologous recombinant HA reference antigens rH5, rH7, rH9 and rH10. HI assay is regarded as the standard method in influenza virus serology [27, 28]. HI titers correlate with protection, while antibodies detected by ELISA can be raised to denatured proteins and do not always predict reactivity in HI assay and protection [27, 29].
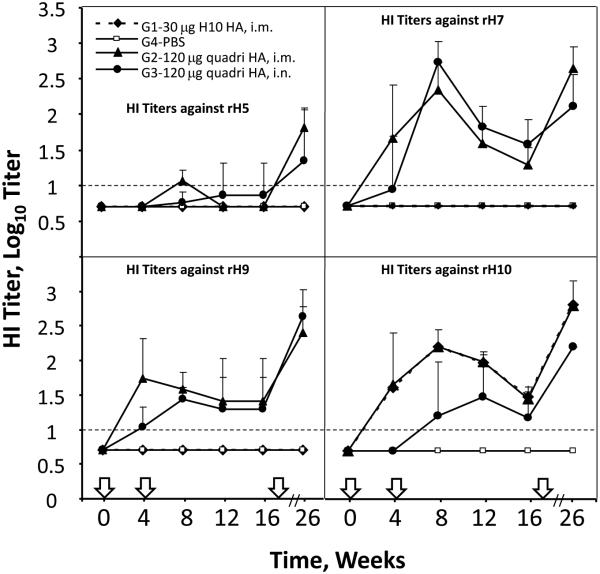
As expected, mock-vaccinated ferrets did not have serum HI antibodies to any of the tested HA. The mono-subtype H10 VLPs elicited HI antibody only against rH10, while the quadri-subtype H5/H7/H9/H10 VLPs elicited HI antibody to H5, H7, H9 and H10 (Figure 3). After i.m. administration of mono-subtype H10 VLPs, HI antibodies we detected after primary vaccination and increased after the boost. Then, HI titers gradually decreased until the second boost. Because a relatively small number of ferrets (4-5/group) were used, we observed considerable variation of responses in the individual animals, especially after the i.n. vaccination. Serum HI antibody titers to rH10 in the mono-subtype group were comparable to the HI titers in the serum of ferrets vaccinated i.m. with the quadri-subtype VLPs, while serum HI titers were lower in ferrets vaccinated i.n. with quadri-subtype VLPs.
Fig. 3.
Immunogenicity of mono-subtype H10 VLP and quadri-subtype H5/H7/H9/H10 VLPs in ferrets. Ferrets were vaccinated as described on weeks 0, 4 and 22 (indicated with arrows on X-axis). Vaccination groups: G1, ferrets inoculated three times (weeks 0, 4 and 22) intramuscularly (i.m.) with H10 VLPs (30 μg of total HA); G2, mock-vaccinated group received PBS as placebo; G3 and G4, two groups of ferrets vaccinated i.m. or intranasally (i.n.), respectively, with 120 μg of HA in quadri-subtype VLPs. Blood was collected on weeks 0, 4, 8, 12, 16 and 26 and serum was analyzed by hemagglutination inhibition (HI) assay using the homologous reference rH5, rH7, rH9, and rH10 antigens. Bars above data points show standard deviations. Dashed horizontal line indicates 1:10 detection limit.
In the quadri-subtype H5/H7/H9/H10 VLP-vaccinated groups, the lowest HI titers were detected to rH5, in which detectable HI titers required at least two vaccinations. This is consistent with the relatively low immunogenicity of the H5 subtype and may reflect low sensitivity of detection method for H5N1 subtype virus [4, 30-33]. In contrast, HI titers to rH7, rH9 and rH10 were detectable after a single vaccination in both i.m. and i.n groups, increased after a boost, and then gradually decreased before a second boost (Figure 3).
