Abstract
Introduction
The therapeutic value of intravenous immunoglobulin (IVIG) as an adjuvant therapy in sepsis remains debatable. We hypothesized that intravenous administration of BT086, a predominantly IgM IVIG solution, would improve host defense in an established rabbit model of endotoxemia and systemic sepsis.
Methods
New Zealand white rabbits were randomized into the following four groups: (1) the negative control group without lipopolysaccharide (LPS, control), (2) the positive control group with LPS infusion (LPS group), (3) the albumin‐treated LPS group (ALB+LPS group), and (4) the BT086‐treated LPS group (BT086 + LPS group). A standardized amount of E. coli was intravenously injected into all of the animals. The vital parameters, the concentration of E. coli in the blood and other organs, the residual granulocyte phagocytosis activity, and the levels of the inflammatory mediators were measured. Histological changes in the lung and liver tissue were examined following autopsy.
Results
The elimination of E. coli from the bloodstream was expedited in the BT086‐treated group compared with the LPS‐ and albumin‐treated groups. The BT086 + LPS group exhibited higher phagocytic activity of polymorphonuclear neutrophils (PMNs) than the control and ALB+LPS groups. The liver energy stores were higher in the BT086 + LPS group than in the other groups.
Conclusion
Our data suggest that the IgM‐enriched IVIG has the potential to improve host defense in a rabbit model of endotoxemia. Studies using different animal models and dosages are necessary to further explore the potential benefits of IgM‐enriched IVIG solutions.
Editorial comment: what this article tells us.
The use of IgM‐enriched Intravenous Immunoglobulin (IVIG) in the treatment of sepsis remains controversial. In this rabbit model of LPS‐induced endotoxemia, administration of an IgM‐enriched IVIG solution improved host defense against E. coli bacteremia. The findings suggest that the IgM‐enriched IVIG solution may improve host defense by neutralizing LPS.
Despite advances in intensive care medicine, the number of sepsis cases continues to increase, and mortality associated with sepsis remains high.1 Bacterial surface proteins and toxins are considered to be key activators of the complex inflammatory cascade in sepsis.2 Antibiotic treatment does not influence the existing bacterial endotoxin load in an animal model of sepsis3 and in humans4 and pathogen disintegration followed antibiosis might even aggravate endotoxin release from bacteria.3, 4 Intravenous immunoglobulin (IVIG) has the potential to scavenge and neutralize endotoxins, which reduces the overall pro‐inflammatory reaction.5 The IgM component of IVIG, in particular, appears to be critical for these properties.6, 7
The beneficial pathobiochemical effects of IVIG have been previously described.8, 9 The role of sepsis therapy using traditional IgG‐enriched IVIGs for improving survival in severe sepsis and septic shock in humans remains controversial. Most clinical IVIG studies in humans are relatively small. One large, multichar, randomized, controlled trial in adult patients (n = 624)10 and one in infants with neonatal sepsis (n = 3493)11 found no survival benefit for patients treated with established IVIG solutions. IgM‐enriched immunoglobulin solutions appear to have a survival benefit in humans.12 There is a discrepancy between the experimental IVIG data5, 8, 9 and the clinical results.13 One possible factor might be the spectrum of immunoglobulin subtypes used in the IVIG solutions.
In this study, we assessed the ability of BT086, an IgM‐enriched IVIG solution (23% of all immunoglobulins are IgM), to eliminate Escherichia coli in anesthetized rabbits with endotoxemia. We further assessed the effects of BT086 administration on bacterial distribution, residual granulocyte phagocytic activity, respiratory burst activity, histopathological changes in lung and liver tissues, and inflammatory mediator plasma levels in endotoxemic rabbits.
Materials and methods
Rabbit model of endotoxemia
The techniques of preparing and conducting experiments in our rabbit endotoxemia model have previously been described in detail.14, 15 All experiments were performed after approval by the commission for animal protection of the local government (AZ 24‐9168.11‐1/2011‐1). Twenty adult New Zealand rabbits were anesthetized and mechanically ventilated via a respirator (Evita, Dräger, Lübeck, Germany) during the entire observation period (240 min). The systemic arterial pressure, rectal temperature, and electrocardiogram were continuously monitored and digitally recorded (“ViPaD”, PCat Computer, Dresden, Germany).
Bacterial culture
A serum‐resistant and non‐hemolytic strain of E. coli (E. coli 018ac:K1:H7, ATCC 700973) with a smooth LPS phenotype was cultivated in tryptic soy broth and frozen in aliquots with glycerol at −80°C until use. Freshly grown E. coli cultures were prepared for the animal experiments. According to our pilot experiments, a standardized E. coli inoculum of 108 CFU was selected to enable the detection of cultivable bacteria in control animals until the end of the experiments.
Experimental protocol
The animals were randomly assigned to one of the following four groups (n = 5 per group): (1) the negative control group without LPS (control group), (2) the positive control group with LPS infusion (LPS group), (3) the albumin‐treated LPS group (ALB + LPS group) to ascertain the protein‐related effects, and (4) the BT086‐treated LPS group (BT086 + LPS group).
