Abstract
In a cohort of β-Thalassemia (β-Thal) transplanted with haploidentical-HSCT we identified one transplanted patient characterized by persistent mixed chimerism (PMC) for several months after HSCT. In this unique β-Thal patient we assessed the donor engraftment overtime after transplantation, the potential loss of the non-shared HLA haplotype, and the presence of CD49b+LAG-3+ T regulatory type 1 (Tr1) cells, previously demonstrated to be associated with PMC after HLA-related HSCT for β-Thal. The majority of the patient's erythrocytes were of donor origin, whereas T cells were initially mostly derived from the recipient, no HLA loss, but an increased frequency of circulating Tr1 cells were observed. For the first time, we showed that when the proportion of residual donor cells decreases, the frequency of CD49b+LAG-3+ Tr1 cells declines, reaching the levels present in healthy subjects. These findings confirm previous results obtained in transplant related settings for β-Thal, and supported the central role of Tr1 cells in promoting and maintaining PMC after allo-HSCT.
Keywords: Haploidentical HSCT, T regulatory type 1 (Tr1) cells, mixed chimerism
Results and Discussion
Haematopoietic stem cell transplant (HSCT) is the only cure for β-thalassemia (β-Thal).1 The recent years have witnessed the development and success of haploidentical (haplo)-HSCT approaches, offering a valuable alternative for most β-Thal patients lacking an HLA-matched donor.2,3 In the transplant related settings for β-Thal, approximately 10% of long-term β-Thal HLA-matched transplanted patients, cured and transfusions independent, developed a status of persistent mixed chimerism (PMC) in which donor- and host-derived cells co-exist for more that 2 years after HSCT. In some PMC pts the donor-derived cell proportion is low, but not in mature erythrocytes, which are all of donor origin.4,5 Although the mechanisms that drive the patient toward the establishment of PMC are still unknown, a strong relationship between the presence of mixed chimerism and immunological tolerance has been observed in other HSCT settings, in pregnancy, or after solid organ transplantation.6-8 Long-term mixed chimerism has been achieved after haplo-HSCT in swine conditioned with non-myeloablative regimen.9 Induction of mixed chimerism was observed in patients conditioned with non-myeloablative regimen underwent HSCT from haploidentical related donors sharing at least one HLA-A, -B, or -DR allele on the mismatched haplotype.10 Moreover, the deliberate establishment of mixed chimerism by reprogramming the immune system of the recipient is an effective way of inducing tolerance and maintaining the graft in solid organ transplants. The achievement of transplantation tolerance has been well-documented in patients who received HLA-mismatched or HLA-matched HSCT with conventional myeloablative conditioning to treat a haematological malignancy followed by an organ transplant from the same donor without needing immunosuppressive therapy.11,12 Interestingly, PMC was induced in the HLA-matched studies, whereas only transient mixed chimerism was seen in the HLA-mismatched transplant study. The mechanisms underlying the induction of tolerance to donor grafts have not been clarified yet; however, it has been suggested a role for host T regulatory cells (Tregs) and NKT cells.12
Our group demonstrated that T regulatory type 1 (Tr1) cells, a discrete population of inducible IL-10-producing Tregs cells, are involved in promoting/maintaining PMC.13,14 Tr1 cells co-express CD49b and LAG-3,15 secrete high levels of IL-10 and minimal amounts of IL-4 and IL-17,16 suppress T cell responses via the secretion of IL-10 and TGF-β,16 and kill myeloid antigen-presenting cells through the release of Granzyme B (GzB).17 Tr1 cells were discovered in a patient with severe combined immunodeficiency who developed PMC after an HLA-mismatched fetal liver HSCT.13 We next demonstrated that a high proportion of Tr1 cell clones were identified in the peripheral blood of β-Thal HLA-matched transplanted patients with PMC; conversely, Tr1 cells were not detected in transplanted patients with full-donor engraftment.14 More recently, we confirmed that a high proportion of Tr1 cells, identified as CD49b+LAG-3+ T cells, is present in the blood of β-Thal HLA-matched transplanted patients with PMC compared to both healthy donors and transplanted patients with full-donor engraftment.15