3.4. Cross-reactive HI responses
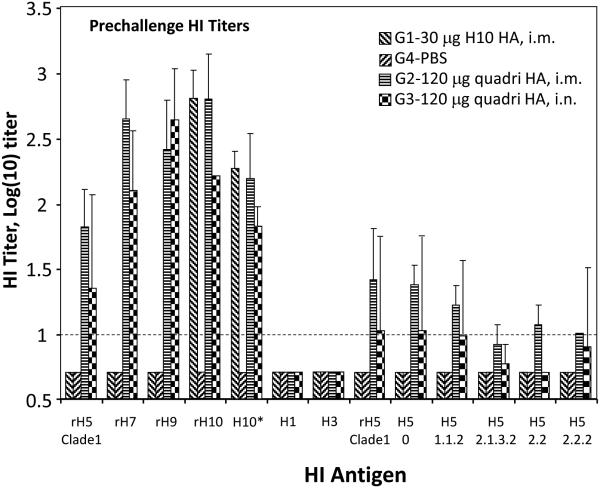
To investigate cross-protective capabilities of VLP vaccinations, pre-challenge sera were tested by HI assay with several influenza isolates of H1, H3, H5 and H10 subtypes (Figure 4). No detectable cross-protective HI antibody to H1 and H3 viruses were detected in sera from either H10 or quadri-subtype VLPs vaccinated ferrets after either i.m. or i.n. vaccinations. However, cross-reactive HI serum antibodies were detected when tested with multiple divergent isolates/clades of H5N1 viruses, as well with divergent H10N1 virus (Figure 4). These results suggest that serum antibody cross-react with multiple antigenic variants of the HA subtypes included in the VLP but not with unrelated subtypes.
Fig. 4.
Summary of HI antibody titer analysis of the pre-challenge sera from vaccinated ferrets. The HI titers are shown on the Y-axis, while HI antigens used in HI assays are shown on X-axis. The HI antigens were the following: rH5 derived from A/Viet Nam/1203/2004 (Clade 1 H5N1); rH9 from A/Hong Kong/33982/2009 (H9N2); rH7 from A/Anhui/1/2013 (H7N9); and rH10 from A/Jiangxi-DongHu/346/2013 (H10N8). BPL-inactivated viruses included H10* A/teal/Egypt/12908-NAMRU3/2005 (H10N1), H1 A/California/07/2009 NYMC X-197A (H1N1), H3 A/Switzerland/9715293/2013 (H3N2) , as well as several H5N1 viruses, A/Hong Kong/156/1997 (clade 0), A/Cambodia/X012331/2013 (clade 1.1.2), A/Indonesia/05/2005 (clade 2.1.3.2), A/bar-headed goose/Qinghai/1A/2005:PR8, (clade 2.2), and A/Bangladesh/3233/2011 (clade 2.2.2). Dashed horizontal line indicates 1:10 detection limit.
3.6. Protection of ferrets from H10N1 virus challenge
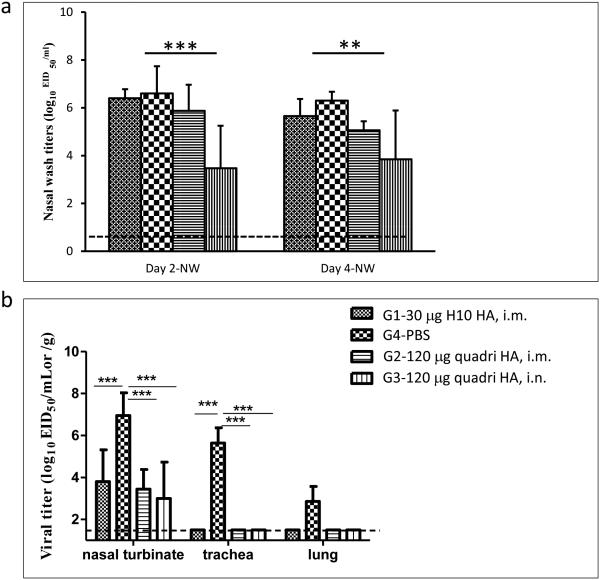
Following vaccination with either H10 VLPs or with quadri-subtype VLPs, ferrets were challenged with H10N1 virus (Figure 5). The A/teal/Egypt/12908-NAMRU3/2005 (H10N1) challenge virus is considered a heterologous challenge, as it is geographically and evolutionarily distinct from the H10 antigen of the A/Jiangxi/IPB13a/2013 (H10N8) virus included into the VLP vaccine. There are 18 amino acid differences (97% identity) between the H10 in the challenge H10N1 virus and the H10 antigen derived from H10N8 virus in the VLP vaccine. Cross-reactive titers were confirmed by HI assay for the H10N1 virus (Figure 4). As expected, the low-pathogenicity H10N1 virus did not cause a lethal or severe disease in ferrets after challenge. No mortality or morbidity was observed in ferrets after challenge. The main measure of protection was the viral titer in nasal washes and in the tissues of the upper (nasal turbinates and trachea) and lower (lungs) respiratory tract following the challenge. In nasal washes on day 2 or day 4 post-challenge, the highest titer was detected in mock-vaccinated animals, while the lowest titers were observed in the animals vaccinated i.m. and i.n. with quadri-subtype VLPs (Figure 5a). The observed reduction in virus titers in the quadri-subtype vaccine groups indicates contributions of H5, H7 and H9 to the protection against H10 challenge, as well as beneficial effect of i.n. route on the protective immunity. Vaccination using i.n. route resulted in the lowest viral titers (Figure 5a). Considering low HI antibody titers to rH10 in the i.n. group (Figures 3, 4) this result suggests a potential role of local mucosal immunity in protection. Interestingly enough, vaccination with mono-subtype H10 VLPs resulted in the reduced virus titer that was not significantly different from the mock-vaccinated animals.