Each experiment lasted 240 min after instrumentation of the rabbits (Fig. 1). Following a 30‐min stabilization period (t ‐30), intravenous LPS (Sigma, Deisenhofen, Germany) was administered continuously at a rate of 40 μg/kg/h to all the rabbits, except the animals in the control group. At t 0, a standardized amount of E. coli was intravenously injected into all of the animals. After 15 min (t ‐15), the control group and the LPS group continuously received a 2 ml/kg/h infusion of balanced electrolyte solution E153 (Serumwerk, Bernburg, Germany). The animals in the albumin treatment group (ALB + LPS group) received 2 ml/kg/h 5% albumin solution (Biotest, Dreieich, Germany), and the animals in the BT086 treatment group (BT086 + LPS group) received 2 ml/kg/h BT086 solution (Biotest), as did a group in a previous study.15 For the analysis of the arterial blood gases, leukocyte counts, hematocrit, hemoglobin concentrations, and bacterial blood clearance, arterial blood was aseptically drawn at the time points t ‐30, t 0, t 1, t 15, t 30, t 90, t 120, and t 180. The polymorphonuclear neutrophil (PMN) oxidative burst, residual phagocytic activity, and inflammatory mediator levels were determined in the arterial blood at t ‐30, t 30, t 90, and t 180.
Figure 1.
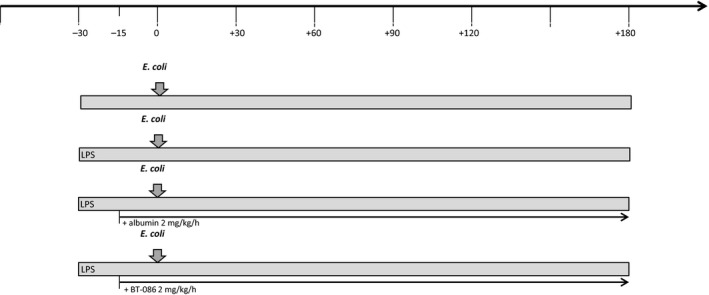
Experimental setup. The animals were randomly assigned to one of the four groups: (1) the negative control group without LPS (control group), (2) the positive control group with LPS infusion (LPS group), (3) the albumin‐treated LPS group (ALB+LPS group) to ascertain the protein‐related effects, and (4) the BT086‐treated LPS group (BT086 + LPS group). Following a 30‐min stabilization period (t ‐30), intravenous LPS was administered continuously at a rate of 40 μg/kg/h to all the rabbits, except the animals in the control group. At t0, a standardized amount of Escherichia coli was intravenously injected into all of the animals. After 15 min (t‐15), the control group and the LPS group continuously received a 2 ml/kg/h infusion of balanced electrolyte solution, the ALB + LPS group received 2 ml/kg/h 5% albumin solution, and BT086 + LPS group received 2 ml/kg/h BT086 solution.
At the end of each experiment, the animals were euthanized. Subsequently, tissue samples from the liver, spleen, kidney, and left lung were removed under aseptic conditions for the bacterial cultures. One sample from a central region of the right fixed lung (adjacent to the main bronchus) and two from a peripheral‐dependent and non‐dependent region of the lung were embedded in paraffin, stained with hematoxylin–eosin, and cut into slices for the morphometric and histological analysis.
Determination of the bacterial load
The blood samples and homogenized organs were serially diluted in normal saline and plated onto blood agar plates. After incubation of the cultures at 37°C for 24 h, the respective E. coli colonies were counted. The final bacterial concentration was calculated as the number of CFU per milliliter of blood or as CFU per gram of tissue.
Quantitative analysis of PMN phagocytosis and PMN burst activity
The phagocytosis test (Phagotest; Glycotope Biotechnology, Heidelberg, Germany) measures the percentage of phagocytes that have ingested FITC‐labeled bacteria and their activity (the number of bacteria per PMN).16 The phagocytosis test was used to evaluate the residual ex vivo phagocytic activity of PMNs in whole blood after the animals already had received a bolus load of unlabeled E. coli at t 0, as described in the experimental protocol. The obtained ex vivo values represent the idle PMN phagocytic capacity in our model, which is an inverse measure of the in vivo PMN phagocytic activity. Single‐cell analysis was performed by flow cytometry (FACS; Becton Dickinson, Heidelberg, Germany). The residual PMN phagocytic capacity was determined by the intracellular content of FITC‐marked E. coli and is expressed as the mean channel fluorescence per cell.15, 16
The extent of the PMN intracellular radical oxygen species production was determined using samples of freshly drawn heparinized blood. The oxidative burst of the PMNs was investigated using a test kit (Bursttest; Glycotope Biotechnology, Heidelberg, Germany). Single‐cell analysis was performed by laser flow cytometry. The method of the quantitative assay for monitoring the oxidative burst has previously been described in detail.15, 17
Histopathological analysis
The histological samples were examined using digitalized photomicrographs of three non‐overlapping fields from lung specimen. The images were digitalized and processed using a computer‐based system and imaging analysis software (AnalySIS, version 3.1; Soft Imaging System, Münster, Germany). Diffuse alveolar damage (DAD) in the blinded samples was quantified systematically by the same investigator using a DAD scoring system. The pathological features of the lung in adult respiratory distress syndrome are characterized under the term DAD.18 The histopathological characteristics of the scoring system used in this study were adapted from the literature19 and modified to include a weighing system similar to that proposed by Broccard et al.20
For the morphometric analysis of the lung tissue, the images were binarized (the color images were converted to black and white images), with the black portions representing parenchyma, edema, or infiltration (non‐aerated), and the white portions representing the aerated areas. The aerated and non‐aerated areas were measured and appraised with imaging analysis software (AnalySIS, version 3.1; Soft Imaging System).
