Abstract
Purpose
To evaluate pregnancy outcomes following onabotulinumtoxinA (US Food and Drug Administration pregnancy category C product) exposure using the Allergan safety database.
Methods
The Allergan Global Safety Database contains reports of onabotulinumtoxinA administration before/during pregnancy, including both prospective (reported before outcome) and retrospective (outcome already known) cases. The database was searched from 1/1/90 to 12/31/13 for eligible cases where treatment occurred during pregnancy or ≤3 months before conception. To minimize reporting bias, prevalence rates were focused on prospective cases.
Results
Of 574 pregnancies with maternal onabotulinumtoxinA exposure, 232 were eligible with known outcomes. Patients received onabotulinumtoxinA most frequently for cosmetic indications (50.5%), movement disorders (16.8%), and pain disorders (14.2%). Of the 137 with dose information, 40.1% received <50U, 14.6% 50U to <100U, 27.7% 100U to <200U, and 17.5% ≥200U. Among 146 cases with known maternal age, 47.9% were ≥35 years. Most (96.0%) fetal exposures occurred during/before the first trimester. Of the 137 prospective cases (139 fetuses), 110 (79.1%) were live births; 29 (20.9%; 95% CI, 14.0–30.0%) ended in fetal loss (21 spontaneous, 8 induced abortions). Among live births, 106 (96.4%) were normal, with four abnormal birth outcomes (1 major fetal defect, 2 minor fetal malformations, 1 birth complication), giving a 2.7% (3/110; 95% CI, 0.6–8.0%) prevalence rate for overall fetal defects.
Conclusions
A 24‐year retrospective review of the Allergan safety database shows that the prevalence of fetal defects in onabotulinumtoxinA‐exposed mothers before/during pregnancy (2.7%) is comparable with background rates in the general population. Pregnancy outcome monitoring in onabotulinumtoxinA‐exposed women continues. © 2015 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Keywords: onabotulinumtoxinA, pregnancy, fetal defects, pharmacoepidemiology
Introduction
OnabotulinumtoxinA (BOTOX®/BOTOX® Cosmetic, Allergan, Inc., Irvine, CA) has been studied and marketed globally for a variety of indications for over 20 years. In the USA, approved indications for onabotulinumtoxinA include strabismus, blepharospasm, cervical dystonia, axillary hyperhidrosis, chronic migraine, upper limb spasticity, neurogenic detrusor overactivity, overactive bladder, and the cosmetic indications of glabellar lines and lateral canthal lines (Table 1).1, 2 OnabotulinumtoxinA is indicated for use in adults in the USA, except for the treatment of blepharospasm and strabismus, in which it is indicated for patients ≥12 years of age. Over the past 24 years (1990–2014), approximately 54 million vials of BOTOX® and BOTOX® Cosmetic have been distributed worldwide (Allergan data on file).
Table 1.