Our group recently reported the outcomes of 31 children with β-Thal who received transplants from haploidentical donors.18,19 As previously reported,19 patients received a pre-conditioning regiment from day −59 to day −11 consisting in Deferoxamine, Hydroxyurea, Azathioprine, and Fludarabine, followed by a conditioning regiment constisting in Busulfan, Cyclophosphamide, Thiotepa, and ATG-Fresenius S. All patients received a megadose of G-CSF-mobilized CD34+ cells, between 4×104 and 15×104, and Cyclosporine for GvHD prophylaxis for the first 2 months post transplantation. Among transplanted patients, 19 developed complete chimerism and are successfully cured, 2 died from transplantation-related causes, 7 rejected their grafts, surviving with β-Thal, and 3 developed PMC and are cured from the disease. Among these 3 PMC patients, 2 showed the presence of few host cells, while the third was characterized by the presence of large amounts of recipient cells for several months after haplo-HSCT. This latter β-Thal patient was haplo-identical with the donor, sharing only one HLA-A-B-C-DR-DQ haplotype, and did not develop GvHD or significant infections complications after transplantation. In this unique β-Thal patient who developed PMC after haplo-HSCT we monitored the donor engraftment and the presence of Tr1 cells at different time points after transplant. White blood cell and T cell counts reached normal levels 3 months after transplant. We detected short-term (+20 and +60 days) after haplo-HSCT full-donor engraftment in peripheral blood mononuclear cells (PBMC) and in bone marrow (BM) that decreased to 62% and 84% at day +172, respectively (Fig. 1A and data not shown). Subsequently, the proportion of donor-derived cells in the BM increased from 89% on day +250 to 97% on day +1334 (data not shown). Conversely, a stable proportion of donor-derived PBMC ranging from 65% to 75% was found till day +723 (Fig. 1A). Afterward the percentage of donor-derived PBMC increased to over 90%, and on day +1334 post haplo-HSCT the patient showed the presence of mixed chimerism, but with a proportion of donor-derived cells of 98% and 97%, in the BM and PBMC, respectively (Fig. 1A, and data not shown). Notably, red blood cells (RBC) were mostly of donor origin, being up to 96% at the time points tested (+221, +546, +1334 days post haplo-HSCT, Fig. 1A). Analysis of the proportion of donor-derived CD3+ T cells isolated over time after haplo-HSCT revealed a progressive increasing from 25% on day +125 to 81% on day +1334 (Fig. 1B). Conversely, CD19+, CD56+ cells, and PMNs, analyzed at the same time points post haplo-HSCT, were mostly of donor origin (range 97–100% for CD19+ cells; 75–86% for CD56+ cells, and 92–100% for PMNs). Although observed in one patient, these findings are in line with results reported in β-Thal patients who develop PMC after sibling allo-HSCT in whom the majority of the patient's erythrocytes were of donor origin, whereas T cells were mostly derived from the recipient.14,20 These findings indicate that the mechanisms underlying the induction of split chimerism in β-Thal patients after HLA-matched HSCT are operational also in haplo-HSCT.
Figure 1.
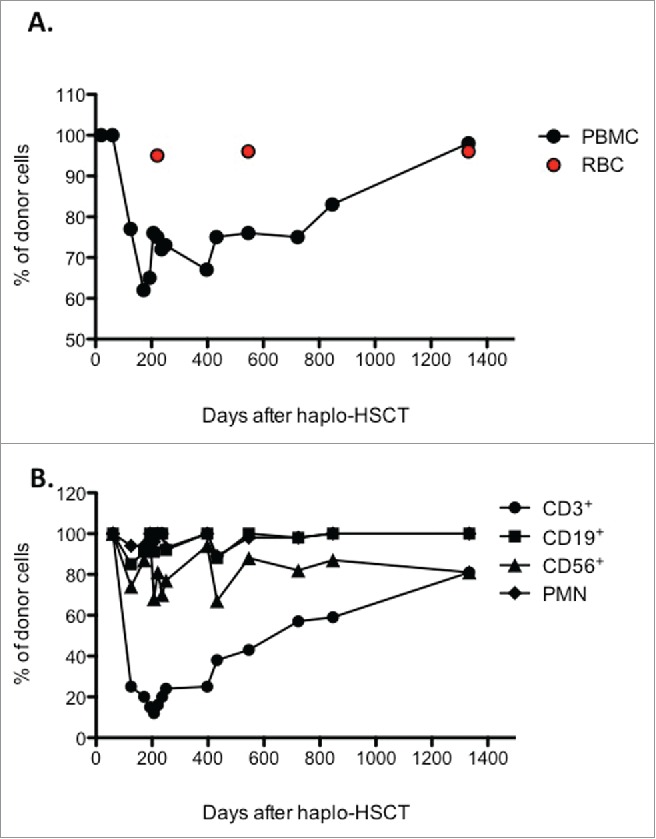
Engraftment evolution in the patient after haplo-HSCT. (A) The frequency of donor-derived cells in peripheral blood mononuclear cells (PBMC) and red blood cells (RBC) of a β-Thal patient underwent haploidentical HSCT was determined by STR (Short Tandem Repeats) at the indicated time points. (B) Donor chimerism was determined in sorted CD3+, CD19+, and CD56+ cells, and CD15+ (PMN) at the indicated time points after haplo-HSCT.