A periodic acid–Schiff stain was used for the detection of glycogen in the hepatocytes. The formalin‐fixed, paraffin‐embedded tissue sections was deparaffinized, hydrated and placed in Schiff reagent (SEV Liquid Solutions, Flintsbach a. Inn, Germany) with following count erstain with hematoxylin. For the quantitative analysis of the glycogen levels, the images were binarized and rated using image analysis software (AnalySIS, version 3.1; Soft Imaging System).
The protein levels of tumor necrosis factor‐α (TNFα), interleukin‐1 (IL‐1), and interleukin‐6 (IL‐6) were measured in the blood plasma at time points t ‐30, t 30, t 90, and t 180 using commercial enzyme‐linked immunosorbent assay kits (R&D Systems, Wiesbaden, Germany), according to the manufacturer's instructions.
Statistical analysis
The power analysis and previous studies using this model14, 15 indicated that a caseload of five animals per group detects a 20% difference in the bacterial CFUs at the 180 min time point with a P value of less than 0.05 and a statistical power of 80%. The single measurements between the groups were compared with an unpaired two‐sided Student's t‐test. The between‐group differences after the repeated measurements were tested by general linear model statistics, according to a two‐way ANOVA. When applicable, the differences in the baseline values were determined by considering the baseline values as covariates within the statistical two‐way ANCOVA. Significance was accepted at P < 0.05. The statistical analysis was performed with SPSS Statistics (release 20.0.0; IBM Corp., Armonk, NY, USA).
Results
Hemodynamic measurements and blood gas analysis
The measurements of the systolic, mean, and diastolic blood pressure, heart rate, rectal temperature, blood gases, and hemoglobin and hematocrit values as well as the differential blood counts and serum lactate levels during the time course of the experiment did not differ among the groups (data not shown).
Bacterial blood clearance and organ colonization
The elimination of E. coli from the bloodstream of the BT086‐treated rabbits with endotoxemia was augmented compared with the untreated (LPS group, P < 0.001) and albumin‐treated rabbits with endotoxemia (ALB + LPS group, P < 0.05, Fig. 2). The first sterile blood culture was determined earlier in the BT086‐treated rabbits with endotoxemia than in the untreated and albumin‐treated animals with endotoxemia (P < 0.05, Fig. 3). No difference was detected between the control group (without LPS) and the BT086 + LPS group (Fig. 3). This finding suggests that the BT086 IgM‐enriched solution improves the host defense by neutralizing the effects of LPS in bloodstream infections. The organ colonization did not differ between the groups.
Figure 2.
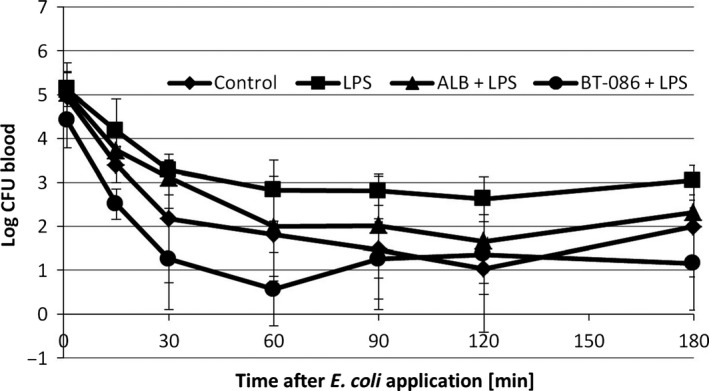
Escherichia coli blood elimination kinetic. The blood clearance of E. coli (mean ± SD) in the animals after injection of 108 CFU E. coli. Control: negative control group without LPS, LPS: positive control group with LPS infusion and no treatment, ALB + LPS: LPS group treated with albumin, BT086 + LPS: LPS group treated with BT086. LPS vs. control P < 0.05, LPS vs. BT086 P = 0.001
Figure 3.
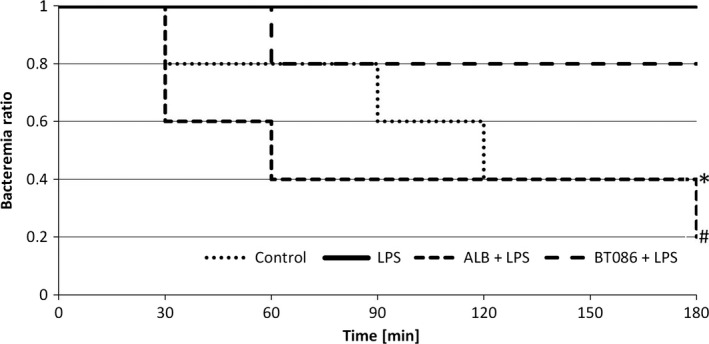
Time to the detection of the first sterile blood culture. Kaplan–Meier estimate. Control group: negative control group without LPS, LPS: a positive control group with LPS infusion, ALB + LPS: LPS group treated with albumin BT086 + LPS: LPS group treated with BT086. *P < 0.05 LPS vs. control group, #P < 0.05 BT086 + LPS vs. LPS group
Phagocytosis and burst activity of PMNs
The IgM effects on the host defense were analyzed by assessing the phagocytosis and burst capacity after the previous bloodstream inoculum. As described, the blood bacterial clearance was assessed by measuring the inoculum of unlabeled E. coli during the in vivo phase of the experiment. At 180 min, the percentage of the additional phagocytic PMNs, indicated by the additional ex vivo uptake of FITC‐marked E. coli, was lower in the BT086 + LPS group than in the LPS and ALB+LPS groups (P < 0.05, Fig. 4). At 180 min, the phagocytic activity, characterized by the number of phagocytized bacteria per leukocyte, was higher in the BT086 + LPS group (P < 0.05, Fig. 5). The oxidative burst of the PMNs did not differ in any of the groups.
