Abstract
Objective
To estimate adherence and persistence with etanercept plus methotrexate (ETN‐MTX) combination therapy and MTX, hydroxychloroquine, and sulfasalazine triple therapy at 1 year following treatment initiation in adults with rheumatoid arthritis (RA).
Methods
This retrospective analysis used data from the Truven Health MarketScan Commercial and Medicare Supplemental databases from January 2009 to July 2013. Adherence was defined as having percentage of days covered >80% for all drugs within each regimen. Persistence was defined as no treatment gap >45 days for any drug and no addition or switching to other disease‐modifying antirheumatic drugs. Multiple logistic regression models were employed in the analyses to control for potential confounders.
Results
A total of 3,724 ETN‐MTX patients and 818 triple therapy patients were eligible. At 1 year, 27.9% who were taking ETN‐MTX and 18.2% using triple therapy were adherent to all agents in their regimen (P < 0.0001), and 29.4% who were taking ETN‐MTX and 23.2% using triple therapy were persistent (P < 0.001). After adjusting for confounders, ETN‐MTX patients had significantly greater odds of being adherent (odds ratio [OR] 1.79, 95% confidence interval [95% CI] 1.47–2.17) and persistent (OR 1.45, 95% CI 1.20–1.72) compared with patients using triple therapy.
Conclusion
Patients with RA initiating treatment with ETN‐MTX combination therapy demonstrated greater adherence and persistence at 1 year than patients initiating triple therapy.
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic, inflammatory, autoimmune disease that requires long‐term treatment. Current guidelines by the American College of Rheumatology (ACR) and European League Against Rheumatism state that the goal of treatment for RA is to achieve low disease activity or remission 1, 2. For patients with RA, monotherapy or combination therapy with nonbiologic disease‐modifying antirheumatic drugs (DMARDs) is recommended as the initial treatment 1, 2. The addition of a biologic DMARD (biologic agents that target molecules involved in RA pathogenesis) to nonbiologic DMARD therapy is recommended for patients who do not achieve low disease activity or remission with nonbiologic DMARD combination therapy and for patients who do not tolerate nonbiologic DMARD therapy.
Box 1. Significance & Innovations.
This was the first published study to assess nationwide treatment adherence and persistence among patients receiving etanercept plus methotrexate (ETN‐MTX) and triple therapy in privately insured US patients with rheumatoid arthritis (RA).
One‐year adherence and persistence of 2 commonly prescribed combination therapies for RA were generally low, and adherence enhancement interventions may be warranted.
Patients with RA using triple therapy had significantly lower 1‐year adherence and persistence compared with the ETN‐MTX arm: >80% using triple therapy were nonadherent and >75% were nonpersistent.
Etanercept (ETN) is a tumor necrosis factor blocker that is indicated for the treatment of moderately to severely active RA, moderately to severely active polyarticular juvenile idiopathic arthritis in patients ages ≥2 years, psoriatic arthritis, active ankylosing spondylitis, and chronic moderate to severe plaque psoriasis 3. Combination therapy with ETN and methotrexate (MTX) has been associated with better clinical outcomes in patients with RA than ETN or MTX monotherapy 4, 5. In a randomized controlled trial, triple combination therapy with the nonbiologic DMARDs sulfasalazine (SSZ), hydroxychloroquine (HCQ), and MTX was shown to be noninferior to ETN plus MTX therapy at 48 weeks in patients with early, poor prognosis RA with active disease 6. Patients without a response to assigned therapy were switched at week 24 to the alternate regimen. Change from baseline to week 48 in Disease Activity Score based on 28 joints assessed (DAS28) and rates of 50% or 70% improvement in ACR criteria (ACR50 and ACR70, respectively) 7 were similar in patients using triple therapy and ETN plus MTX, and there was no significant difference in adverse events associated with the medications 6. In another randomized, double‐blind clinical trial, DAS28 scores were similar after 2 years of treatment in patients receiving ETN plus MTX and patients receiving triple therapy, but patients taking ETN and MTX had less radiographic progression than patients using triple therapy (change from baseline to week 102 in modified Sharp score of 0.64 versus 1.69; P = 0.05) 8.
Patients enrolled in clinical trials are carefully monitored to ensure that they receive treatment at the protocol‐specified dose at scheduled intervals, whereas in clinical practice clinicians must rely on patients to take their prescribed doses. Although clinical trials provide important information about efficacy and safety of different treatments, they generally cannot account for adherence or persistence to therapy in clinical practice.