Approved indications for onabotulinumtoxinA in the USA and Europe
| Year of FDA approval | Indication | Typical age ranges* | Indication statement approved by FDA1, 2 | Year of EU approval† |
|---|---|---|---|---|
| 1989 | Strabismus | Commonly in first decade of life35 | Treatment of strabismus in patients ≥12 years of age | N/A |
| 1989 | Blepharospasm | Onset in 50s,36 with prevalence increasing with age37 | Treatment of blepharospasm associated with dystonia in patients ≥12 years of age | 1994 |
| 2000 | Cervical dystonia | Onset in early 40s38, 39, 40 | Treatment of cervical dystonia in adult patients, to reduce the severity of abnormal head position and neck pain | 1995 |
| 2002‡ | Glabellar lines | Mean age of onset, 46.4 ± 9.9 years41 | Temporary improvement in the appearance of moderate to severe glabellar lines associated with corrugators and/or procerus muscle activity in adult patients | 2003 |
| 2004 | Primary axillary hyperhidrosis | Onset in mid‐20s, with highest prevalence of 3.5–4.5% among those aged 25–64 years42 | Treatment of severe axillary hyperhidrosis that is inadequately managed by topical agents in adult patients | 2001 |
| 2010 | Focal upper limb spasticity | Prevalence of stroke increases with age: 4.8% among those aged 65–84 years and 7.1% in those >75 years.43 At 12 months post‐stroke, prevalence of upper limb spasms is 17–19% | Treatment of upper limb spasticity in adult patients | 2001 |
| 2010 | Chronic migraine | Peak prevalence in 40s at 1.9% for females; 0.8% for males44 | Prophylaxis of headaches in adult patients with chronic migraine (≥15 days per month with headache lasting 4 h or longer per day) | 2010 |
| 2011 | Detrusor overactivity associated with a neurologic condition | Mean age 62.5 years among NDO patients in a US claims database45 | Treatment of urinary incontinence because of detrusor overactivity associated with a neurologic condition (e.g., spinal cord injury, multiple sclerosis) in adults who have an inadequate response to or are intolerant of an anticholinergic medication | 2011 |
| 2013 | Overactive bladder | Age group with highest prevalence: ≥60 years (19.1% in females; 8.2% in males)46 | Treatment of overactive bladder with symptoms of urgency urinary incontinence, urgency, and frequency in adults who have an inadequate response to or are intolerant of an anticholinergic medication | 2013 |
| 2013‡ | Lateral canthal lines | Mean age of onset, 46.4 ± 9.9 years41 | Temporary improvement in the appearance of moderate to severe lateral canthal lines associated with orbicularis oculi activity in adult patients | 2014 |
| N/A | Juvenile cerebral palsy dynamic equinus foot deformity | Appears in infancy or childhood | N/A | 1997 |
| N/A | Lower limb spasticity | Prevalence of stroke increases with age: 4.8% among those aged 65–84 years and 7.1% in those >75 years.43 At 12 months post‐stroke, prevalence of lower limb spasms is 11–32% | N/A | 2014 |
EU = European Union, FDA = Food and Drug Administration, N/A = not applicable, NDO = neurogenic detrusor overactivity.
Table is adapted from Brin MF, Blitzer A. History of onabotulinumtoxinA therapeutic. In Botulinum Toxin. Carruthers J and Carruthers A (eds). 3rd edition. London: Elsevier Saunders, 2013:6–12.
The mean age of onset for the cosmetic indications is based on treatment data.41 For all other indications, the data are as published in the literature, and not necessarily representative of the population being treated.
First EU approval in UK or Ireland /France. (Reference Member State of the Mutual Recognition Process for Therapeutic/Aesthetic respectively). Specific indications often vary from those licensed in the USA.
As BOTOX® Cosmetic.
OnabotulinumtoxinA is designated as a US Food and Drug Administration (FDA) pregnancy category C pharmaceutical product, as there are no adequate and well controlled studies in pregnant women, and it should only be used during pregnancy if the benefits outweigh the potential risks.3 While women who are pregnant, nursing, or planning a pregnancy are excluded from clinical trials on botulinum toxin (BoNT), many women being treated with onabotulinumtoxinA (eg, chronic migraine, axillary hyperhidrosis, and cosmetic use) are of reproductive age. Several of the indications for which onabotulinumtoxinA is approved are more prevalent in women and overall use is greater in females. For example, the prevalence of chronic migraine is 2.5 to 6.5 times higher in women than in men,4 >60% of hyperhidrosis patients are reported to be female,5 and in the USA, almost 90% of the onabotulinumtoxinA injections for cosmetic use are for women.6 In addition, pregnancy rates for women in their 30s and early 40s have increased in the developed countries.7
Given the high frequency of onabotulinumtoxinA use among women, especially those of fertile age, we have analyzed birth outcomes of women exposed to onabotulinumtoxinA prior to or during pregnancy.