Figure 4.
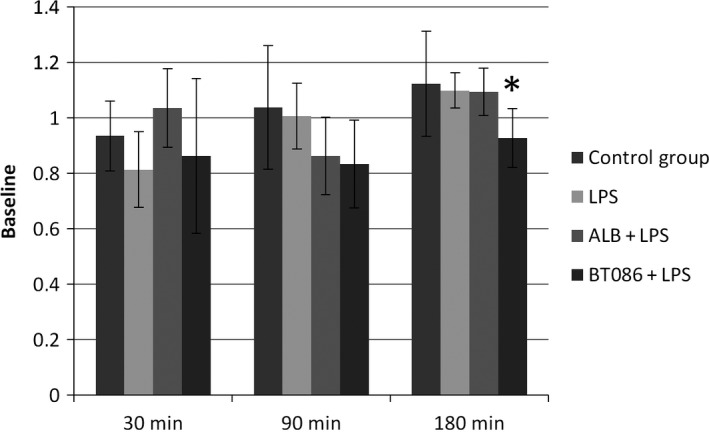
Polymorphonuclear neutrophil residual phagocytosis. The percentage of additionally phagocytic PMNs. The percentage of additional phagocytic PMNs, indicated by the additional uptake of FITC‐marked Escherichia coli, as a percent of the baseline measurements (t ‐30 min). At 180 min, the percentage of additional phagocytic PMNs was lower in the BT086 + LPS group compared with the LPS and ALB + LPS groups (*P < 0.05). All data are the mean ± SD
Figure 5.
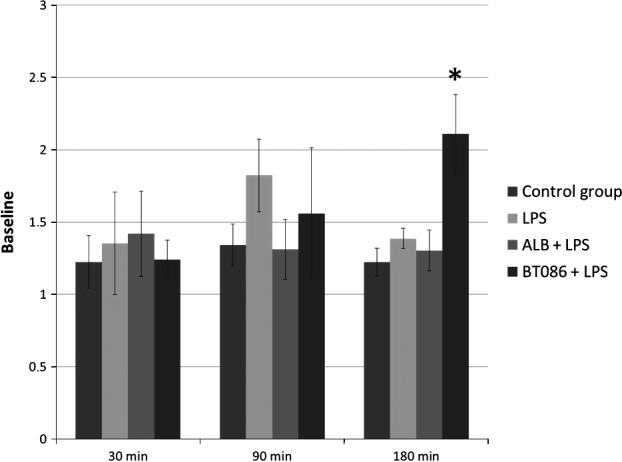
Polymorphonuclear neutrophil residual phagocytosis. Phagocytes activity. The activity of the phagocytes, characterized by the number of phagocytized FITC‐marked bacteria per leukocyte, as a percentage of the baseline measurements (t ‐30 min). At 180 min, the phagocytic activity was higher in the BT086 + LPS group (*P < 0.05). All data are the mean ± SD.
Histopathological analysis
The DAD score analysis as well the morphometric analysis of the lung tissue did not differ between the groups (Fig. 6). The percentage of glycogen was higher in the BT086 + LPS group than in the other groups (P < 0.001, Figs. 7 and 8).
Figure 6.
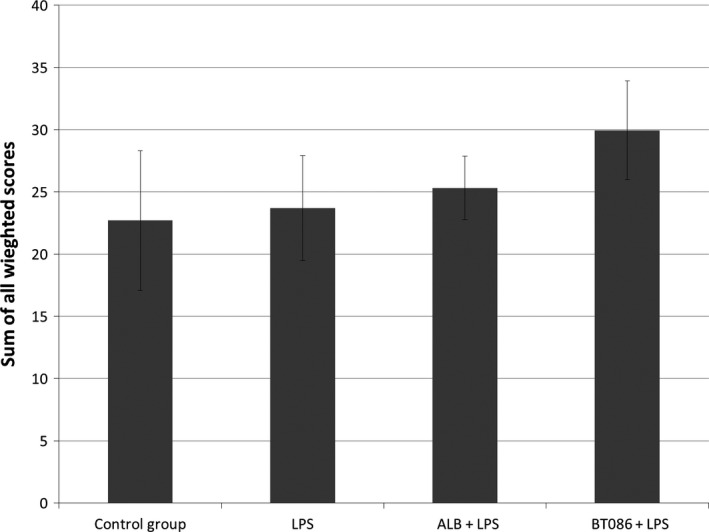
The sum of all weighted diffuse alveolar damage scores. The sum of all of the weighted diffuse alveolar damage scores showed no difference between the groups. All data are the mean ± SD.
Figure 7.