Fig. 5.
Challenge of vaccinated ferrets with H10N1 virus. (a) Titers of replicating virus on days 2 and 4 p.c. in the nasal washes. (b) Titers of replicating virus on day 4 p.c. in the tissues of nasal turbinates, trachea and lungs. Statistically significant differences are indicated with asterisks, with two asterisks indicating p<0.01 and three asterisks indicating p<0.001.
Reduction of replicating virus titers was more apparent in the nasal turbinates and in the tissues of trachea and lung tissues (Figure 5b). In trachea and lung tissues, viral titers were reduced below detectable levels in all vaccinated ferrets that received either mono-subtype H10 VLPs or quadri-subtype H5/H7/H9/H10 VLPs by either route. Viral titers in nasal turbinate tissues were also significantly reduced in all vaccinated animals demonstrating the protective efficacy of these VLP vaccines against a heterologous H10N1 challenge. Again, quadri-subtype VLP vaccination resulted in the detectable reduction of viral titers in the nasal turbinate tissues, as compared to the mono-subtype H10 VLP vaccine, although the observed differences were within standard error.
4. Discussion
Pandemic influenza of avian origin represents a serious concern for the U.S. and world public health. Phylogenetically, there are 18 subtypes of HA that are subdivided into two major antigenic groups, 1 and 2 [34, 35]. Because of genetic drift, influenza viruses accumulate mutations and continuously change. Seasonal vaccines need to be regularly updated to match changing circulating virus strains [36, 37]. Novel influenza variants including vaccine strains can be made by genetic engineering [38]. Protection against multiple viruses is important for pandemic preparedness strategies [37, 39]. Multivalence is a well-known method to prepare broadly protective vaccines against many viral and bacterial agents including influenza, HPV, and pneumococcus. A multivalent influenza vaccine is more likely to protect against an emerging pandemic virus and/or to improve probability of survival in the event of infection. Although a multivalent vaccine may not provide complete protection against all newly emerging strains, the vaccine is expected to reduce the severity of disease and to prevent lethal cases from emerging pathogenic viruses until a specific vaccine is made. An H5N1 vaccine has been prepared and stockpiled with this goal in mind [39]. Presumably, a trivalent or a quadrivalent vaccine can be made by using current vaccine technology. In this case, each virus of interest is grown separately in embryonated hen’s eggs or in cultured cells, processed, and mixed with other strains to generate blended vaccine formulations [37, 40]. However, the need for separate production of highly pathogenic viruses raises biosafety concerns and increases the cost of vaccine.
Promising new approaches for the development of broadly-protective vaccines are being evaluated including conserved epitope approaches [41]. We recently showed that multivalency can be achieved by co-localizing within a VLP protective HA antigens derived from distinct viruses. In this novel vaccine design, HA subtypes were co-localized on a multi-subtype VLP, which resulted in experimental vaccines with broader protective characteristics [20, 21, 42]. Recombinant multi-subtype VLPs vaccines do not depend on eggs for production, have inherent safety features, and provide efficient protection against multiple subtypes and strains, as required for a pandemic vaccine [37, 43]. Multi-subtype VLPs can be produced in a single manufacturing process, with no need for individual preparation of each vaccine or blending, which may decrease the cost of vaccine production. Expression of VLPs from rBV vectors is stable for at least five passages of rBV [15, 42]. Thus, multi-HA VLPs can represent a promising alternative to blended vaccines in order to prepare multivalent formulations including pandemic influenza vaccines and potentially, seasonal influenza vaccines. Potentially, quadri-subtype VLPs can result in the higher HA content in the envelope of VLPs as compared to the monovalent VLPs. Assuming equivalent transcription from the multiple polyhedrin promoters, each gene within the six expression cassettes (quadri-subtype VLP) is calculated to express at 16.7% of the combined polyhedrin expression, with the total 66.8% for four HA subtypes. In contrast, each gene within three expression cassette (H10 VLP) is expected to express at 33.3%. This is in agreement with experimental data (Figure 1a,b; top left panels). However, other factors can affect VLP production including efficiency of VLP assembly and secretion, which require additional research out of scope of the current study.