It is well known that haploidentical T cells are able to recognize and eliminate residual patient leukemic cells by an allo-reaction against the patient-specific HLA molecules encoded on the mismatched haplotype. However, Vago et al.21 showed that leukemic cells are frequently able to evade this potent graft-versus-leukemia effect by a permanent loss of the non-shared HLA haplotype. In the described patient, we did not observe the loss of the non-shared HLA haplotype in any of the different lymphoid cell subsets analyzed after haplo-HSCT. Thus, despite CD3+ and CD8+ T cells of the recipient were present in the patient in an extremely high frequency over time after haplo-HSCT, the 2 recipient HLA haplotypes co-exist together with the non-shared HLA haplotype of the donor in the T cells. Based on this observation, it can be postulated that the mechanism of escape from allo-recognition of the CD3+ cells in the haplo-HSCT setting for haemoglobinopathies might not be considered similar to those proposed for oncological settings.21,22
To investigate whether Tr1 cells are involved in the induction and maintenance of PMC in the described patient, we took the advantage of the use of the newly identified biomarkers of Tr1 cells, the CD49b and the LAG-3.15 The proportion of CD4+CD45RA−CD49b+LAG-3+ Tr1 cells was 11% and 16%, on day +125 and +221 after haplo-HSCT, respectively (Table 1), and resulted higher as compared to that observed in healthy donors (1.62±0.55%, mean±StD, n=5), and in transplanted β-Thal patients who rejected the graft (1.63±0.32, mean±StD, n=3). Interestingly, on day +1334 after haplo-HSCT, when a significantly higher proportion of donor-derived CD3+ T cells was observed (81%, Fig. 1B), indicative of the evolution of mixed chimerism toward full-donor engraftment, the proportion of CD4+CD45RA−CD49b+LAG-3+ Tr1 cells declined to 2.1%, reaching the levels generally detected in healthy donors. Although observed in one patient, these results support our previous conclusion that Tr1 cells are involved in establishing and maintaining PMC after allo-HSCT, not only in β-Thal patients transplanted with HLA-related donors,14,15 but also in the haplo-HSCT setting. Moreover, these findings support the hypothesis that Tr1 cells are induced and/or expanded in the presence of persistent chronic (allo) antigenic stimulation,23 and that can be used as biomarkers of mixed chimerism induction and maintenance after allo-HSCT.
Table 1.
Proportion of Tr1 cells.
| Days after Haplo-HSCT | % of donor-derived T cells | % of (CD45RA−CD49b+LAG-3+) Tr1 cells |
|---|---|---|
| +125 | 25% | 11% |
| +221 | 16% | 16% |
| +1334 | 81% | 2.1% |
The frequency of donor-derived T cells in peripheral blood of a β-Thal patient underwent haplo-identical HSCT was determined by STR (Short Tandem Repeats) at the indicated time points. In parallel, the frequency of CD4+ T cells co-expressing CD49b and LAG-3 in the peripheral blood was analyzed by FACS.
To further characterize the immunological status of the haplo-HSCT transplanted patient, we assessed the proliferative responses and cytokine profile of T cells isolated from the peripheral blood on day +125, when the patient showed the co-existence of donor and recipient cells, and on day +1334, when the proportion of residual host T cells drastically decreased. Independently from the time point analyzed, T cells isolated from the patient were hypo-responsive to polyclonal stimulation (i.e. anti-CD3/CD28 mAbs) as demonstrated by the low percentages of CD4+KI67+ T cells (7.7% and 22%, respectively, Table 2). In line with these results, no cytokines were detected in culture supernatant of peripheral blood cells of the patient on day +125, while only limited levels of IFN-γ were secreted by T cells isolated on day +1334 (Table 2). As expected, the proliferation of T cells isolated from peripheral blood of healthy donors or patients who rejected the graft, tested in parallel, were significantly higher compared to those of the patient (92±2.83% and 89±4.24% of CD4+KI67+ T cells, mean±StD, n=2, respectively). Moreover, peripheral blood T cells from healthy donors and from patients with rejection secreted significant and almost comparable levels of IL-2, IFN-γ, TNF-α, IL-17, and limited amounts of IL-10 upon polyclonal stimulation (Table 2). Of note, T cells of the patient on day +1334 after haplo-HSCT showed an increased proliferative capacity and ability to secrete IFN-γ compared to T cells isolated on day +125 post haplo-HSCT. Thus, it can be postulated that the presence of Tr1 cells at high frequency in the peripheral blood might inhibit the proliferative response of effector T cells allowing the co-existence of donor and recipient cells.