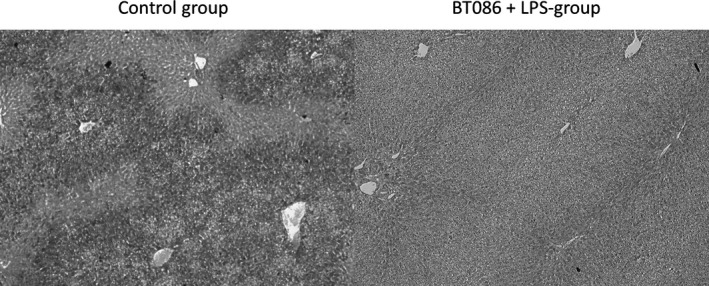
Periodic acid–Schiff stain of the hepatocytes. The periodic acid–Schiff stains demonstrate the glycogen content of the hepatocytes. The glycogen content of the hepatic cells from the BT086 + LPS group (right) is considerably higher compared to all other groups (the control group is shown on the left).
Figure 8.
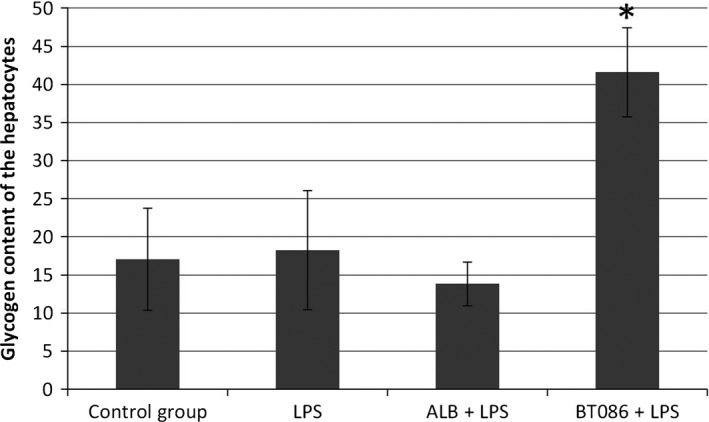
The glycogen content of hepatocytes. The periodic acid–Schiff stain of the hepatocytes in the BT086 + LPS group showed a significantly higher glycogen content compared to the control group and the ALB + LPS group (*P < 0.001). All data are the mean ± SD.
Inflammatory Mediators
The measurements of the TNFα, IL‐1, and IL‐6 plasma levels did not differ among the groups (Table 1).
Table 1.
The plasma level of TNFα, IL‐1, and IL‐6 before and after the induction of endotoxemia in anesthetized rabbits
| Control | LPS | ALB | BT086 | |
|---|---|---|---|---|
| TNFα, pg/ml | ||||
| −30 min | 87 ± 196 | 0 | 0 | 0 |
| +30 min | 640 ± 994 | 1934 ± 2043 | 1449 ± 467 | 632 ± 384 |
| +90 min | 7179 ± 2080 | 4766 ± 3668 | 6913 ± 4046 | 5289 ± 6561 |
| +180 min | 61 ± 36 | 70 ± 91 | 118 ± 123 | 331 ± 564 |
| IL‐1, pg/ml | ||||
| −30 min | 82.0 ± 25.6 | 68.0 ± 37.4 | 79.0 ± 35.5 | 95.3 ± 12.8 |
| +30 min | 80.7 ± 30.6 | 70.3 ± 36.8 | 74.4 ± 31.4 | 93.8 ± 5.3 |
| +90 min | 91.5 ± 20.8 | 87.8 ± 41.9 | 88.4 ± 28.2 | 114.6 ± 6.7 |
| +180 min | 110.2 ± 26.7 | 91.9 ± 37.1 | 100.5 ± 27.1 | 123.5 ± 16.2 |
| IL‐6, pg/ml | ||||
| −30 min | 80.0 ± 34.2 | 56.3 ± 35.1 | 68.5 ± 20.5 | 97.4 ± 38.6 |
| +30 min | 78.8 ± 51.2 | 60.6 ± 32.9 | 75.4 ± 34.6 | 91.0 ± 26.5 |
| +90 min | 91.4 ± 39.9 | 79.7 ± 45.6 | 88.8 ± 28.0 | 102.5 ± 26.8 |
| +180 min | 101.2 ± 46.9 | 83.5 ± 41.3 | 95.6 ± 42.8 | 115.6 ± 45.7 |
The measurements of TNFα, IL‐1, and IL‐6 levels did not differ among the groups (all the data are the mean ± SD).
Discussion
In this study, we present the first experimental data using BT086, a modified IgM‐enriched solution, in an established animal model of experimental endotoxemia and demonstrate that BT086 improved the host defense and preserved the energy stores in the liver in experimental endotoxemia in rabbits.