Here we showed that quadri-subtype VLPs elicit HI antibody against four subtypes including divergent variants within the subtypes included in the VLP. HI antibody titers correlate with protection and represent a gold standard in evaluation of influenza vaccines [27, 28]. The result suggests that quadri-subtype H5/H7/H9/H10 VLPs can induce immunity to all four avian-origin influenza subtypes that are known to infect humans. Protection against heterologous H10 challenge was demonstrated using vaccination with either mono- or quadri-subtype VLPs. Other strategies to protect from H10 influenza include HA stalk- and NA-specific monoclonal antibodies, as well as a 6:2 reassortant virus expressing the surface glycoproteins of A/Jiangxi-Donghu/346/13 (H10N8) on an A/Puerto Rico/8/34 backbone [44, 45]. Although in the current study we did not challenge vaccinated animals with multiple influenza viruses, we previously showed that VLPs displaying three HA subtypes are capable of protecting against multiple homologous virus challenges [20, 21, 42]. It has been recently reported that vaccination with VLPs displaying multiple individual HA subtypes can elicit broad protection in a mouse model [46] suggesting that simultaneous vaccination with multiple subtypes has advantage for broader protection. In the current study, we observed detectable reduction in virus titers in the quadri-subtype vaccine groups, as compared to the H10 vaccine group (Figure 5), which suggests contributions of H5, H7 and H9 to the protection against H10 challenge, although the role of heterosubtypic immunity requires additional studies. Vaccination by the i.n. route can potentially provide an additional advantage. It has been reported previously that i.n. vaccination can result in a broader protection against divergent viruses [11]. Beneficial effects of i.n. vaccination was also confirmed in the current study (Figure 5), although protective effects using i.n. route were less apparent than in our previous study [11]. Here we showed that i.n. vaccination results in the lower serum HI titers, yet provides more efficient clearance of the virus after i.n. challenge suggesting the role of local immunity. Taken together, these results warrant further research of recombinant, multi-subtype VLPs as intrinsically safe recombinant vaccines with potentially broader protective characteristics against influenza viruses.
Highlights.
Mono-subtype H10 VLPs and Quadri-subtype H5/H7/H9/H10 VLPs were prepared in Sf9 cells and evaluated as experimental vaccines in a ferret H10 challenge model.
Immunogenicity of VLPs was confirmed including serum HI antibody reactions with divergent HA subtypes.
Both mono-subtype and quadri-subtype VLPs induced protection against heterologous H10N1 virus challenge.
Acknowledgements
This research was supported in part by grant R01AI111532 from the NIH NIAID. The authors declare no competing financial interests. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015 Mar;14(3):167–82. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- [2].Centers for Disease C, Prevention Estimates of deaths associated with seasonal influenza --- United States, 1976-2007. MMWR Morb Mortal Wkly Rep. 2010 Aug 27;59(33):1057–62. [PubMed] [Google Scholar]
- [3].Trock SC, Burke SA, Cox NJ. Development of Framework for Assessing Influenza Virus Pandemic Risk. Emerg Infect Dis. 2015 Aug;21(8):1372–8. doi: 10.3201/eid2108.141086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kang SM, Pushko P, Bright RA, Smith G, Compans RW. Influenza virus-like particles as pandemic vaccines. Curr Top Microbiol Immunol. 2009;333:269–89. doi: 10.1007/978-3-540-92165-3_14. [DOI] [PubMed] [Google Scholar]
- [5].Xiao C, Ma W, Sun N, Huang L, Li Y, Zeng Z, et al. PB2-588 V promotes the mammalian adaptation of H10N8, H7N9 and H9N2 avian influenza viruses. Sci Rep. 2016;6:19474. doi: 10.1038/srep19474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, Van Hoeven N, et al. Contemporary North American influenza H7 viruses possess human receptor specificity: Implications for virus transmissibility. Proceedings of the National Academy of Sciences of the United States of America. 2008 May 27;105(21):7558–63. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005 Dec 30;23(50):5751–9. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- [8].Garcia-Sastre A, Schmolke M. Avian influenza A H10N8--a virus on the verge? Lancet. 2014 Feb 22;383(9918):676–7. doi: 10.1016/S0140-6736(14)60163-X. [DOI] [PubMed] [Google Scholar]