Table 2.
T cell proliferation and cytokine production.
| % of CD4+Ki67+ cells | pg/ml |
|||||
|---|---|---|---|---|---|---|
| IL-2 | IFN-γ | TNF-α | IL-17 | IL-10 | ||
| Pt at day +125 | 7.7 | <31 | <31 | <31 | <62 | <15 |
| Pt at day +1335 | 22 | <31 | 248 | <31 | <62 | <15 |
| HDs | 92±2.83 | 175±30 | 3055±245 | 1185±8 | 461±298 | 94±36 |
| Pts with Rejection | 89±4.24 | 317±404 | 1367±1631 | 717±260 | 95±46 | <15 |
Total PBMC of the β-Thal patient underwent haplo-identical HSCT at the indicated time points after haplo-HSCT and of healthy donors (HDs), and of patients who rejected the graft (Pts with Rejection) were stimulated with coated with anti-CD3 and anti-CD28 mAbs for 5 days. After culture, percentage of CD4+Ki67+ T cells and levels of the indicated cytokines in culture supernatants after 24h (IL-2) and 48h (IFN-γ, TNF-α, IL-17, IL-10) were measured. Mean±StD of n=2 HDs and pts with rejection tested in parallel are shown.
In conclusion, we report for the first time the presence of PMC in a β-Thal patient who received transplant from haploidentical donors. Moreover, in this unique haplo-identical transplanted β-Thal patient we show that Tr1 cells are highly represented in peripheral blood during the induction of PMC and that, as soon as the proportion of residual host cells drastically decreases, the frequency of Tr1 cells declines reaching levels normally observed in healthy individuals. Although highly preliminary, our findings support the hypothesis that chronic allogeneic stimulation by mismatched antigen-presenting cells plays a role in Tr1 cell induction after allo-HSCT, and that these cells actively control allogeneic T cell responses leading to the establishment and maintenance of mixed chimerism. Finally, we confirm that CD49b and LAG-3 can be used to trace Tr1 cells in vivo and we propose that the frequency of circulating CD49b+LAG-3+ Tr1 cells can be used as a biomarker for monitoring the induction and maintenance of PMC.
Material and Methods
Signed informed consent was received before transplantation in accordance with the Declaration of Helsinki, and all procedures were performed according to our center's established protocols. The study protocol was approved by the institutional review board of the Mediterranean Institute of Hematology.
Monitoring of Engraftment
Peripheral blood and bone marrow samples were collected in EDTA from patient at different time points after transplantation. CD3, CD19 and CD56 positive cells and granulocytes were obtained by rosette centrisep separation and donor engraftment was established according to the protocol described in.20 Chimerism in red blood cells (RBC) was quantified as previously described.20
HLA Typing
Peripheral blood samples were typed for HLA-A, -B, -C, -DRB1 and -DQB1 genes by PCR-SSO method using Luminex technology (One Lambda - Canoga Park) and, when needed by PCR-SSP (Olerup SSP AB).
Flow Cytometric Analysis
Peripheral blood mononuclear cells were stained with anti-CD4 (SK3, BD PharMingen), anti-CD45RA (HI100, Biolegend), anti-CD49b (clone REA188, Miltenyi Biotech), and anti-LAG-3 (FAB2319P, R&D Systems) mAbs. The staining was performed at 37 °C for 15 min. Samples were acquired using a BD FACS Canto II flow cytometer (Becton Dickinson, CA), and data were analyzed with Flowjo software. Quadrant markers were set accordingly to unstained controls.