The potential use of IgG‐enriched IVIG solutions to combat bacterial endotoxin and inflammatory dysregulation in sepsis has been of major interest in recent decades. Despite the promising effects of human IgG‐enriched IVIG solutions in animal experiments,21 conventional class G‐based immunoglobulin therapy did not improve clinical outcome of critically ill patients with sepsis.22 The spectrum of the immunoglobulin subtypes used in the IVIG solutions might be important to the clinical effect. Two meta‐analyses demonstrated a trend toward superior results with IgM‐enriched solutions compared with those enriched with IgG.12, 23 The rationale for enrichment of IVIGs with IgM‐type immunoglobulins is the unique ability of IgM to neutralize LPS in the early stage of antibody‐dependent host defense.12 Specifically, IgM contributes to the opsonization of bacteria,7 inhibits the LPS‐triggered release of TNFα in monocytes,6 and stimulates the production of antibodies against LPS,7 which is one of the key activators of the complex anti‐inflammatory cascade.12, 24
In this study, we evaluated an IVIG solution with an increased portion of IgM. Using the present model of endotoxemia in rabbits, our group previously examined the effects of IgG21‐ and IgM‐enriched solutions.15 The E. coli dose was determined in a pilot study to ensure that bacteremia could be detected in healthy animals until the end of the experiments. Additionally, we continuously administered intravenous LPS in the previously identified dose14, 25 in all the groups except the control. We achieved endotoxemia with bacteremia, allowing for the detection of the in vivo effects on early phase of bacterial clearance. To control for non‐specific protein‐related effects, we established an albumin‐treated LPS group (ALB + LPS group). In this study, the time to the first sterile blood culture was shorter in the BT086 + LPS group than in the LPS and ALB + LPS groups, without a significant difference compared to the control group (Fig. 3). Treatment with BT086 appeared to neutralize the LPS effect concerning bacterial clearance. Previous findings by our group showed no significant effects on the bacterial blood elimination kinetics in animals receiving an IgM‐enriched solution15 possibly due to another composition of the immunoglobulins in the BT086 compared to Pentaglobin©, which was used in earlier studies. In the previous study, the examination of the IgM effects on the E. coli organ concentrations showed that IgM reduced the E. coli counts in the liver and spleen of rabbits. This observation was interpreted as an improved reticuloendothelial system clearance effect of the IgM‐enriched solution.15 In the present study, we found no differences in the organ colonization of E. coli, which is most likely a consequence of the enhanced bacterial blood clearance. Our findings are in agreement with those of Fabrizio et al., who found that a single dose of IgM led to a reduction in the bacterial blood load.26 As a possible explanation, the authors discussed a promotion of in vivo killing. Phagocytosis by competent cells and the production of reactive oxygen species play a crucial role in the innate immune response to eliminate the invading microorganism.27 We found a significantly higher phagocytic activity in the BT086 + LPS group at the end of experiment, without a difference in the oxidative burst of PMNs in any group. The higher number of phagocytized bacteria per leukocyte might be one of the reasons for the improved bacterial clearance observed in the BT086 + LPS group. These data agree with our previous study,15 in which we demonstrated an enhanced in vivo phagocytosis as depicted in electron microscopy. Gille et al. reported similar results in experiments using human cord blood and peripheral blood monocytes.28 The authors found that the addition of immunoglobulins to freshly harvested monocytes increased their phagocytic capacity, measured via the number of phagocytic monocytes and the amount of bacteria per cell. In agreement with our results, Tinguely et al. found that excess immunoglobulin preparations promoted in vitro phagocytosis, more so for monocytes than for PMNs alone.29 Danikas et al. showed that the phagocytic activity of peripheral blood PMNs in severe sepsis patients at the time of admission was lower in the patients who later died.30 This finding is in agreement with the well‐described fact that the immune system becomes hyporeactive during the later stages of sepsis, which is characterized by several hallmarks including increased anti‐inflammatory cytokine release31 and ablated blood leukocyte responsiveness.32, 33 It appears that BT086 might hamper the immunosuppression of peripheral blood PMNs or increase their activity because our endotoxemia and sepsis model demonstrated enhanced phagocytosis after the administration of BT086. This finding highlights the possible clinical benefit of BT086 for septic patients, at least in the early phase of sepsis.
We found no differences in the plasma levels of inflammatory mediators such as TNFα, IL‐1, and IL‐6 between the groups. Some authors have described the anti‐inflammatory effects of intravenous immunoglobulins such as a neutralizing effect on microbial toxins and pro‐inflammatory cytokines,34 the inactivation of the potent pro‐inflammatory anaphylatoxins, C3a and C5a,35 and significantly reduced levels of keratinocyte‐derived chemokine, IL‐6, and macrophage inflammatory protein‐2 expression.26 Barrat‐Due et al. documented early inflammatory responses to Pentaglobin in a porcine model of E. coli‐induced sepsis.36 In our study, neither a pro‐inflammatory nor an anti‐inflammatory effect of the selected dose of was discernible, based on the evaluation of the inflammatory mediators, the diffuse alveolar damage, and the morphometric lung tissue analysis. We found no effects of BT086 on the hemodynamic responses. Possibly, the absence of significant effects of BT086 treatment on the hemodynamic parameters and inflammation is a consequence of the relatively short observation period in our model of endotoxemia and early sepsis. In addition, the failure to demonstrate any difference between groups on inflammatory markers could potentially be a relatively small group size and large SD on cytokine data.
In humans, glycogen in the muscles and the liver represents one form of energy storage. During sepsis, patients frequently enter a hypermetabolic state, marked by several alterations including increased glucose production and depressed gluconeogenesis,37 with consequential depletion of the glycogen stores. To our knowledge, no data exist on the effects of immunoglobulins on the glycogen content in hepatocytes. We demonstrated a significantly higher concentration of glycogen in hepatic cells after BT086 treatment, as opposed to that in the other groups. The clinical importance of this finding remains unclear; however, it is possible that the increased energy stores in the form of glycogen have a beneficial effect during critical illness.
In conclusion, in this study we demonstrate improvements in bacterial blood clearance and phagocytic activity, as well a higher glycogen content in hepatocytes, after application of the IgM‐enriched solution, BT086 (Biotest, Dreieich, Germany). Our data suggest that IgM‐enrichment of IVIGs has the potential to improve the host defense by neutralizing the effects of LPS on bloodstream infection in a rabbit model of endotoxemia and sepsis. Additional animal studies using different models of sepsis and inflammation, as well as using a different dosage of BT086, are necessary to further explore the potential beneficial effects of BT086. With these findings, a positive influence of IgM‐based immunoglobulin therapy on host defense in endotoxemia could be suggested.