- [9].To KK, Tsang AK, Chan JF, Cheng VC, Chen H, Yuen KY. Emergence in China of human disease due to avian influenza A(H10N8)--cause for concern? J Infect. 2014 Mar;68(3):205–15. doi: 10.1016/j.jinf.2013.12.014. [DOI] [PubMed] [Google Scholar]
- [10].Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18(1):244–51. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- [11].Perrone LA, Ahmad A, Veguilla V, Lu X, Smith G, Katz JM, et al. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009 Jun;83(11):5726–34. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007 May 10;25(19):3871–8. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- [13].Quan FS, Vunnava A, Compans RW, Kang SM. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PloS one. 5(2):e9161. doi: 10.1371/journal.pone.0009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ross TM, Mahmood K, Crevar CJ, Schneider-Ohrum K, Heaton PM, Bright RA. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PloS one. 2009;4(6):e6032. doi: 10.1371/journal.pone.0006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Tretyakova I, Hidajat R, Hamilton G, Horn N, Nickols B, Prather RO, et al. Preparation of quadri-subtype influenza virus-like particles using bovine immunodeficiency virus gag protein. Virology. 2016 Jan;487:163–71. doi: 10.1016/j.virol.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pushko P, Pujanauski LM, Sun X, Pearce M, Hidajat R, Kort T, et al. Recombinant H7 hemagglutinin forms subviral particles that protect mice and ferrets from challenge with H7N9 influenza virus. Vaccine. 2015 Jul 21; doi: 10.1016/j.vaccine.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cox MM, Izikson R, Post P, Dunkle L. Safety, efficacy, and immunogenicity of Flublok in the prevention of seasonal influenza in adults. Ther Adv Vaccines. 2015 Jul;3(4):97–108. doi: 10.1177/2051013615595595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pushko P, Tumpey TM, Van Hoeven N, Belser JA, Robinson R, Nathan M, et al. Evaluation of influenza virus-like particles and Novasome adjuvant as candidate vaccine for avian influenza. Vaccine. 2007 May 22;25(21):4283–90. doi: 10.1016/j.vaccine.2007.02.059. [DOI] [PubMed] [Google Scholar]
- [19].Pushko P, Kort T, Nathan M, Pearce MB, Smith G, Tumpey TM. Recombinant H1N1 virus-like particle vaccine elicits protective immunity in ferrets against the 2009 pandemic H1N1 influenza virus. Vaccine. 2010 Jul 5;28(30):4771–6. doi: 10.1016/j.vaccine.2010.04.093. [DOI] [PubMed] [Google Scholar]
- [20].Pushko P, Pearce MB, Ahmad A, Tretyakova I, Smith G, Belser JA, et al. Influenza virus-like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine. 2011 Aug 11;29(35):5911–8. doi: 10.1016/j.vaccine.2011.06.068. [DOI] [PubMed] [Google Scholar]
- [21].Tretyakova I, Pearce MB, Florese R, Tumpey TM, Pushko P. Intranasal vaccination with H5, H7 and H9 hemagglutinins co-localized in a virus-like particle protects ferrets from multiple avian influenza viruses. Virology. 2013 Jul 20;442(1):67–73. doi: 10.1016/j.virol.2013.03.027. [DOI] [PubMed] [Google Scholar]
- [22].Liu YV, Massare MJ, Pearce MB, Sun X, Belser JA, Maines TR, et al. Recombinant virus-like particles elicit protective immunity against avian influenza A(H7N9) virus infection in ferrets. Vaccine. 2015 Mar 12; doi: 10.1016/j.vaccine.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, et al. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol. 2005 Sep;79(18):11788–800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013 May 16;368(20):1888–97. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- [25].Molecular characterization of H9N2 isolated in Hong Kong from 2008 to 2009. 2010. Cheng PKCaL, W.L. Unpublished. Hong Kong, GenBank Acc. No. CY055137.