Methods
The Allergan Global Safety Database was used to identify pregnancy cases, in which the patient was exposed to onabotulinumtoxinA or BoNT type A (manufacturer unspecified). This database contains individual case safety reports received from pre‐ and post‐approval sources (approved indications shown in Table 1), including Allergan‐sponsored and partner‐sponsored clinical trials (serious adverse events and pregnancies), spontaneous sources (serious and non‐serious events reported by consumers and healthcare providers), post‐authorization studies, health authorities, and published literature. Women enrolled in Allergan‐sponsored clinical trials who had a positive pregnancy test during the trial period were withdrawn from the studies and followed for pregnancy outcome. The database includes cases with pregnancy outcomes that are prospectively (pregnancy case reported before outcome was known) or retrospectively (outcome known when pregnancy case was reported) collected. When a pregnancy exposure is reported to Allergan, a minimum of three attempts are made, by phone, email, and/or letter to gather information on the pregnancy exposure and to obtain follow‐up information on the pregnancy outcome. Additional follow‐up is based upon the reporter's willingness to provide more information, receipt of consent to access medical information, and provision of the doctor's contact information. Whenever possible, the patient's physician is contacted for additional information about the pregnancy.
For this analysis, the database was queried from January 1, 1990 through December 31, 2013 for case reports involving an identifiable patient exposed to onabotulinumtoxinA or BoNT type A who was confirmed pregnant, and the patient age was unknown or was under the age of 65 years. Additionally, case reports concerning accidental topical exposure to onabotulinumtoxinA were excluded.
Only cases in which onabotulinumtoxinA injection occurred during pregnancy or up to 3 months prior to the estimated date of conception were retained; these are considered eligible pregnancies for the purpose of this analysis. OnabotulinumtoxinA is not expected to be present in peripheral blood at measurable levels following intramuscular or intradermal injection at the recommended doses, and preclinical studies8, 9 predict that any potentially systemic onabotulinumtoxinA would be completely eliminated from the systemic circulation within 48 h post‐injection in rodents. Therefore, it was decided to review cases with maternal exposure of onabotulinumtoxinA 3 months prior to conception, because this is the approximate duration of action of the drug in striated muscle.10
All cases were reviewed by a medical safety physician, who determined if the outcome was a normal live birth or fetal loss (which included both spontaneous and elective abortions, as well as other cases of fetal loss), or in the case of a live birth, whether there were any fetal defects. Elective abortion cases included the birth type of “elective termination” or Medical Dictionary for Regulatory Activities preferred term of “abortion induced” or “abortion.” Fetal defects were assigned to the following categories11: major, which have serious medical and/or social implications and often require surgical repair; minor, which are of mostly cosmetic significance and are rarely medically significant or require surgical repair; birth complication, which results from the birthing process; genetic abnormality, which results from a genetic disorder; and significant adverse event, which is another event considered to be significant by the reviewer. Outcomes of interest were fetal defects and fetal loss. Additional evaluated variables were maternal age, timing of exposure, indication, and dose.
Retrospective pregnancy cases (ie, reported after the pregnancy outcome is known) have an inherent reporting bias because they may be influenced by the outcome itself; for example, abnormal outcomes are more likely to be reported than normal outcomes. Such bias may lead to a higher proportion of abnormal outcomes that does not reflect the “true” prevalence rate. As noted by the FDA12 and other published reports,13, 14, 15 retrospective reports will not provide an accurate risk calculation. For completeness, however, the proportions of abnormal pregnancy outcomes among retrospective cases are provided. In cases of prospective pregnancies, reporting bias regarding outcome is not anticipated because the outcome has not yet occurred. Consistent with FDA guidance12 and other reports13, 14, 15 only prospective pregnancy cases are used to estimate prevalence rates of abnormal pregnancy outcomes, and their 95% confidence intervals (CI), based on a Poisson distribution, are provided.
Results
Distribution of cases
Overall, 574 pregnancy cases were retrieved from the Allergan Global Safety Database for the time period of January 1, 1990 through December 31, 2013 (Figure 1). Out of these, 276 pregnancies (48.1%) had a known outcome, and of these 276 cases, 232 pregnancies (137 prospective and 95 retrospective) were eligible and encompassed 237 fetal records (139 prospective and 98 retrospective). Six of the overall 574 pregnancy cases (two prospective and four retrospective) were exposed to unspecified BoNT.