Adherence to therapy for RA has been reported to be low with both nonbiologic DMARDs 9, 10 and biologic agents 11, 12. However, no studies have reported on adherence to triple DMARD therapy in clinical practice. Additionally, studies to evaluate adherence to therapy for RA vary in the methods used to assess adherence, including self‐reporting, pill counts, electronic monitoring, laboratory assays, and physician assessments 13. A literature review spanning 10 years of published rates of adherence with nonbiologic DMARDs reported 1‐year rates of adherence ranging from 30% to 81% 10. Similarly, 2 systematic reviews of adherence and persistence to biologic DMARDs for RA reported 1‐year adherence rates ranging from 32% to 91% 11 and 32% to 81% 12. Additionally, rates of adherence decrease with an increasing number of medications 14, 15, 16. Nonadherence to RA medications has a negative effect on clinical outcomes: patients with good adherence have lower disease activity 17, with major and sustained outcomes 18, whereas nonadherence is associated with disease flares 19.
Adherence and persistence to combination nonbiologic and/or biologic DMARDs used in RA have not been clearly demonstrated in real‐world clinical practice. Compared with patients taking monotherapy, patients using combination therapies may be more susceptible to treatment nonadherence, and thus subject to greater likelihood of unrealized therapeutic benefits. We used pharmacy and medical claims from commercial databases to evaluate real‐world adherence to combination therapies used in RA. Specifically, the objective of this study was to evaluate adherence and persistence to triple therapy consisting of MTX, HCQ, and SSZ and to combination therapy with ETN plus MTX in adult US patients with RA.
PATIENTS AND METHODS
Data source
Data for this retrospective analysis were extracted from the Truven Health MarketScan Commercial Claims and Encounters database and the Medicare Supplemental and Coordination of Benefits database. These de‐identified databases represent patients with employer‐provided health insurance and their dependents and include fully adjudicated medical and pharmaceutical claims for over 30 million patients annually in approximately 130 commercial insurance plans across the US. Information in the databases includes inpatient and outpatient diagnoses (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] format), procedures (ICD‐9‐CM or Current Procedural Terminology, Fourth Edition, and Healthcare Common Procedure Coding System formats), and both retail and mail‐order prescription records, including National Drug Code and date and quantity of medication dispensed.
Study design
This study was a retrospective analysis of health care claims for commercially insured individuals with RA initiating ETN‐MTX combination therapy or triple therapy with MTX, HCQ, and SSZ between January 1, 2009 and June 30, 2012 (see Supplementary Figure 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22638/abstract). Patients were required to have a 6‐month preindex period and were followed for 1 year. Treatment adherence, persistence, and criteria suggesting poor treatment response, including switching or adding a biologic or nonbiologic DMARD, increasing glucocorticoid usage, or having multiple intraarticular injections were assessed.
Patients
Commercially insured individuals or retirees with RA initiating ETN‐MTX combination therapy or triple therapy between January 1, 2009 and July 1, 2012 were included in the study. Patients had to have ≥1 claim for ETN and oral MTX or ≥1 claim for oral MTX, HCQ, and SSZ (triple therapy) during the study period. The index date was defined separately for each treatment regimen.
For the ETN‐MTX regimen, the index date was the date ETN was initiated. Patients could initiate ETN and MTX concurrently or could add ETN to MTX. Additionally, 1 of the following events was required to ensure that the patient was starting combination therapy, rather than switching from MTX to ETN: another prescription refill for MTX without a >30‐day refill gap between the adjacent prescriptions, or an overlap of days’ supply of ETN and MTX of ≥28 days.
For the triple therapy regimen the index date was defined as the date the last drug in the regimen was initiated, and that agent was the index agent. To ensure that the patient was not switching DMARDs, patients had to initiate the third agent (MTX, HCQ, or SSZ) while still taking the other 2 drugs, based on days’ supply of prescription claims (Figure 1). Additionally, 1 of the following events was required: another prescription refill for the first 2 drugs without a >30‐day refill gap between the adjacent prescriptions for each drug, or an overlap of days’ supply between the 3 agents of 28 days.
Figure 1.
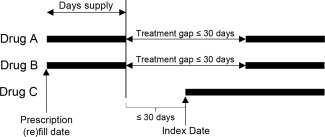
Index date definition for triple therapy regimen for patients with a treatment gap for either or both of the first 2 agents. If the days supply for all drugs in triple therapy regimen overlapped ≥28 days, the patient qualified as a triple therapy user, and the date that the last drug in the series was prescribed was the index date. If a patient did not have overlap of ≥28 days for all drugs, a patient could qualify as a triple therapy user if a subsequent prescription for either of the first two drugs occurred ≤30 days after the end of the days supply for the first claim for that drug.