- [26].Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006 Mar 30;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- [27].Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccine Immunol. 2010 Jul;17(7):1055–65. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kumar S, Henrickson KJ. Update on influenza diagnostics: lessons from the novel H1N1 influenza A pandemic. Clin Microbiol Rev. 2012 Apr;25(2):344–61. doi: 10.1128/CMR.05016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Oshansky CM, Wong SS, Jeevan T, Smallwood HS, Webby RJ, Shafir SC, et al. Seasonal influenza vaccination is the strongest correlate of cross-reactive antibody responses in migratory bird handlers. MBio. 2014;5(6):e02107. doi: 10.1128/mBio.02107-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brady RC, Treanor JJ, Atmar RL, Keitel WA, Edelman R, Chen WH, et al. Safety and immunogenicity of a subvirion inactivated influenza A/H5N1 vaccine with or without aluminum hydroxide among healthy elderly adults. Vaccine. 2009 Aug 13;27(37):5091–5. doi: 10.1016/j.vaccine.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zangwill KM, Treanor JJ, Campbell JD, Noah DL, Ryea J. Evaluation of the safety and immunogenicity of a booster (third) dose of inactivated subvirion H5N1 influenza vaccine in humans. J Infect Dis. 2008 Feb 15;197(4):580–3. doi: 10.1086/526537. [DOI] [PubMed] [Google Scholar]
- [32].Stephenson I, Heath A, Major D, Newman RW, Hoschler K, Junzi W, et al. Reproducibility of serologic assays for influenza virus A (H5N1) Emerg Infect Dis. 2009 Aug;15(8):1252–9. doi: 10.3201/eid1508.081754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vemula SV, Zhao J, Liu J, Wang X, Biswas S, Hewlett I. Current Approaches for Diagnosis of Influenza Virus Infections in Humans. Viruses. 2016 Apr;8(4):96. doi: 10.3390/v8040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Medina RA, Garcia-Sastre A. Influenza A viruses: new research developments. Nat Rev Microbiol. 2011 Aug;9(8):590–603. doi: 10.1038/nrmicro2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tong S, Zhu X, Li Y, Shi M, Zhang J, Bourgeois M, et al. New world bats harbor diverse influenza A viruses. PLoS Pathog. 2013;9(10):e1003657. doi: 10.1371/journal.ppat.1003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cox MM. Cell-based protein vaccines for influenza. Current opinion in molecular therapeutics. 2005 Feb;7(1):24–9. [PubMed] [Google Scholar]
- [37].Palese P. Making better influenza virus vaccines? Emerg Infect Dis. 2006 Jan;12(1):61–5. doi: 10.3201/eid1201.051043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fouchier RA, Garcia-Sastre A, Kawaoka Y, Barclay WS, Bouvier NM, Brown IH, et al. Science. New York, NY: Jan 20, 2012. Pause on Avian Flu Transmission Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Oxford JS, Lambkin R, Elliot A, Daniels R, Sefton A, Gill D. Scientific lessons from the first influenza pandemic of the 20th century. Vaccine. 2006 Nov 10;24(44-46):6742–6. doi: 10.1016/j.vaccine.2006.05.101. [DOI] [PubMed] [Google Scholar]
- [40].Subbarao K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat Rev Immunol. 2007 Mar 16;7(4):267–78. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Krammer F, Palese P, Steel J. Advances in universal influenza virus vaccine design and antibody mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol. 2015;386:301–21. doi: 10.1007/82_2014_408. [DOI] [PubMed] [Google Scholar]
- [42].Kapczynski DR, Tumpey TM, Hidajat R, Zsak A, Chrzastek K, Tretyakova I, et al. Vaccination with virus-like particles containing H5 antigens from three H5N1 clades protects chickens from H5N1 and H5N8 influenza viruses. Vaccine. 2016 Mar 18;34(13):1575–81. doi: 10.1016/j.vaccine.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Vemula SV, Ahi YS, Swaim AM, Katz JM, Donis R, Sambhara S, et al. Broadly protective adenovirus-based multivalent vaccines against highly pathogenic avian influenza viruses for pandemic preparedness. PLoS One. 2013;8(4):e62496. doi: 10.1371/journal.pone.0062496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wohlbold TJ, Chromikova V, Tan GS, Meade P, Amanat F, Comella P, et al. Hemagglutinin Stalk- and Neuraminidase-Specific Monoclonal Antibodies Protect against Lethal H10N8 Influenza Virus Infection in Mice. J Virol. 2016 Jan;90(2):851–61. doi: 10.1128/JVI.02275-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wohlbold TJ, Hirsh A, Krammer F. An H10N8 influenza virus vaccine strain and mouse challenge model based on the human isolate A/Jiangxi-Donghu/346/13. Vaccine. 2015 Feb 25;33(9):1102–6. doi: 10.1016/j.vaccine.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schwartzman LM, Cathcart AL, Pujanauski LM, Qi L, Kash JC, Taubenberger JK. An Intranasal Virus-Like Particle Vaccine Broadly Protects Mice from Multiple Subtypes of Influenza A Virus. MBio. 2015;6(4):e01044. doi: 10.1128/mBio.01044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]