Figure 1.
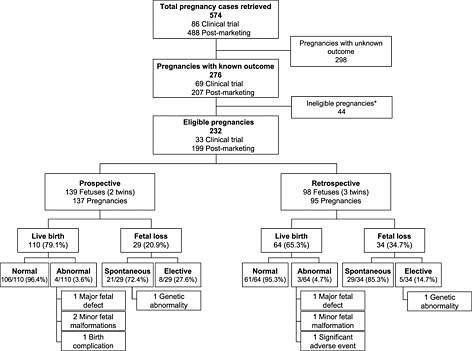
Distribution of pregnancy cases. *Pregnancies in which onabotulinumtoxinA injection occurred >3 months before the estimated date of conception
Maternal characteristics
Maternal ages were known for 146 (62.9%) of the total known outcome cases (Table 2). Of the patients with known age, 47.9% were of advanced maternal age (≥35 years); 42.3% of prospective patients were ≥35 years and 59.2% of retrospective patients were ≥35 years. The most commonly reported condition for which patients received onabotulinumtoxinA injection was cosmetic use (50.5%), followed by movement disorders (16.8%), pain disorders (14.2%), and hyperhidrosis (11.6%) (Table 2). The majority (82.6%) of fetal exposures occurred during the first trimester (Table 2). Of the total patients with a known dose (total dose), 40.1% received <50U, 14.6% received 50U to <100U, and 45.3% received ≥100U (Table 2).
Table 2.
Characteristics of prospective and retrospective pregnancy cases with known outcome*
| Prospective pregnancies (n = 137) | Retrospective pregnancies (n = 95) | Total (N = 232) | |
|---|---|---|---|
| Maternal age | |||
| Age known, n | 97 | 49 | 146 |
| <35 years | 56 (57.7) | 20 (40.8) | 76 (52.1) |
| ≥35 years | 41 (42.3) | 29 (59.2) | 70 (47.9) |
| BOTOX indication MedDRA preferred term | |||
| Indication known, n | 128 | 62 | 190 |
| Cosmetic | 70 (54.7) | 26 (41.9) | 96 (50.5) |
| Skin cosmetic procedure | 1 | 0 | 1 |
| Skin wrinkling | 69 | 26 | 95 |
| Gastrointestinal disorders | 1 (0.8) | 2 (3.2) | 3 (1.6) |
| Anal fissure | 1 | 0 | 1 |
| Esophageal achalasia | 0 | 2 | 2 |
| Hyperhidrosis | 12 (9.4) | 10 (16.1) | 22 (11.6) |
| Hyperhidrosis | 12 (9.4) | 10 (16.1) | 22 (11.6) |
| Movement disorders | 15 (11.7) | 17 (27.4) | 32 (16.8) |
| Blepharospasm | 1 | 0 | 1 |
| Dystonia | 4 | 0 | 4 |
| Hemiparesis | 1 | 0 | 1 |
| Muscle contracture | 0 | 1 | 1 |
| Spasmodic dysphonia | 1 | 2 | 3 |
| Strabismus | 0 | 1 | 1 |
| Torticollis | 8 | 13 | 21 |
| Pain disorders | 22 (17.2) | 5 (8.1) | 27 (14.2) |
| Headache | 2 | 0 | 2 |
| Migraine | 17 | 5 | 22 |
| Muscle spasms | 2 | 0 | 2 |
| Pelvic pain | 1 | 0 | 1 |
| Spasticity | 3 (2.3) | 1 (1.6) | 4 (2.1) |
| Muscle spasticity | 3 | 1 | 4 |
| Urological disorders | 5 (3.9) | 1 (1.6) | 6 (3.2) |
| Cystitis interstitial | 1 | 0 | 1 |
| Hypertonic bladder | 1 | 0 | 1 |
| Neurogenic bladder | 3 | 1 | 4 |
| Timing of exposure | |||
| Timing known, n | 124 | 77 | 201 |
| Prior to conception | |||
| >2–3 months | 2 (1.6) | 1 (1.3) | 3 (1.5) |
| >1–2 months | 7 (5.6) | 2 (2.6) | 9 (4.5) |
| 0–1 month | 12 (9.7) | 3 (3.9) | 15 (7.5) |
| First trimester | 98 (79.0) | 68 (88.3) | 166 (82.6) |
| Second trimester | 5 (4.0) | 1 (1.3) | 6 (3.0) |
| Third trimester | 0 (0) | 2 (2.6) | 2 (1.0) |
| BOTOX dose | |||
| Dose known, n | 95 | 42 | 137 |
| <50U | 40 (42.1) | 15 (35.7) | 55 (40.1) |
| 50U to <100U | 13 (13.7) | 7 (16.7) | 20 (14.6) |
| 100U to <150U | 17 (17.9) | 9 (21.4) | 26 (19.0) |
| 150U to <200U | 10 (10.5) | 2 (4.8) | 12 (8.8) |
| 200U to <250U | 6 (6.3) | 3 (7.1) | 9 (6.6) |
| 250U to <300U | 1 (1.1) | 0 (0) | 1 (0.7) |