Patients in both arms had to be ages ≥18 years on the index date, have a diagnosis of RA (ICD‐9‐CM code 714.0) within 6 months prior to or on the index date, and be continuously enrolled 6 months prior to and 12 months after the index date (see Supplementary Figure 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22638/abstract). Patients could not have claims for any RA‐related biologic DMARD (abatacept, adalimumab, anakinra, certolizumab pegol, ETN, golimumab, infliximab, rituximab, tocilizumab) or the nonbiologic DMARD tofacitinib in the 6‐month preindex period for the ETN‐MTX arm or during the preindex period and 30‐day postindex period (to ensure that patients were not waiting for authorization to initiate a biologic agent) for the triple therapy arm; claim for their index agent in the 6‐month preindex period; claim for a new nonbiologic DMARD in the 30 days before or after the index date; or days’ supply of another nonbiologic DMARD in the 30 days postindex. In addition, we excluded patients with a diagnosis of juvenile idiopathic arthritis (ICD‐9‐CM code 714.3x), plaque psoriasis (696.1x), psoriatic arthritis (696.0x), ankylosing spondylitis (720.0x), Crohn's disease (555.xx), or ulcerative colitis (556.xx) in the 6 months before or 30 days after the index date or any J‐codes for MTX during the 12‐month postindex period.
Outcomes
Study outcomes included rates of adherence and persistence to treatment regimen and individual drugs and criteria that might suggest poor treatment response, including switching or adding a biologic or nonbiologic DMARD, increasing glucocorticoid usage, or having multiple intraarticular injections.
Adherence was assessed using percentage of days covered (PDC). PDC is the percentage of days based on days’ supply of prescription claims during which a patient has medication available during the 1‐year postindex period. PDC differs from medication possession ratio in that gaps in coverage earlier in the year cannot be filled in by subsequent early refills. Patients with a PDC >80% for each drug within a treatment regimen were considered adherent to the regimen, independently of whether the last available prescription dates for drugs within each regimen were identical or different 20. The 80% cutoff has been used in studies to examine adherence to medications 21, 22, 23, 24 and was used in our study for consistency.
A gap of 45 days in days’ supply was used to define nonpersistence with treatment. Gaps were measured following the runout of the previous days’ supply, appending for early refills. For the ETN‐MTX arm, nonpersistence was defined as a 45‐day gap in ETN or MTX, switching biologic agents, or adding a nonbiologic DMARD. For the triple therapy arm, nonpersistence was defined as a 45‐day gap in MTX, HCQ, or SSZ, initiating a biologic agent, or adding a nonbiologic DMARD. Sensitivity analyses were conducted using gap lengths of 15, 30, and 60 days to assess the impact of tolerable gap length on persistence rates.
Criteria that suggest poor treatment response were based on those used to estimate effectiveness of RA therapy using claims data 25; however, that algorithm has not been validated for use with these specific regimens, and thus overall effectiveness was not assessed. The criteria used in the effectiveness algorithm were based on factors that could be expected to be associated with a suboptimal response to RA therapies 25. The following criteria were assessed: switching or adding a biologic or nonbiologic DMARD, increasing glucocorticoid usage, or having multiple intraarticular injections after the first 90 days of therapy. Increased glucocorticoid use was defined as use of oral glucocorticoid therapy for >30 days between 90 days after the index date and 1 year postindex for patients who did not use glucocorticoid therapy during the preindex period, or >120% increase in oral glucocorticoid dose during months 7–12 postindex compared with the preindex period for patients who did use glucocorticoid therapy during the preindex period.
Statistical analysis
Descriptive comparisons were made using 2‐sample t‐tests for continuous measures and chi‐square tests for categorical measures. Multiple logistic regression models were used to control for risk factors for nonadherence and nonpersistence for ETN‐MTX and triple therapy users, controlling for age (categories of 18–34, 35–44, 45–54, 55–64, and ≥65 years), sex (male, female), urban status (urban, rural), region (northeast, south, west, or midwest), index year (2009, 2010, 2011, or 2012), health plan type (comprehensive/indemnity, health maintenance organizations, exclusive provider organizations/preferred provider organizations, point‐of‐service, consumer‐directed health plans/high‐deductible health plans, others/unknown), preindex rheumatologist visits (yes, no), preindex total RA‐related costs, preindex comorbidity level (as measured by the Charlson‐Deyo comorbidity index), preindex glucocorticoid use (yes, no), and number of preindex distinct National Drug Code codes (as a proxy for medication use). Patients who met the selection criteria for both cohorts were only included in the triple therapy arm for multivariate modeling. Analyses were performed using Stata software, version 12.1.
