Abstract
Ultrasound probes used in endocavitary procedures have been shown to be contaminated with high‐risk HPV after routine use and HPV is also known to be resistant to some high level disinfectants (HLDs). This study compared efficacy of two leading ultrasound probe HLD methods; liquid ortho‐phthalaldehyde (Cidex® OPA) and an automated device using sonicated hydrogen peroxide (trophon® EPR) against HPV16 and HPV18 in a hard‐surface carrier test. Native HPV16 and HPV18 virions were generated in organotypic epithelial raft cultures. Viral lysates were dried onto carriers with a 5% (v/v) protein soil. Efficacy tests were performed against the automated device at 35% and 31.5% H2O2 and 0.55% OPA in quadruplicate with matched input, neutralization, and cytotoxicity controls. Hypochlorite was included as a positive control. Infectivity was determined by the abundance (qRT‐PCR) of the spliced E1^E4 transcript in infected recipient cells. The automated HLD device showed excellent efficacy against HPV16 and HPV18 (>5 log10 reductions in infectivity) whereas OPA showed minimal efficacy (<0.6 log10 reductions). While HPV is highly resistant to OPA, sonicated hydrogen peroxide offers an effective disinfection solution for ultrasound probes. Disinfection methods that are effective against HPV should be adopted where possible. J. Med. Virol. 88:1076–1080, 2016. © 2015 The Authors. Journal of Medical Virology Published by Wiley Periodicals, Inc.
Keywords: high level disinfection (HLD), trophon EPR, ortho‐phthalaldehyde, virucidal
INTRODUCTION
High risk human papillomavirus (HPV) is the causative agent of cervical cancer, and plays an important role in anogenital and oropharyngeal cancers [Broker and Botchan, 1986; Taichman and LaPorta, 1986; Pfister, 1987a, 1987b; zur Hausen and Schneider, 1987; Howley, 1990; Chaturvedi et al., 2008, 2011]. In particular, the subtypes HPV16 and HPV18 are associated with the majority of HPV induced tumors. Reusable medical devices such as ultrasound probes are routinely used in endocavitary procedures such as transvaginal, transrectal, and transesophogeal ultrasound coinciding with those body sites where HPV exhibits its most carcinogenic effects. Appropriate reprocessing including high‐level disinfection is critically important to maintain patient safety and reduce HPV transmission risk and that of other potentially transmissible organisms. Several studies have shown residual HPV DNA on intracavity ultrasound probes following routine use, highlighting the need for appropriate disinfection measures [Casalegno et al., 2012; Ma et al., 2013; M'Zali et al., 2014].
Current guidelines require high‐level disinfection of ultrasound probes used in semi‐critical applications including procedures that may involve contact with mucous membranes or broken skin [Centers for Disease Control (CDC), 2008]. By definition, high‐level disinfection refers to the complete elimination of all viruses and microorganisms, with the exception of bacterial endospores, some of which are permitted to remain [Centers for Disease Control (CDC), 2008]. It has only recently become possible to specifically test the efficacy of high‐level disinfectants against native HPV virions, due to a lack of an adequate culture system for virion production and an appropriate infectivity assay [Meyers et al., 2014]. Our earlier study showed that aldehyde‐based high‐level disinfectants including glutaraldehyde (GTA) and ortho‐phthalaldehyde (OPA) showed minimal activity against HPV16 even when tested at extended contact times in a liquid suspension [Meyers et al., 2014].
In this study, we used a more stringent hard surface carrier test method in line with Federal Drug Administration (FDA) guidelines for assessing virucidal efficacy of high‐level disinfectants [Federal Drug Administration, 2000; ASTM International, 2011]. This test involves drying virus onto carriers in the presence of a protein soil before recovery and an assay for infectivity. We compared two leading ultrasound probe disinfectant methodologies, liquid soaking in OPA and the use of an automated high level disinfection system using sonicated hydrogen peroxide, (trophon® EPR). We show here that OPA has minimal efficacy against HPV and that the automated system is effective in completely inactivating native, infectious HPV16 and HPV18 under normal use parameters.