| 300U to <350U | 6 (6.3) | 1 (2.4) | 7 (5.1) |
| 350U to <400U | 2 (2.1) | 0 (0) | 2 (1.5) |
| >400U | 0 (0) | 5 (11.9) | 5 (3.6) |
MedDRA = Medical Dictionary for Regulatory Activities.
Data are expressed as n or n (% among those with known information).
Fetal outcomes
Overview
There were 137 prospective pregnancies, comprising 139 fetuses because of two sets of twins (Figure 1). Of these, 110 (79.1%) resulted in a live birth. Most (96.4%) of the live births were normal. There were four abnormal birth outcomes, including one major fetal defect, two minor fetal malformations, and one birth complication (Table 3). The prevalence rate for major fetal defects was 0.9% (1/110; 95% CI, 0.02–5.1%) and for overall fetal defects it was 2.7% (3/110; 95% CI, 0.6–8.0%). Of the prospective fetuses, 20.9% (29/139; 95% CI, 14.0–30.0%) ended in fetal loss. Twenty‐one fetal losses (72.4%) were spontaneous, one of which included a genetic abnormality, and eight (27.6%) were elective abortions.
Table 3.
Summary of fetal defects in prospective and retrospective cases of live births and abortions
| Adverse event | Outcome | BOTOX indication | Time of exposure | BOTOX dose | Maternal age |
|---|---|---|---|---|---|
| Prospective fetal defects in live births | |||||
| Major fetal defects (n = 1) | |||||
| Ventricular septal defect | C‐section; asymptomatic, no intervention required | Axillary hyperhidrosis | 37 days pre‐conception | 100U | 28 years |
| Minor fetal malformations (n = 2) | |||||
| Metatarsus adductus | Induced vaginal delivery (decreased fetal movement); no other abnormalities | Chronic migraine | 15 days post‐conception | 155U | 19 years |
| Innocent asymptomatic cardiac murmur* | Family history of cardiac murmur | Blepharospasm | Trimesters 1, 2, 3 | 8U, 12U, 14U | 23 years |
| Birth complication (n = 1) | |||||
| Horner syndrome | C‐section, placenta previa, and uterine varicosities; no abnormalities reported 11 months later | Axillary hyperhidrosis | “Few days” before conception | 100U (50U each axilla) | Unknown |
| Prospective fetal defect in abortions | |||||
| Genetic abnormalities (n = 1) | |||||
| Down syndrome | Miscarriage at gestation month 5 | Skin wrinkling (glabella) | 40 days post‐conception | 12U | 38 years |
| Retrospective fetal defects in live births | |||||
| Major fetal defects (n = 1) | |||||
| Tracheoesophageal fistula, esophagealatresia | Pre‐term labor at ~33 weeks resulting in C‐section; surgical repair successful | Facial wrinkles | 3 days post‐conception | 36U | 30 years |
| Minor fetal malformations (n = 1) | |||||
| Laryngomalacia | Planned C‐section at 38 weeks; settled spontaneously over several months | Facial wrinkles | 1 week pre‐conception and 2 weeks post‐conception | Not reported | 38 years |
| Significant adverse event (n = 1) | |||||
| Brain tumor (unspecified)† | Successful surgical resection | Cervical dystonia | Within 1 week before conception | 100U | 25 years |
| Retrospective fetal defects in abortions | |||||
| Genetic abnormality (n = 1) | |||||
| Down syndrome | Elective abortion at week 20 | Skin wrinkling (glabella) | “Immediately after injection” | 25U | 40 years |
Diagnosed at 7 days.