RESULTS
Differences between patients
A total of 818 patients were included in the triple therapy arm and 3,724 were included in the ETN‐MTX arm (Table 1 and Supplementary Tables 1, 2, 3, and 4, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22638/abstract). Twenty‐nine patients met the criteria for both arms (all qualified as triple therapy users first) and were included in both arms. Patients in the triple therapy arm had a higher baseline Charlson‐Deyo comorbidity score than the ETN‐MTX arm (1.38 versus 1.30; P = 0.008) and received more outpatient pharmacy medications than patients in the ETN‐MTX arm (12.8 versus 11.5; P < 0.001) in the 6‐month preindex period. Patients in the ETN‐MTX arm were more likely to have seen a rheumatologist than patients in the triple therapy arm (65.5% versus 56.4%; P < 0.001) and had more RA‐related outpatient visits than patients in the triple therapy arm (4.6 versus 3.5 visits; P < 0.001) in the 6‐month preindex period.
Table 1.
Demographic and clinical characteristics at baselinea
| Characteristic | ETN‐MTX (n = 3,724)b | MTX‐HCQ‐SSZ (n = 818)b | P |
|---|---|---|---|
| Age, years | 51.9 ± 11.8 | 55.2 ± 12.5 | < 0.001 |
| Age category, no. (%), years | < 0.001 | ||
| 18–34 | 301 (8.1) | 44 (5.4) | |
| 35–44 | 603 (16.2) | 103 (12.6) | |
| 45–54 | 1,178 (31.6) | 231 (28.2) | |
| 55–64 | 1,244 (33.4) | 276 (33.7) | |
| ≥65 | 398 (10.7) | 164 (20.0) | |
| Female, no. (%) | 2,894 (77.7) | 608 (74.3) | 0.037 |
| Geographic region, no. (%) | < 0.001 | ||
| Northeast | 583 (15.7) | 118 (14.4) | |
| North central | 977 (26.2) | 266 (32.5) | |
| South | 1,395 (37.5) | 246 (30.1) | |
| West | 690 (18.5) | 172 (21.0) | |
| Unknown | 79 (2.1) | 16 (2.0) | |
| Charlson‐Deyo comorbidity index | 1.30 ± 0.75 | 1.38 ± 0.98 | 0.008 |
| Rheumatology visit preindex, no. (%) | 2,438 (65.5) | 461 (56.4) | < 0.001 |
| RA‐related inpatient visits preindex | 0.00 ± 0.07 | 0.00 ± 0.06 | 0.738 |
| RA‐related outpatient visits preindex | 4.6 ± 5.2 | 3.5 ± 3.6 | < 0.001 |
| Outpatient pharmacy medications preindex | 11.5 ± 6.2 | 12.8 ± 6.2 | < 0.001 |
Values are mean ± SD unless indicated otherwise. ETN = etanercept; MTX = methotrexate; HCQ = hydroxychloroquine; SSZ = sulfasalazine; RA = rheumatoid arthritis.
Twenty‐nine patients met the criteria for both study arms (qualified for triple therapy first) and were included in both arms for descriptive analyses.
Adherence
Bivariate analyses indicated that more patients in the ETN‐MTX arm (27.9%) than in the triple therapy arm (18.2%) were adherent at 1 year postindex (P < 0.0001) (Table 2). After controlling for baseline demographic and clinical characteristics, treatment with triple therapy was associated with significantly lower odds of being adherent at 1 year (odds ratio [OR] 0.56, 95% confidence interval [95% CI] 0.46–0.68, P < 0.001) compared to treatment with ETN‐MTX.
Table 2.
Crude adherence with individual drugs and combination therapies at 1 yeara
| ETN‐MTX (n = 3,724) | MTX‐HCQ‐SSZ (n = 818) | ||||||
|---|---|---|---|---|---|---|---|
| Adherence | ETN | MTX | Between ETN‐MTXb | MTX | HCQ | SSZ | Between MTX‐HCQ‐SSZb |
| Individual drug | 1,851 (49.7) | 1,690 (45.4) | – | 456 (55.7) | 430 (52.6) | 268 (32.8) | – |
| Regimen | – | – | 1,040 (27.9) | – | – | – | 149 (18.2) |
Values are the number (percentage). Adherence was defined as having percentage of days covered (number of days covered by the prescription fills during the 1‐year postindex period) >80%. ETN = etanercept; MTX = methotrexate; HCQ = hydroxychloroquine; SSZ = sulfasalazine.
P < 0.0001 between ETN‐MTX and MTX‐HCQ‐SSZ arms.
At 1 year, the mean ± SD PDC for patients taking ETN‐MTX was 67% ± 26% for combined ETN and MTX, 68% ± 33% for ETN, and 65% ± 33% for MTX. For patients using triple therapy, the mean ± SD PDC was 63% ± 25% for all 3 agents, 70% ± 34% for MTX, 51% ± 37% for SSZ, and 69% ± 34% for HCQ.