MATERIALS AND METHODS
Study Design
The hard surface carrier test method utilized in this study was based on the ASTM E1053‐11 standard test method suitable for assessing virucidal activity on non‐porous surfaces [ASTM International, 2011]. This standard meets the Environmental Protection Agency (EPA) efficacy data requirements for virucides which are in turn referenced by the FDA guidance for 510(k) submissions for high‐level disinfectants [Federal Drug Administration, 2000; U.S. Department Of Health And Human Services, 2000].
Cell Culture and Virus Production
HaCaT cells were maintained in DMEM supplemented with 10% FBS, 0.025 mg/ml Gentamicin, and 0.11 mg/ml sodium pyruvate. Primary human keratinocytes from newborn foreskin circumcision were isolated as previously described [Biryukov et al., 2014]. Keratinocytes were maintained in 154 medium supplemented with Human Keratinocyte Growth Supplement Kit (Cascade Biologics, Inc., Portland, OR). Immortalized keratinocytes stably maintaining HPV episomes were cultured in E‐medium with J2‐3T3 feeder cells and grown in raft culture to produce virus as previously described. Mature virus particles were harvested from tissues after 20 days. Rafts were harvested and virus was isolated by homogenization in phosphate buffer (5 mM Na‐phosphate, pH 8, 2 mM MgCl2) as previously described [Biryukov et al., 2014]. All virus preps for concentration and infectivity assays were treated with benzonase (375 U) at 37°C for one hour to remove any un‐encapsidated viral genomes. Samples were adjusted to 1 M NaCl and centrifuged at 4°C for 10 min at 10,500 rpm to remove cellular debris.
Virus Titers
To release the viral genomes, 10 μl of a virus prep was resuspended in a 200 μl HIRT DNA extraction buffer (400 mM NaCl/10 mM Tris‐HCl, pH 7.4/10 mM EDTA, pH 8.0), with 2 μl 20 mg/ml Proteinase K, and 10 μl 10% SDS for 2 hr at 37°C. The DNA was purified by phenol‐chloroform extraction followed by ethanol precipitation and re‐suspended in 20 μl TE. Titers were determined using a qPCR‐based DNA encapsidation assay utilizing a Qiagen Quantitect SYBR Green PCR Kit. Amplification of the viral genome target was performed using previously described E2 primers against a standard curve of 10‐fold serial dilutions from 108 to 104 copies per ml [Biryukov et al., 2014].
Infections, Neutralizations, and Inhibition Assays
HaCaT cells were seeded in 24‐well plates, 50,000 cells per well 2 days prior to infection. Compounds were mixed with virus and media in a total volume of 500 μl prior to addition to cells. An MOI of 10 particles per cell was used unless otherwise noted. Virus was incubated with the cells for 48 hr at 37°C and mRNA was harvested using a Qiagen RNAeasy Kit.
Carrier Preparation
Carriers were 50 × 3 mm circular discs of acrylonitrile butadiene styrene, a plastic used in ultrasound transducer construction. Carriers were prepared by soaking in 10% hydrogen peroxide for 15 min, neutralization in sterile water containing 200 U/ml of catalase for 10 min and rinsing in sterile water for 10 min before being dried in a sterile petri dish. An organic load of 5% FBS was added to the virus suspension and 200 μl of this was spread onto a single carrier side with a sterile pipette tip. The inoculated carriers were allowed to dry in a laminar flow cabinet for 30 min or until dry.
Disinfectants
The two ultrasound probe disinfection methods tested included liquid OPA (0.55%) (Cidex® OPA, Advanced Sterilization Products) and an automated device using sonicated hydrogen peroxide (35%) (trophon® EPR, Nanosonics). The device uses sonication to create an ultrafine mist which disinfects the probe in an enclosed chamber in an automated cycle. Both products are FDA‐cleared for the high‐level disinfection of ultrasound probes. Hypochlorite (0.87%) (Pure Bright Germicidal Ultra Bleach, KIK International) was used as a positive control based on its previously demonstrated efficacy against HPV16 in suspension tests [Meyers et al., 2014]. All disinfectant products were used according to the manufacturer's instructions for use.