Diagnosed at 13 months.
There were 95 retrospective pregnancies and 98 fetuses because of three sets of twins (Figure 1), 64 (65.3%) of which resulted in a live birth. Out of the 64 live births, 61 (95.3%) were normal. The three abnormal birth outcomes included one major fetal defect, one minor fetal malformation, and one significant adverse event (Table 3). Of the 98 fetuses from retrospective pregnancies, 34 (34.7%) ended in fetal loss, 29 (85.3%) were spontaneous losses, and 5 (14.7%) were elective abortions, one of which included a genetic abnormality.
Fetal loss
Overall, there were 50 spontaneous abortions in the 47 prospective and retrospective pregnancies (Table S1). The most commonly reported condition for which patients experienced spontaneous fetal loss was cosmetic (64.4%), followed by movement disorders (13.3%), pain disorders (13.3%), and hyperhidrosis (6.7%). The most common dose of onabotulinumtoxinA among patients who experienced spontaneous fetal loss was <50U, and the majority (94.3%) of fetal exposure occurred during the first trimester. The most common gestational ages at the time of spontaneous loss were between 1 and 2 months (43.2%) and 2 and 3 months (43.2%). Most (26 [70.3%]) of the spontaneous abortions had the documented risk factor of a maternal age >35 years.16 One spontaneous abortion involved a genetic abnormality (Down syndrome); more information on the case is provided in Table 3.
Among the prospective pregnancy cases, a significantly higher proportion of patients with spontaneous abortions (68.8%) were of advanced maternal age (ie, ≥35 years) compared with those experiencing live births (39.5%; p = .032) (Table 4). A majority of the patients with spontaneous abortions (89.5%) reported receiving onabotulinumtoxinA for cosmetic use, which was comparable to the overall rate of cosmetic (vs. therapeutic) use of 85.8% on a per‐patient basis in the US population (data on file). Cosmetic use of onabotulinumtoxinA was reported in 50.5% of the patients with live births. It is notable, however, that the timing of exposure to onabotulinumtoxinA was comparable in both the spontaneous abortions and live birth cases (Table 4).
Table 4.
Comparison of characteristics between prospective pregnancy cases with live births and spontaneous abortions*
| Pregnancies with live births (n = 109) | Pregnancies with spontaneous abortions (n = 20) | p‐values | |
|---|---|---|---|
| Maternal age | |||
| Age known, n | 76 | 16 | 0.032 |
| <35 years | 46 (60.5) | 5 (31.3) | |
| ≥35 years | 30 (39.5) | 11 (68.8) | |
| BOTOX indication | |||
| Indication known, n | 103 | 19 | 0.002 |
| Cosmetic | 52 (50.5) | 17 (89.5) | |
| Therapeutic | 51 (49.5) | 2 (10.5) | |
| Timing of exposure | |||
| Timing known, n | 99 | 17 | 0.518 |
| Prior to conception | 18 (18.2) | 2 (11.8) | |
| First/Second trimester | 81 (81.8) | 15 (88.2) | |
| BOTOX dose | |||
| Dose known, n | 80 | 12 | 0.141 |
| <50U | 32 (40.0) | 8 (66.7) | |
| 50U to <100U | 11 (13.8) | 2 (16.7) | |
| ≥100U | 37 (46.3) | 2 (16.7) | |
Pregnancy cases with unknown maternal age, BOTOX indication, timing of exposure or BOTOX dose were not included in the individual analyses.