Persistence
Bivariate analyses indicated that more patients in the ETN‐MTX arm were persistent than patients in the triple therapy arm (29.4% versus 23.2%; P = 0.005 at 1 year) (Table 3). For patients in the ETN‐MTX arm, persistence was similar between ETN and MTX through month 5; persistence for ETN was higher than persistence for MTX from month 6 through 1 year (Figure 2). For patients in the triple therapy arm, persistence was highest throughout the study for MTX, followed by HCQ and SSZ (Figure 2). After controlling for baseline demographic and clinical characteristics, treatment with triple therapy was associated with significantly lower odds of being persistent at 1 year (OR 0.69, 95% CI 0.58–0.83, P < 0.001) compared to treatment with ETN‐MTX. Among patients who added or switched to another biologic or nonbiologic DMARD, the mean time to addition/switch was similar between ETN‐MTX and triple therapy patients (mean 162 days; P = 0.963). Sensitivity analyses showed that 1‐year persistence ranged from 12.5% to 34.6% for ETN‐MTX and from 10.3% to 26.7% for triple therapy using gap lengths of 15, 30, and 60 days.
Table 3.
Crude persistence on treatment regimensa
| Patients persistent | ETN‐MTX (n = 3,724) | MTX‐HCQ‐SSZ (n = 818) | P |
|---|---|---|---|
| 3 months | 2,746 (73.7) | 625 (76.4) | 0.114 |
| 6 months | 1,816 (48.8) | 341 (41.7) | < 0.001 |
| 9 months | 1,336 (35.9) | 241 (29.5) | < 0.001 |
| 1 year | 1,096 (29.4) | 190 (23.2) | < 0.001 |
Values are the number (percentage) unless indicated otherwise. ETN = etanercept; MTX = methotrexate; HCQ = hydroxychloroquine; SSZ = sulfasalazine.
Figure 2.
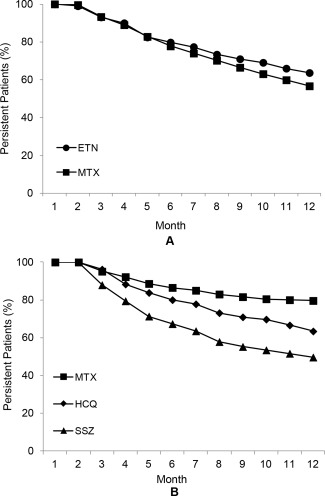
Crude persistence on individual agents for patients who did not switch from their index treatment regimen. The percentages of patients who were persistent on individual agents of (A) ETN‐MTX combination therapy and (B) triple therapy through 1 year are shown. ETN = etanercept; MTX = methotrexate; HCQ = hydroxychloroquine; SSZ = sulfasalazine.
Criteria suggesting poor treatment response
Patients in the triple therapy arm were significantly more likely to add a biologic DMARD than were patients in the ETN‐MTX arm to switch biologic DMARDs (25.8% versus 19.7%; P < 0.001). Conversely, patients in the ETN‐MTX arm were more likely to add a new nonbiologic DMARD than patients in the triple therapy arm (14.4% versus 8.7%; P < 0.001). In addition, patients in the triple therapy arm were more likely to add an oral glucocorticoid than patients in the ETN‐MTX arm (7.6% versus 3.7%; P < 0.001). A similar percentage of patients in each arm had an increase in glucocorticoid dose and received multiple joint injections (Table 4).
Table 4.
Criteria suggesting poor treatment responsea
| Individual reasons | ETN‐MTX (n = 3,724) | MTX‐HCQ‐SSZ (n = 818) | P |
|---|---|---|---|
| Biologic agent switch | 733 (19.7) | 211 (25.8) | < 0.001 |
| New DMARD | 536 (14.4) | 71 (8.7) | < 0.001 |
| Oral glucocorticoid use | 138 (3.7) | 62 (7.6) | < 0.001 |
| Glucocorticoid dose increase | 315 (8.5) | 78 (9.5) | 0.321 |
| Multiple joint injections | 256 (6.9) | 63 (7.7) | 0.402 |
Values are the number (percentage) unless indicated otherwise. ETN = etanercept; MTX = methotrexate; HCQ = hydroxychloroquine; SSZ = sulfasalazine; DMARD = disease‐modifying antirheumatic drug.
DISCUSSION
In this analysis of commercial claims data, RA patients initiating treatment with ETN‐MTX had greater adherence and persistence at 1 year than patients initiating triple therapy. A hierarchy of persistence with individual agents was observed: for patients following the triple therapy regimen, persistence was greatest for MTX, followed by HCQ and SSZ. Rates of adding or switching DMARDs differed between groups, with patients using triple therapy more likely to add a biologic agent and patients taking ETN‐MTX more likely to add another DMARD.