Disinfection Procedure
Carriers were either disinfected by adding liquid disinfectant or utilizing the automated device. For device disinfection, carriers were transferred onto a rack which suspended the carriers within the disinfection chamber. A standard cycle was run in accordance with the manufacturer's instructions for use. The device uses a disinfectant cartridge which contains hydrogen peroxide (35%). A cartridge with a reduced hydrogen peroxide concentration (31.5%), corresponding to the minimum effective concentration of the system, was also tested.
For the liquid disinfectants, 2 ml of 0.55% OPA or 0.87% hypochlorite was added to the upward facing surface of the carrier so that the liquid formed a droplet covering the entire carrier surface. Carriers were then incubated at room temperature for 12 min (OPA) and 5 min (hypochlorite) (Table I). Treated viral films were resuspended in 2 ml of cell medium and were collected in a 15 ml 100K Amicon filter tube. Two milliliters of the appropriate neutralizer was added (7% glycine for OPA, base neutralizer for hypochlorite, and medium containing catalase for the automated device). The sample was filtered and assayed for infectivity as previously described [Meyers et al., 2014]. All disinfection efficacy tests were conducted in quadruplicate.
Table I.
Disinfectants and Parameters Used in This Study
| Method | Concentration | Contact time | Soil | High‐level disinfectant | Setup |
|---|---|---|---|---|---|
| Automated device (sonicated H2O2) | 35% H2O2 | 2 min a | 5% FCS | Yes | Carriers suspended in disinfection chamber |
| 31.5% H2O2 | |||||
| Liquid OPA | 0.55% OPA | 12 min | Liquid disinfectant applied to carrier | ||
| Positive control (liquid hypochlorite) | 0.87% hypochlorite | 5 min | No |
Comprised 2 cycles consisting of 30 sec of delivery and 30 sec of dwell time for a total time of 2 min.
HPV Infectivity Assay
Infection was analyzed using a previously described RT‐qPCR‐based infectivity assay for E1^E4 transcript levels [Biryukov et al., 2014]. The E1^E4 spliced transcript was amplified using primers specific for the spliced transcript that do not amplify viral genomic DNA. HPV16 infectivity assays were performed as previously described. HPV18 infectivity assays were performed in the same manner. The HPV18 E1^E4 primers used were: forward 5′ GGCTGATCCAGAAACCAGTGAC 3′ and reverse 5′ CTGGCCGTAGGTCTTTGCGGTG 3′ at final concentrations of 4 μM. A fluorogenic, dual‐labeled, HPV18 E1^E4 probe (5′‐6‐FAM‐CCTCACCGTATTCCAGCACCGTGTCCGTGBHQ‐1‐3′) was utilized at a final concentration of 0.2 μM to detect HPV18 E1^E4 cDNA. Complete viral inactivation was considered achieved when post‐disinfection infectivity assays showed equivalent or higher Ct values compared to uninfected controls.
RESULTS
In order to satisfy the requirements of the hard surface carrier test based on ASTM E1053‐11 and FDA and EPA guidelines, disinfectants must achieve at least a 4 log10 reduction in infectivity and also must achieve complete inactivation of the virus. The acceptance criteria also require that cytotoxicity and neutralization controls show less than a 0.5 log10 reduction. Across all tests completed, all matched controls showed no cytotoxicity and less than 0.5 log10 reductions after neutralization thereby satisfying the acceptance criteria (Fig. 1). All RT‐PCR Ct results used to derive the data presented in Figure 1 are given in Supplementary Table S1. No signal was detected by qRT‐PCR on uninfected control wells across the primer sets.
Figure 1.