Acknowledgements
The authors thank Arkadi Beloiartsev for critical revision of the text and Maria Feilmeier for excellent technical assistance.
Shmygalev S, Damm M, Knels L, Strassburg A, Wünsche K, Dumke R, Stehr SN, Koch T, Heller AR. IgM‐enriched solution BT086 improves host defense capacity and energy store preservation in a rabbit model of endotoxemia. Acta Anaesthesiologica Scandinavica 2016.
Location where the work was carried out: Laboratory of Medical Theoretical char, Faculty of medicine Carl Gustav Carus, TU Dresden.
Conflicts of interest
The authors state that they have no conflict of interest to declare.
Funding
This study was supported by Biotest AG, Dreieich, Germany. Biotest AG had no role in study design, in the collection, analysis and interpretation of data, and in the decision to submit the paper for publication.
References
- 1. Soares MO, Welton NJ, Harrison DA, Peura P, Shankar‐Hari M, Harvey SE, Madan JJ, Ades AE, Palmer SJ, Rowan KM. An evaluation of the feasibility, cost and value of information of a multicentre randomised controlled trial of intravenous immunoglobulin for sepsis (severe sepsis and septic shock): incorporating a systematic review, meta‐analysis and value of information analysis. Health Technol Assess 2012;16:1–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Medzhitov R. Origin and physiological roles of inflammation. Nature 2008; 454: 428–35. [DOI] [PubMed] [Google Scholar]
- 3. Vianna RC, Gomes RN, Bozza FA, Amancio RT, Bozza PT, David CM, Castro‐Faria‐Neto HC. Antibiotic treatment in a murine model of sepsis: impact on cytokines and endotoxin release. Shock 2004; 21: 115–20. [DOI] [PubMed] [Google Scholar]
- 4. Mignon F, Piagnerelli M, Van Nuffelen M, Vincent JL. Effect of empiric antibiotic treatment on plasma endotoxin activity in septic patients. Infection 2014; 42: 521–8. [DOI] [PubMed] [Google Scholar]
- 5. McCuskey RS, Nishida J, McDonnell D, Baker GL, Urbaschek R, Urbaschek B. Effect of immunoglobulin G on the hepatic microvascular inflammatory response during sepsis. Shock 1996; 5: 28–33. [DOI] [PubMed] [Google Scholar]
- 6. Raponi G, Lun MT, Gaeta A, Ghezzi MC, Nazzari C, Mancini C, Filadoro F, Bartolazzi A, Natali P, Rozenberg‐Arska M. Differential effect of human and murine polyclonal and monoclonal antisera on TNF‐alpha production by human monocytes. J Chemother 1993; 5: 317–24. [DOI] [PubMed] [Google Scholar]
- 7. Trautmann M, Held TK, Susa M, Karajan MA, Wulf A, Cross AS, Marre R. Bacterial lipopolysaccharide (LPS)‐specific antibodies in commercial human immunoglobulin preparations: superior antibody content of an IgM‐enriched product. Clin Exp Immunol 1998; 111: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med 2012; 367: 2015–25. [DOI] [PubMed] [Google Scholar]
- 9. Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol 2013; 13: 176–89. [DOI] [PubMed] [Google Scholar]
- 10. Werdan K, Pilz G, Bujdoso O, Fraunberger P, Neeser G, Schmieder RE, Viell B, Marget W, Seewald M, Walger P, Stuttmann R, Speichermann N, Peckelsen C, Kurowski V, Osterhues HH, Verner L, Neumann R, Muller‐Werdan U. Score‐Based Immunoglobulin Therapy of Sepsis Study G. Score‐based immunoglobulin G therapy of patients with sepsis: the SBITS study. Crit Care Med 2007; 35: 2693–701. [PubMed] [Google Scholar]
- 11. Brocklehurst P, Farrell B, King A, Juszczak E, Darlow B, Haque K, Salt A, Stenson B, Tarnow‐Mordi W. Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med 2011; 365: 1201–11. [DOI] [PubMed] [Google Scholar]
- 12. Norrby‐Teglund A, Haque KN, Hammarstrom L. Intravenous polyclonal IgM‐enriched immunoglobulin therapy in sepsis: a review of clinical efficacy in relation to microbiological aetiology and severity of sepsis. J Intern Med 2006; 260: 509–16. [DOI] [PubMed] [Google Scholar]
- 13. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric S . Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013; 39: 165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heller S, Weber K, Heller A, Urbaschek R, Koch T. Pentoxifylline improves bacterial clearance during hemorrhage and endotoxemia. Crit Care Med 1999; 27: 756–63. [DOI] [PubMed] [Google Scholar]
- 15. Stehr SN, Knels L, Weissflog C, Schober J, Haufe D, Lupp A, Koch T, Heller AR. Effects of IGM‐enriched solution on polymorphonuclear neutrophil function, bacterial clearance, and lung histology in endotoxemia. Shock 2008; 29: 167–72. [DOI] [PubMed] [Google Scholar]
- 16. Instructions PHAGOTEST . [www document] Available at: http://www.glycotope.com/wp-content/uploads/documents/phagotst.pdf (accessed 07 January 2012).