Proliferative Response and Cytokine Determination
Peripheral blood mononuclear cells were stimulated at a concentration of 106 cells/ml in 96-well plates coated with anti-CD3 (OKT3, Miltenyi Biotec-10 μg/ml) and anti-CD28 (CD28.2, BD Pharmigen-1 μg/ml) mAbs for 5 days. To determine T cell proliferation, cells were stained with anti-human Ki67 (Biolegend,USA) mAbs according to the manufacturer's instructions. Samples were acquired using a BD FACS Canto II flow cytometer (Becton Dickinson, CA, USA), and data were analyzed with Flowjo software. Concentration levels of cytokines were determined by capture ELISA according to the manufacturer's instruction (BD Biosciences) in culture supernatants after 24h (IL-2) and 48h (IFN-γ, TNF-α, IL-17, IL-10) of culture. The limits of detection were as follows: IL-2: 31 pg/ml; IL-10: 15 pg/ml; IFN-γ: 31 pg/ml; IL-17: 62 pg/ml and TNF- α: 31 pg/ml.
Authorship Contributions
A. M. provided the clinical samples, conceived the scientifc idea, supervised the project, and wrote the manuscript; M. E. G. performed some of the experiments; T.M. analyzed data and contributed to the preparation of the manuscript; B. MR. performed some of the experiments; G. T. performed some of the experiments; M. A., S. P. and L. G. provided the clinical samples, M. G.-R. provided scientific advises; S. G. conceived the scientifc idea, supervised the project, and wrote the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by Telethon Italy grant number TGT11E02, by the IME Foundation (Rome), and by the European grant for European cooperation in science and technology (Action to Focus and Accelerate Cell-based Tolerance-inducing Therapies).
References
- 1.Lucarelli G, Isgro A, Sodani P, Gaziev J. Hematopoietic stem cell transplantation in thalassemia and sickle cell anemia. Cold Spring Harb Perspect Med 2012. May; 2(5):a011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isgro A, Marziali M, Sodani P, Gaziev J, Erer B, Polchi P, Paciaroni K, Roveda A, De Angelis G, Gallucci C, et al.. Immunohematologic reconstitution in pediatric patients after T cell-depleted HLA-haploidentical stem cell transplantation for thalassemia. Biol Blood Marrow Transplant 2010. Nov; 16(11):1557-66; http://dx.doi.org/ 10.1016/j.bbmt.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 3.Gaziev J, Lucarelli G. Hematopoietic stem cell transplantation for thalassemia. Curr Stem Cell Res Ther 2011. Jun; 6(2):162-9; http://dx.doi.org/ 10.2174/157488811795495413 [DOI] [PubMed] [Google Scholar]
- 4.Andreani M, Testi M, Gaziev J, Condello R, Bontadini A, Tazzari PL, Ricci F, De Felice L, Agostini F, Fraboni D, et al.. Quantitatively different red cell/nucleated cell chimerism in patients with long-term, persistent hematopoietic mixed chimerism after bone marrow transplantation for thalassemia major or sickle cell disease. Haematologica 2011. Jan; 96(1):128-33; http://dx.doi.org/ 10.3324/haematol.2010.031013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreani M, Testi M, Lucarelli G. Mixed chimerism in haemoglobinopathies: from risk of graft rejection to immune tolerance. Tissue Antigens 2014. Mar; 83(3):137-46; http://dx.doi.org/ 10.1111/tan.12313 [DOI] [PubMed] [Google Scholar]
- 6.Gammill HS, Adams Waldorf KM, Aydelotte TM, Lucas J, Leisenring WM, Lambert NC, Nelson JL. Pregnancy, microchimerism, and the maternal grandmother. PLoS One 2011; 6(8):e24101; PMID:21912617; http://dx.doi.org/ 10.1371/journal.pone.0024101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graves SS, Mathes DW, Georges GE, Kuhr CS, Chang J, Butts TM, Storb R. Long-term tolerance to kidney allografts after induced rejection of donor hematopoietic chimerism in a preclinical canine model. Transplantation. 2012. Sep 27; 94(6):562-8; http://dx.doi.org/ 10.1097/TP.0b013e3182646bf1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strober S, Spitzer TR, Lowsky R, Sykes M. Translational studies in hematopoietic cell transplantation: treatment of hematologic malignancies as a stepping stone to tolerance induction. Seminars Immunol. 2011. Aug; 23(4):273-81; http://dx.doi.org/ 10.1016/j.smim.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cina RA, Wikiel KJ, Lee PW, Cameron AM, Hettiarachy S, Rowland H, Goodrich J, Colby C, Spitzer TR, Neville DM Jr, et al.. Stable multilineage chimerism without graft vs. host disease following nonmyeloablative haploidentical hematopoietic cell transplantation. Transplantation 2006. Jun 27; 81(12):1677-85; http://dx.doi.org/ 10.1097/01.tp.0000226061.59196.84 [DOI] [PubMed] [Google Scholar]