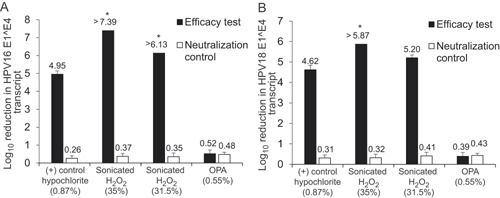
Differing efficacy profiles of disinfectants against HPV. HPV16 (A) or HPV18 (B) virions were subjected to hard surface carrier tests based on the ASTM E1053‐11 standard test method against the disinfectants indicated. Virus films were dried onto 50 mm diameter ABS carriers in the presence of a 5% fetal bovine serum soil before being disinfected according to the disinfectant or device manufacturer's instructions. Viral films were assayed for infectivity using a quantitative RT‐PCR based method detecting the spliced E1^E4 transcript in infected recipient cells. Post‐disinfection infectivity was compared to input to determine log10 reductions. Each efficacy test was conducted in quadruplicate and was paired with a matched neutralization control. Data is expressed as an average of n = 4; error bars indicate standard deviation. *=complete inactivation of the virus as shown by a lack of detectable infectivity relative to the uninfected control; OPA, ortho‐phthalaldehyde.
HPV16 was shown to be highly resistant to OPA disinfection with only a 0.52 log10 reduction in viral infectivity (Fig. 1). This reduction was only marginally higher than the log10 reduction seen in the neutralization control of 0.48. The automated system managed to achieve complete inactivation of HPV16 with a >7.39 and a >6.13 log10 reduction, using 35% hydrogen peroxide and the minimum effective concentration of 31.5% hydrogen peroxide, respectively. The difference in these values reflects slightly different starting infectivities. These results indicate that the automated high‐level disinfection device was virucidal according to the standard test method efficacy criteria. The positive control (hypochlorite) achieved a 4.95 log10 reduction in line with previous results [Meyers et al., 2014].
Efficacy against HPV18 was also tested. Again, HPV18 was shown to be highly resistant to OPA treatment with a minimal 0.39 log10 reduction in infectivity (Fig. 1). The automated device using 35% peroxide reduced viral infectivity by >5.87 log10, achieving total inactivation. When 31.5% peroxide was used, the device achieved a 5.20 log10 reduction in infectivity but did not achieve complete inactivation. The positive control (hypochlorite) showed a 4.62 log10 reduction in infectivity. Again, no cytotoxicity was observed and neutralization controls showed less than 0.5 log10 reductions.
DISCUSSION
Here, we present the first results of carrier‐based efficacy tests for common ultrasound probe high level disinfectants against native HPV. We observed that HPV16 and 18 were highly resistant to OPA disinfection when used under recommended conditions. This is cause for serious concern given the very widespread use of OPA (along with GTA) as a high‐level disinfectant, particularly for the disinfection of ultrasound probes used in endocavitary procedures. Given that these probes are known to harbor HPV after routine use [Casalegno et al., 2012; Ma et al., 2013; M'Zali et al., 2014], it seems possible that the use of GTA or OPA as per recommended guidelines, for reprocessing of these medical devices may potentially lead to inadvertent exposure of some patients to HPV, despite compliance with guidelines. While it is difficult to prove direct transmission, the potential for non‐sexual transmission of HPV has been recognized [Ryndock et al., 2014]. We also note that other guideline recommendations are based on theoretical transmission risk [Rutala et al., 2008].
Resistance to aldehydes such as GTA and OPA is not unprecedented. A number of Mycobacterium sp. exhibit aldehyde resistance and have led to cases of patient to patient transmission via medical devices reprocessed with aldehyde chemistries [Fisher et al., 2012]. Additionally, it is well understood that aldehydes have a specific mechanism of action that involves crosslinking of specific functional groups of proteins, glycoproteins, nucleic acids, and polysaccharides. The most reactive sites in proteins include exposed primary amines (e.g., lysine) and thiols (e.g., cysteine) [Feldman, 1973]. This leads to a variable ability to crosslink proteins depending on the specific amino acid side chains exposed in the protein structure. Presumably, the capsid of HPV is not conducive to crosslinking by aldehydes, or the crosslinks do not substantially affect infectivity, although more work needs to be done to fully understand how available functional groups may affect OPA susceptibility.