Nonadherence to medications is a substantial problem in the treatment of RA 13. No consistent risk factors for nonadherence to RA medications have been identified 26, 27, but frequent visits to the rheumatologist, satisfaction with the health care provider, and receipt of sufficient information about RA treatment correlate with better adherence 26, 28. Overall, interventions to improve medication adherence have had limited effects 26. Of the published randomized controlled trials of education interventions to improve adherence to nonbiologic DMARDs, 3 found modest 29 or no increases 30, 31 in adherence with group sessions versus handing out brochures, and 1 found no statistically significant difference between group counseling versus individual counseling 32. Importantly, the number of medications has been shown to impact medication adherence 15, 16. A single‐institution analysis of a cohort of patients with a variety of diseases found that medication adherence was significantly lower in patients who were taking 5 or more medications (P < 0.0001) 16. A logistic regression model constructed by Salt and Frazier 15 showed that the number of medications taken for RA was an independent predictor of nonadherence (OR 1.7, P < 0.05). Despite this relationship, we have found that patients using triple therapy as compared with ETN‐MTX had significantly lower odds of being persistent and adherent after adjusting for number of medications used. Given this finding, treatment effectiveness of triple therapy would less likely be realized in real‐world clinical scenarios.
A strength of this analysis was the use of objective claims data to evaluate rates of adherence and persistence, which does not rely on subjective patient‐reported compliance or prescription patterns; adherence and persistence were based on processed health care claims. As such, “adherence” in this study may be more accurately described as “refill adherence.” Assessing the adherence and persistence of triple therapy versus ETN‐MTX combination therapy may not necessarily affect physician prescribing choice between the 2 regimens from an efficacy and safety standpoint. However, these data can provide a proxy indication in regards to the likelihood of realizing treatment effectiveness.
This study had a number of limitations that are general to claims database analyses as well as some that are specific to this study. A diagnosis of RA was identified using ICD‐9‐CM diagnosis codes, which are subject to potential misclassification, although the added requirement of a claim for either a biologic agent or triple therapy should have increased the likelihood that the RA diagnosis was correct. Additionally, by using claims data we could only determine whether patients obtained, but not whether they actually took the drugs. As this study was a retrospective cohort study, the findings may not indicate any causal relationships between the exposure and outcome variables 33. Patients may have been nonadherent or nonpersistent to their index treatment regimen for reasons other than lack of efficacy or safety issues, such as drug costs or alleviated disease severity. Other unmeasured confounders (e.g., race/ethnicity, education, socioeconomic status, etc.) may have affected the relationships identified between treatment regimen and outcomes (adherence and persistence). While risk factors for nonadherence and nonpersistence were considered in this analysis, patient‐ or physician‐reported reasons for nonadherence and nonpersistence were not documented in the study database. This analysis was restricted to only 2 treatment regimens, and evaluation of other regimens is warranted. Another limitation of claims databases is that the prescribers’ intent to prescribe is not known. Thus, patient eligibility involving assessment of prescription claims data (especially for combination therapies) may require extra caution to reduce the likelihood of including false cases. This caution is especially the case for triple therapy users, as many patients may have simply switched from 1 DMARD therapy to another without applying any gatekeeper criteria to identify the patients (Figure 1 and Supplementary Figure 1, available on the Arthritis Care & Research web site at http://onlinelibrary.wiley.com/doi/10.1002/acr.22638/abstract). A conservative approach to define the combination therapies was deemed necessary to ensure that study findings that involved treatment comparisons had sufficient internal validity. Conversely, the strict eligibility requirements for identification of patients using triple therapy may have led to the omission of some patients who actually qualified to be included in the cohort (i.e., external validity).
Patients were identified by their treatment regimen and were not randomized to a treatment arm, and patients in each regimen may therefore represent different populations. The study was conducted using data from adult patients enrolled in a US health plan, and the data may not be generalizable to non‐US plans, pediatric patients, or uninsured or underinsured patients. Because we restricted eligible patients using triple therapy (but not those taking ETN‐MTX) to those who did not have a claim for biologic agents during the 30‐day postindex period to ensure prior authorization of biologic agents did not occur, this single‐arm exclusion criterion may overestimate the adherence and persistence of triple therapy, thus underestimating the differences between ETN‐MTX and triple therapy.
In conclusion, adherence was poor for at least 1 agent in patients taking ETN‐MTX or using triple therapy. Adherence was lowest for MTX in the ETN‐MTX regimen and for SSZ in the triple therapy regimen. This analysis also revealed that patients with RA taking ETN‐MTX had significantly higher adherence and persistence compared with patients using triple therapy. Given the substantial economic and clinical burden associated with nonadherence, it may be necessary to develop programs to improve the adherence of biologic and nonbiologic DMARD combination therapies in patients with RA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Bonafede had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Bonafede, Johnson, Tang, Shah, Harrison, Collier.
Acquisition of data
Bonafede, Johnson.