Data are expressed as n or n (% among those with known information).
There were a total of 13 elective abortions from both prospective and retrospective cases, which occurred with similar frequency among patients treated for various indications (Table S2). The majority (81.8%) of fetal exposures in these cases occurred during the first trimester. Of the 10 cases for which the time of abortion was known, nine occurred in the first trimester. The reasons for elective abortions were known in the following nine cases: five personal/social, one fetal disorder, one high risk because of age, one blighted ovum, and one gestational sac with no embryo. One elective abortion involved the genetic abnormality Down syndrome (Table 3).
Fetal defects
Among all live births (n = 174), there were a total of seven abnormalities (Table 3). Two fetal cases were categorized as a major malformation: ventricular septal defect, which was prospective, asymptomatic, and did not require medical intervention, and tracheoesophageal fistula/esophageal atresia, a retrospective case that was successfully surgically repaired. The single prospective case of major malformation gave a 0.9% (1/110; 95% CI, 0.02–5.1%) prevalence rate of major fetal defects.
There were three minor fetal malformations: laryngomalacia, which resolved spontaneously over several months, and a reportedly asymptomatic innocent cardiac murmur in an infant with a family history of cardiac murmur. One prospective case with metatarsus adductus was provisionally classified as having a minor fetal defect, although data regarding the severity and need for interventions were not known for this case.
The one birth complication was Horner syndrome, which spontaneously resolved after 11 months. The one significant adverse event was a benign brain tumor identified at 13 months, which was successfully surgically resected.
Discussion
This analysis examined over 20 years of data reported to Allergan on pregnancy outcomes in women exposed to onabotulinumtoxinA or unspecified BoNT. We observed that the rates of spontaneous abortion and fetal defects among pregnant women treated with onabotulinumtoxinA are similar to those reported for the general population. Among prospective cases, the observed prevalence rates of elective and spontaneous abortion were 5.8% (8/139; 95% CI, 2.5–11.3%) and 15.1% (21/139; 95% CI, 9.4–23.1%), respectively, compared with rates of 18.4 and 17.0% for elective and spontaneous abortions in the US general population.7 As expected, the retrospectively collected prevalence rates of elective and spontaneous abortion outcomes were higher, owing to the inherent reporting bias seen in a retrospective subset. As noted by the FDA12 and other authors,13, 14, 15 the retrospective data cannot be compared with prevalence rates in the general population.
In this analysis, the observed prevalence rate of any fetal defects in prospective cases is 2.7% (95% CI, 0.6–8.0%), and the prevalence rate of major fetal defects is 1.8%, which is comparable to large population‐based studies. For example, the Centers for Disease Control and Prevention found a 3% overall prevalence rate of live births with major fetal defects from 1978 to 2005,17 and a UK study reported a 2% prevalence rate of major fetal defects diagnosed before 1 year of age in children born between 1990–2009.18 The March of Dimes estimates a birth prevalence rate of approximately 6% of the annual births worldwide for serious fetal defects of genetic or partially genetic origin; about 5.5% in high‐income countries.19
In addition, these data are consistent with observations from preclinical registration studies conducted by Allergan, in which onabotulinumtoxinA was not selectively toxic to fetal development, and fetal effects were observed only in association with maternal toxicity. No adverse effects on fetal development were observed when pregnant rats received single intramuscular injections of onabotulinumtoxinA 1, 4, or 16U/kg prior to implantation, at implantation, or during organogenesis.1 Intramuscular daily injections of onabotulinumtoxinA to pregnant rats or rabbits during the period of organogenesis (total of 12 doses in rats, 13 doses in rabbits) resulted in reduced fetal body weights and decreased fetal skeletal ossification at the two highest doses in rats (4U/kg, 8U/kg) and at the highest dose in rabbits (0.5 U/kg).1 These doses were also associated with maternal toxicity, including abortions, early deliveries, and maternal death. The developmental no‐effect doses in these studies were lower than the average high dose used in humans. In addition to the studies on rats and rabbits, onabotulinumtoxinA did not appear to have an effect on neural tube development in a chick embryo model.20
Reports in the literature of exposure to BoNT from botulism show low levels of adverse effects on the fetus (Table S3). Clinical botulism did not appear to directly cause any adverse effects on the pregnancy or fetus in six published case reports.21, 22, 23, 24, 25, 26 In one case, the patient was paralyzed and the fetus was the only visible motion, indicating that peripherally circulating BoNT does not cross the placenta.22 Indeed, this conclusion was confirmed by an in vivo rabbit study,27 and is consistent with the notion that should any onabotulinumtoxinA have been present in the systemic circulation after treatment, the large molecular weight makes it unlikely to passively diffuse across the placenta.