In this study, we have demonstrated the first ultrasound probe high‐level disinfection system which is able to fully inactivate HPV16 and HPV18 under normal use conditions (concentration, time, temperature). The sonicated H2O2 device achieved complete inactivation of HPV16 when used at both normal (35%) and reduced (31.5%) concentrations. Similarly, the device completely inactivated HPV18 with 35% peroxide, however some residual HPV18 infectivity remained after disinfection with the reduced 31.5% concentration, despite a 5.20 log10 reduction. It seems likely that the device could meet the virucidal criterion of complete inactivation at the reduced 31.5% concentration, if an inoculum closer to 104 infective units was used. This device offers a potential solution for ultrasound probe users concerned about HPV transmission.
Hypochlorite was included as a positive control based on previously demonstrated efficacy in suspension tests [Meyers et al., 2014]. While hypochlorite was fairly effective at reducing infectivity of HPV16 and HPV18, complete inactivation was not achieved. Hypochlorite is less suitable for medical device disinfection as it is not considered a high‐level disinfectant and also suffers from issues with material compatibility.
These laboratory tests have been performed according to standards meant to simulate worst‐case clinical conditions (high protein soil, high viral titer and absence of cleaning) in accordance with FDA testing requirements. While there is precedent in generalizing these results to the clinical setting, it is always possible that factors beyond these may also have an impact.
This study and our previous study have made use of native, infectious HPV virions produced in organotypic culture. We showed previously that quasivirions, spontaneously assembled from artificially expressed proteins and DNA, showed vastly different resistance profiles to common disinfectants when compared to native HPV virions [Meyers et al., 2014]. It is important that efficacy claims be supported by data from native HPV virions as there is a clear difference in efficacy profiles versus quasivirions. Surrogate viruses such as the polyoma virus SV40 have also been used to claim HPV efficacy in some regions. However, SV40 is from a different family of viruses relative to HPV, and no testing has been done to compare their relative sensitivities to disinfectants. Until surrogates have been compared to native HPV directly, care should be used in interpreting their resistance profiles as an indicator of HPV efficacy.
Furthermore, caution should be exercised in generalizing these results to other disinfection chemistries, contact times and concentrations. The sonicated hydrogen peroxide used in the device tested here is delivered as a nebulized mist, meaning that it is not directly comparable to liquid or vapor phase hydrogen peroxide. Additionally, liquid HLD chemistries that use hydrogen peroxide are used at much lower concentrations. Specific testing with those disinfectants would be required to determine whether they are effective.
Finally, these results suggest that for some disinfectant chemistries, HPV may be one of the most difficult agents to inactivate. Other authors have drawn attention to the fact that there are a number of atypically resistant organisms that do not fit within the standard paradigm ranking bacterial endospores as most difficult to disinfect [McDonnell and Burke, 2011]. Given the well understood cancer‐risk associated with HPV infection and patient expectation that their safety is paramount, healthcare providers need to give careful consideration to their disinfection practices and how they manage risk in regard to HPV in relation to endocavity ultrasound probe reprocessing.
FUNDING
Nanosonics Ltd provided funding for this study and provided the trophon EPR device for testing. The authors were responsible for the study design, data collection, data analysis, data interpretation, and writing of the manuscript. Approval from the funding organization for submission was not required.
DECLARATION OF INTEREST
R. R. has received speaker honoraria from Nanosonics. C. M. has received speaker honoraria from Merck, Quest Diagnostics, GSK, Wyeth, Bristol‐Myers Squibb and Nanosonics and has performed research funded by Merck, The Phillip Morris External Research Program, NexMed, GSK, OriGenix and Interferon Sciences Inc. All other authors: none to declare.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supporting Information.
REFERENCES
- ASTM International. 2011. ASTM E1053‐11 Standard Test Method to Assess Virucidal Activity of Chemicals Intended for Disinfection of Inanimate, Nonporous Environmental Surfaces.