Analysis and interpretation of data
Bonafede, Johnson, Tang, Shah, Harrison, Collier.
ROLE OF THE STUDY SPONSOR
Immunex/Amgen Inc. and Wyeth/Pfizer had no role in the study design or in the collection, analysis, or interpretation of the data, the writing of the manuscript, or the decision to submit the manuscript for publication. Publication of this article was not contingent upon approval by Immunex/Amgen Inc. or Wyeth/Pfizer.
Supporting information
Supplementary Figure 1. Study design schema and cohort identification. ETN, etanercept; MTX, methotrexate; HCQ, hydroxychloroquine; SSZ, sulfasalazine; RA, rheumatoid arthritis; JIA, juvenile idiopathic arthritis; PsO, psoriasis; PsA, psoriatic arthritis; AS, ankylosing spondylitis; CD, Crohn's disease; UC, ulcerative colitis.
Supplementary Table 1. ETN‐MTX cohort attrition
Supplementary Table 2. MTX in triple therapy cohort attrition
Supplementary Table 3. HCQ in triple therapy cohort attrition
Supplementary Table 4. SSZ in triple therapy cohort attrition
ACKNOWLEDGMENTS
Julie Wang (Amgen Inc.) and Julia R. Gage (Gage Medical Writing, LLC on behalf of Amgen Inc.) provided medical writing support.
REFERENCES
- 1. Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease‐modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smolen JS, Landewe R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Enbrel (etanercept) prescribing information. Thousand Oaks (CA): Immunex Corporation; 2013. [Google Scholar]
- 4. Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340:253–9. [DOI] [PubMed] [Google Scholar]
- 5. Kremer JM, Weinblatt ME, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Jackson CG, et al. Etanercept added to background methotrexate therapy in patients with rheumatoid arthritis: continued observations. Arthritis Rheum 2003;48:1493–9. [DOI] [PubMed] [Google Scholar]
- 6. O'Dell JR, Mikuls TR, Taylor TH, Ahluwalia V, Brophy M, Warren SR, et al. Therapies for active rheumatoid arthritis after methotrexate failure. N Engl J Med 2013;369:307–18. [DOI] [PubMed] [Google Scholar]
- 7. Felson DT, Anderson JJ, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American College of Rheumatology preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. [DOI] [PubMed] [Google Scholar]
- 8. Moreland LW, O'Dell JR, Paulus HE, Curtis JR, Bathon JM, St Clair EW, et al. A randomized comparative effectiveness study of oral triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis: the treatment of Early Aggressive Rheumatoid Arthritis Trial. Arthritis Rheum 2012;64:2824–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van den Bemt BJ, van den Hoogen FH, Benraad B, Hekster YA, van Riel PL, van Lankveld W. Adherence rates and associations with nonadherence in patients with rheumatoid arthritis using disease modifying antirheumatic drugs. J Rheumatol 2009;36:2164–70. [DOI] [PubMed] [Google Scholar]
- 10. De Achaval S, Suarez‐Almazor ME. Treatment adherence to disease‐modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int J Clin Rheumatol 2010;5:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blum MA, Koo D, Doshi JA. Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther 2011;33:901–13. [DOI] [PubMed] [Google Scholar]
- 12. Fidder HH, Singendonk MM, van der Have M, Oldenburg B, van Oijen MG. Low rates of adherence for tumor necrosis factor‐alpha inhibitors in Crohn's disease and rheumatoid arthritis: results of a systematic review. World J Gastroenterol 2013;19:4344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harrold LR, Andrade SE. Medication adherence of patients with selected rheumatic conditions: a systematic review of the literature. Semin Arthritis Rheum 2009;38:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Agarwal S, Zaman T, Handa R. Retention rates of disease‐modifying anti‐rheumatic drugs in patients with rheumatoid arthritis. Singapore Med J 2009;50:686–92. [PubMed] [Google Scholar]
- 15. Salt E, Frazier SK. Predictors of medication adherence in patients with rheumatoid arthritis. Drug Dev Res 2011;72:756–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lyles A, Culver N, Ivester J, Potter T. Effects of health literacy and polypharmacy on medication adherence. Consult Pharm 2013;28:793–9. [DOI] [PubMed] [Google Scholar]