- 10.Sykes M, Preffer F, McAfee S, Saidman SL, Weymouth D, Andrews DM, Colby C, Sackstein R, Sachs DH, Spitzer TR. Mixed lymphohaemopoietic chimerism and graft-versus-lymphoma effects after non-myeloablative therapy and HLA-mismatched bone-marrow transplantation. Lancet 1999. May 22; 353(9166):1755-9; http://dx.doi.org/ 10.1016/S0140-6736(98)11135-2 [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V, et al.. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008. Jan 24; 358(4):353-61; http://dx.doi.org/ 10.1056/NEJMoa071074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li HW, Sykes M. Emerging concepts in haematopoietic cell transplantation. Nature Rev Immunol 2012. Jun; 12(6):403-16; http://dx.doi.org/ 10.1038/nri3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, de Waal Malefyt R, de Vries JE, Roncarolo MG. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med 1994. Feb 1; 179(2):493-502; http://dx.doi.org/ 10.1084/jem.179.2.493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serafini G, Andreani M, Testi M, Battarra M, Bontadini A, Biral E, Fleischhauer K, Marktel S, Lucarelli G, Roncarolo MG, et al.. Type 1 regulatory T cells are associated with persistent split erythroid/lymphoid chimerism after allogeneic hematopoietic stem cell transplantation for thalassemia. Haematologica 2009. Oct; 94(10):1415-26; http://dx.doi.org/ 10.3324/haematol.2008.003129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, et al.. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 2013. Jun; 19(6):739-46; http://dx.doi.org/ 10.1038/nm.3179 [DOI] [PubMed] [Google Scholar]
- 16.Roncarolo MG, Gregori S, Bacchetta R, Battaglia M. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr Topics Microbiol Immunol 2014; 380:39-68; PMID:25004813 [DOI] [PubMed] [Google Scholar]
- 17.Magnani CF, Alberigo G, Bacchetta R, Serafini G, Andreani M, Roncarolo MG, Gregori S. Killing of myeloid APCs via HLA class I, CD2 and CD226 defines a novel mechanism of suppression by human Tr1 cells. Eur J Immunol 2011. Jun; 41(6):1652-62; http://dx.doi.org/ 10.1002/eji.201041120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sodani P, Isgro A, Gaziev J, Polchi P, Paciaroni K, Marziali M, Simone MD, Roveda A, Montuoro A, Alfieri C, et al.. Purified T-depleted, CD34+ peripheral blood and bone marrow cell transplantation from haploidentical mother to child with thalassemia. Blood 2010. Feb 11; 115(6):1296-302; http://dx.doi.org/ 10.1182/blood-2009-05-218982 [DOI] [PubMed] [Google Scholar]
- 19.Sodani P, Isgro A, Gaziev J, Paciaroni K, Marziali M, Simone MD, Roveda A, De Angelis G, Gallucci C, Torelli F, et al.. T cell-depleted hla-haploidentical stem cell transplantation in thalassemia young patients. Pediatr Rep 2011. Jun 22; 3 Suppl 2:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andreani M, Testi M, Battarra M, Lucarelli G. Split chimerism between nucleated and red blood cells after bone marrow transplantation for haemoglobinopathies. Chimerism 2011. Jan; 2(1):21-2; http://dx.doi.org/ 10.4161/chim.15057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MT, Perrelli NF, Cosentino C, Torri F, Angius A, et al.. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med 2009. Jul 30; 361(5):478-88; http://dx.doi.org/ 10.1056/NEJMoa0811036 [DOI] [PubMed] [Google Scholar]
- 22.Toffalori C, Cavattoni I, Deola S, Mastaglio S, Giglio F, Mazzi B, Assanelli A, Peccatori J, Bordignon C, Bonini C, et al.. Genomic loss of patient-specific HLA in acute myeloid leukemia relapse after well-matched unrelated donor HSCT. Blood 2012. May 17; 119(20):4813-5; http://dx.doi.org/ 10.1182/blood-2012-02-411686 [DOI] [PubMed] [Google Scholar]
- 23.Roncarolo MG, Gregori S, Lucarelli B, Ciceri F, Bacchetta R. Clinical tolerance in allogeneic hematopoietic stem cell transplantation. Immunol Rev 2011. May; 241(1):145-63; http://dx.doi.org/ 10.1111/j.1600-065X.2011.01010.x [DOI] [PubMed] [Google Scholar]