- Biryukov J, Cruz L, Ryndock EJ, Meyers C. 2014. Native human papillomavirus production, quantification, and infectivity analysis. Methods Mol Biol 1249:317–331. [DOI] [PubMed] [Google Scholar]
- Broker T, Botchan M. 1986. Papillomaviruses: retrospectives and prospectives. Cancer Cells 4:17–36. [Google Scholar]
- Casalegno JS, Le Bail Carval K, Eibach D, Valdeyron ML, Lamblin G, Jacquemoud H, Mellier G, Lina B, Gaucherand P, Mathevet P, Mekki Y. 2012. High risk HPV contamination of endocavity vaginal ultrasound probes: an underestimated route of nosocomial infection? PLoS ONE 7:e48137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC). 2008. Guideline for Disinfection and Sterilization in Healthcare Facilities. CDC.
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. 2008. Incidence trends for human papillomavirus‐related and ‐unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 26:612–619. [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug‐Saber M, Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S, Anderson WF, Rosenberg PS, Gillison ML. 2011. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29:4294–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Drug Administration. 2000. Guidance for Industry and FDA Reviewers: Content and Format of Premarket Notification [510(k)] Submissions for Liquid Chemical Sterilants/High Level Disinfectants.
- Feldman MY. 1973. Reactions of nucleic acids and nucleoproteins with formaldehyde. Prog Nucleic Acid Res Mol Biol 13:1–49. [DOI] [PubMed] [Google Scholar]
- Fisher CW, Fiorello A, Shaffer D, Jackson M, McDonnell GE. 2012. Aldehyde‐resistant mycobacteria bacteria associated with the use of endoscope reprocessing systems. Am J Infect Control 40:880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley PM. 1990. Papillomavirinae and their replication In: Fields BN, Knipe DM, Chanock RMea, editors. Fields Virology. New York: Raven Press, pp 1625–1650. [Google Scholar]
- M'Zali F, Bounizra C, Leroy S, Mekki Y, Quentin‐Noury C, Kann M. 2014. Persistence of microbial contamination on transvaginal ultrasound probes despite low‐level disinfection procedure. PLoS ONE 9:e93368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ST, Yeung AC, Chan PK, Graham CA. 2013. Transvaginal ultrasound probe contamination by the human papillomavirus in the emergency department. Emerg Med J 30:472–475. [DOI] [PubMed] [Google Scholar]
- McDonnell G, Burke P. 2011. Disinfection: is it time to reconsider Spaulding? J Hosp Infect 78:163–170. [DOI] [PubMed] [Google Scholar]
- Meyers J, Ryndock E, Conway MJ, Meyers C, Robison R. 2014. Susceptibility of high‐risk human papillomavirus type 16 to clinical disinfectants. J Antimicrob Chemother 69:1546–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H. 1987a. Human papillomaviruses and genital cancer. Adv Cancer Res 48:113–147. [DOI] [PubMed] [Google Scholar]
- Pfister H. 1987b. Relationship of papillomaviruses to anogenital cancer. Obstet Gynecol Clin North Am 14:349–361. [PubMed] [Google Scholar]
- Ryndock EJ, Conway MJ, Alam S, Gul S, Murad S, Christensen ND, Meyers C. 2014. Roles for human papillomavirus type 16 l1 cysteine residues 161, 229, and 379 in genome encapsidation and capsid stability. PLoS ONE 9:e99488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutala WA, Weber DJ, HICPAC. 2008. Guideline for Disinfection and Sterilization in Healthcare Facilities. USA: Centers for Disease Control.
- Taichman LP, LaPorta RF. 1986. The expression of papillomaviruses in epithelial cells In: Salzman N, Howley PM, editors. The Papillomaviruses. New York: Plenum Press, pp 109–139. [Google Scholar]
- U.S. Department of Health And Human Services FDA, Center for Devices and Radiological Health, Infection Control Devices Branch, Division of Dental, Infection Control and General Hospital Devices, Office of Device Evaluation. 2000. Content and Format of Premarket Notification [510(k)] Submissions for Liquid Chemical Sterilants/High Level Disinfectants.
- zur Hausen H, Schneider A. 1987. The role of papillomaviruses in human anogenital cancer In: Salzman N, Howley PM, editors. The Papillomaviruses. New York: Plenum Press, pp 245–263. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supporting Information.