- 17. Waimann CA, Marengo MF, de Achaval S, Cox VL, Garcia‐Gonzalez A, Reveille JD, et al. Electronic monitoring of oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis: consequences of low adherence. Arthritis Rheum 2013;65:1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Contreras‐Yanez I, Cabiedes J, Villa AR, Rull‐Gabayet M, Pascual‐Ramos V. Persistence on therapy is a major determinant of patient‐, physician‐ and laboratory‐reported outcomes in recent‐onset rheumatoid arthritis patients. Clin Exp Rheumatol 2010;28:748–51. [PubMed] [Google Scholar]
- 19. Contreras‐Yanez I, Ponce De Leon S, Cabiedes J, Rull‐Gabayet M, Pascual‐Ramos V. Inadequate therapy behavior is associated to disease flares in patients with rheumatoid arthritis who have achieved remission with disease‐modifying antirheumatic drugs. Am J Med Sci 2010;340:282–90. [DOI] [PubMed] [Google Scholar]
- 20. Choudhry NK, Shrank WH, Levin RL, Lee JL, Jan SA, Brookhart MA, et al. Measuring concurrent adherence to multiple related medications. Am J Manag Care 2009;15:457–64. [PMC free article] [PubMed] [Google Scholar]
- 21. Monane M, Bohn RL, Gurwitz JH, Glynn RJ, Levin R, Avorn J. The effects of initial drug choice and comorbidity on antihypertensive therapy compliance: results from a population‐based study in the elderly. Am J Hypertens 1997;10:697–704. [DOI] [PubMed] [Google Scholar]
- 22. Avorn J, Monette J, Lacour A, Bohn RL, Monane M, Mogun H, et al. Persistence of use of lipid‐lowering medications: a cross‐national study. JAMA 1998;279:1458–62. [DOI] [PubMed] [Google Scholar]
- 23. Gurwitz JH, Yeomans SM, Glynn RJ, Lewis BE, Levin R, Avorn J. Patient noncompliance in the managed care setting: the case of medical therapy for glaucoma. Med Care 1998;36:357–69. [DOI] [PubMed] [Google Scholar]
- 24. Shrank WH, Hoang T, Ettner SL, Glassman PA, Nair K, DeLapp D, et al. The implications of choice: prescribing generic or preferred pharmaceuticals improves medication adherence for chronic conditions. Arch Intern Med 2006;166:332–7. [DOI] [PubMed] [Google Scholar]
- 25. Curtis JR, Baddley JW, Yang S, Patkar N, Chen L, Delzell E, et al. Derivation and preliminary validation of an administrative claims‐based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther 2011;13:R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Salt E, Frazier SK. Adherence to disease‐modifying antirheumatic drugs in patients with rheumatoid arthritis: a narrative review of the literature. Orthop Nurs 2010;29:260–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van den Bemt BJ, Zwikker HE, van den Ende CH. Medication adherence in patients with rheumatoid arthritis: a critical appraisal of the existing literature. Expert Rev Clin Immunol 2012;8:337–51. [DOI] [PubMed] [Google Scholar]
- 28. Muller R, Kallikorm R, Polluste K, Lember M. Compliance with treatment of rheumatoid arthritis. Rheumatol Int 2012;32:3131–5. [DOI] [PubMed] [Google Scholar]
- 29. Hill J, Bird H, Johnson S. Effect of patient education on adherence to drug treatment for rheumatoid arthritis: a randomised controlled trial. Ann Rheum Dis 2001;60:869–75. [PMC free article] [PubMed] [Google Scholar]
- 30. Brus HL, van de Laar MA, Taal E, Rasker JJ, Wiegman O. Effects of patient education on compliance with basic treatment regimens and health in recent onset active rheumatoid arthritis. Ann Rheum Dis 1998;57:146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zwikker HE, van den Ende CH, van Lankveld WG, den Broeder AA, van den Hoogen FH, van de Mosselaar B, et al. Effectiveness of a group‐based intervention to change medication beliefs and improve medication adherence in patients with rheumatoid arthritis: a randomized controlled trial. Patient Educ Couns 2014;94:356–61. [DOI] [PubMed] [Google Scholar]
- 32. Homer D, Nightingale P, Jobanputra P. Providing patients with information about disease‐modifying anti‐rheumatic drugs: individually or in groups? A pilot randomized controlled trial comparing adherence and satisfaction. Musculoskeletal Care 2009;7:78–92. [DOI] [PubMed] [Google Scholar]
- 33. Cox E, Martin BC, Van Staa T, Garbe E, Siebert U, Johnson ML. Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report–Part II. Value Health 2009;12:1053–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Study design schema and cohort identification. ETN, etanercept; MTX, methotrexate; HCQ, hydroxychloroquine; SSZ, sulfasalazine; RA, rheumatoid arthritis; JIA, juvenile idiopathic arthritis; PsO, psoriasis; PsA, psoriatic arthritis; AS, ankylosing spondylitis; CD, Crohn's disease; UC, ulcerative colitis.
Supplementary Table 1. ETN‐MTX cohort attrition
Supplementary Table 2. MTX in triple therapy cohort attrition
Supplementary Table 3. HCQ in triple therapy cohort attrition
Supplementary Table 4. SSZ in triple therapy cohort attrition