- 17. Stehr SN, Weber S, Heller SC, Weikel J, Hubler M, Koch T, Heller AR. N(omega)‐nitro‐L‐arginine methyl ester effects on neutrophil function and bacterial clearance. Shock 2004; 22: 180–5. [DOI] [PubMed] [Google Scholar]
- 18. Katzenstein AL, Bloor CM, Leibow AA. Diffuse alveolar damage–the role of oxygen, shock, and related factors. A review. Am J Pathol 1976; 85: 209–28. [PMC free article] [PubMed] [Google Scholar]
- 19. Ichikado K, Suga M, Gushima Y, Johkoh T, Iyonaga K, Yokoyama T, Honda O, Shigeto Y, Tomiguchi S, Takahashi M, Itoh H, Ikezoe J, Muller NL, Ando M. Hyperoxia‐induced diffuse alveolar damage in pigs: correlation between thin‐section CT and histopathologic findings. Radiology 2000; 216: 531–8. [DOI] [PubMed] [Google Scholar]
- 20. Broccard AF, Shapiro RS, Schmitz LL, Ravenscraft SA, Marini JJ. Influence of prone position on the extent and distribution of lung injury in a high tidal volume oleic acid model of acute respiratory distress syndrome. Crit Care Med 1997; 25: 16–27. [DOI] [PubMed] [Google Scholar]
- 21. Koch T, Heller S, Weber K, Heller A, Urbaschek R. [Effects of human i.v. immunoglobulin on bacterial clearance and granulocyte function in endotoxinemia]. Anasthesiol Intensivmed Notfallmed Schmerzther 1997; 32: 420–5. [DOI] [PubMed] [Google Scholar]
- 22. Alejandria MM, Lansang MA, Dans LF, Mantaring JB III. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev 2013; 9: CD001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kreymann KG, de Heer G, Nierhaus A, Kluge S. Use of polyclonal immunoglobulins as adjunctive therapy for sepsis or septic shock. Crit Care Med 2007; 35: 2677–85. [PubMed] [Google Scholar]
- 24. Morrison DC, Silverstein R, Luchi M, Shnyra A. Structure‐function relationships of bacterial endotoxins. Contribution to microbial sepsis. Infect Dis Clin North Am 1999; 13: 313–40. [DOI] [PubMed] [Google Scholar]
- 25. Heller AR, Heller SC, Borkenstein A, Stehr SN, Koch T. Modulation of host defense by hydrocortisone in stress doses during endotoxemia. Intensive Care Med 2003; 29: 1456–63. [DOI] [PubMed] [Google Scholar]
- 26. Fabrizio K, Groner A, Boes M, Pirofski LA. A human monoclonal immunoglobulin M reduces bacteremia and inflammation in a mouse model of systemic pneumococcal infection. Clinical and vaccine immunology: CVI 2007; 14: 382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gomes RN, Teixeira‐Cunha MG, Figueiredo RT, Almeida PE, Alves SC, Bozza PT, Bozza FA, Bozza MT, Zimmerman GA, Castro‐Faria‐Neto HC. Bacterial clearance in septic mice is modulated by MCP‐1/CCL2 and nitric oxide. Shock 2013; 39: 63–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gille C, Dreschers S, Spring B, Tarnok A, Bocsi J, Poets CF, Orlikowsky TW. Differential modulation of cord blood and peripheral blood monocytes by intravenous immunoglobulin. Cytometry B Clin Cytom 2012; 82: 26–34. [DOI] [PubMed] [Google Scholar]
- 29. Tinguely C, Schaller M, Nydegger UE. Mononuclear cells ingest E. coli opsonized by investigational intravenous immunoglobulin preparations in the absence of complement more efficiently than polymorphonuclear phagocytes. Transfus Apheres Sci 2001; 25: 43–50. [DOI] [PubMed] [Google Scholar]
- 30. Danikas DD, Karakantza M, Theodorou GL, Sakellaropoulos GC, Gogos CA. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin Exp Immunol 2008; 154: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosas‐Ballina M, Olofsson PS, Ochani M, Valdes‐Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine‐synthesizing T cells relay neural signals in a vagus nerve circuit. Science 2011; 334: 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369: 2063. [DOI] [PubMed] [Google Scholar]
- 33. van der Poll T, Opal SM. Host‐pathogen interactions in sepsis. Lancet Infect Dis 2008; 8: 32–43. [DOI] [PubMed] [Google Scholar]
- 34. Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 2001; 345: 747–55. [DOI] [PubMed] [Google Scholar]
- 35. Basta M, Van Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, Szebeni J, Alving CR, Carroll MC, Berkower I, Stojilkovic SS, Metcalfe DD. F(ab)'2‐mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat Med 2003; 9: 431–8. [DOI] [PubMed] [Google Scholar]
- 36. Barratt‐Due A, Sokolov A, Gustavsen A, Hellerud BC, Egge K, Pischke SE, Lindstad JK, Pharo A, Castellheim A, Thorgersen EB, Mollnes TE. Polyvalent immunoglobulin significantly attenuated the formation of IL‐1beta in Escherichia coli‐induced sepsis in pigs. Immunobiology 2013; 218: 683–9. [DOI] [PubMed] [Google Scholar]
- 37. Mizock BA. Alterations in carbohydrate metabolism during stress: a review of the literature. Am J Med 1995; 98: 75–84. [DOI] [PubMed] [Google Scholar]