It is believed that little systemic distribution of therapeutic doses of onabotulinumtoxinA occurs. OnabotulinumtoxinA is not expected to be present in the peripheral blood at measurable levels following intramuscular or intradermal injection at the recommended doses. The recommended quantities of neurotoxin administered at each treatment session are not expected to result in systemic, overt, distant clinical effects (ie, muscle weakness) in patients without other neuromuscular dysfunction.
Literature reports of BoNT exposure from cosmetic or therapeutic use also show low levels of adverse effects on the fetus. Of the 30 cases of BoNT exposure for cosmetic or therapeutic purposes during pregnancy reported in the literature, two resulted in miscarriages (one of which is included in this analysis28) and one in elective abortion with no report of fetal defects.26, 28, 29, 30, 31, 32, 33, 34
This study has several limitations, which are consistent with other safety database analyses. It is restricted to cases reported to Allergan, and case reports may not include full data on factors such as dose and timing of exposure, which limits detailed analysis on risk factors in this population. In addition, outcomes at the time of birth were examined, but there could also be more subtle developmental effects identified at later postnatal visits.
Conclusions
Based on a 24‐year retrospective review of the Allergan safety database, the prevalence of abnormal pregnancy outcomes in mothers exposed to onabotulinumtoxinA prior to or during pregnancy is consistent with background rates in the general population. No consistent types of malformation by organ were observed, and no new safety concerns were identified. Allergan will continue to monitor the pregnancy outcomes in onabotulinumtoxinA‐exposed women through routine pharmacovigilance.
Conflict of Interest
MFB, MMM, LP, and IY are employees of Allergan, Inc. HY was an employee of Allergan, Inc. at the time the analysis was conducted. RSK and AS have previously served as consultants for Allergan, Inc.
Key Points.
Several indications for which onabotulinumtoxinA is approved are more prevalent in women, and many women being treated are of reproductive age
This analysis of reported pregnancies during 24 years of onabotulinumtoxinA utilization shows that the prevalence of abnormal pregnancy outcomes in women exposed to onabotulinumtoxinA is comparable with background rates in the general population
No consistent types of malformation by organ were observed, and no new safety concerns were identified
Monitoring of pregnancy outcomes in onabotulinumtoxinA‐exposed women is ongoing
Supporting information
Supporting info item
Ethics statement
The authors state that no ethical approval was needed.
Acknowledgements
Writing assistance for manuscript development was provided by Jennifer Giel, PhD, and Jaya Kolipaka, MS, of Evidence Scientific Solutions, and was funded by Allergan, Inc.
Brin, M. F. , Kirby, R. S. , Slavotinek, A. , Miller‐Messana, M. A. , Parker, L. , Yushmanova, I. , and Yang, H. (2016) Pregnancy outcomes following exposure to onabotulinumtoxinA. Pharmacoepidemiol Drug Saf, 25: 179–187. doi: 10.1002/pds.3920.
An earlier analysis of the database was presented at the International Neurotoxin Association Conference, January 14–17, 2015.
References
- 1. BOTOX® [package insert]. Irvine, CA: Allergan, Inc.; 2015. http://www.allergan.com/assets/pdf/botox_pi.pdf [13 November 2015].
- 2. BOTOX® Cosmetic [package insert]. Irvine, CA: Allergan, Inc.; 2015. http://www.allergan.com/assets/pdf/botox_cosmetic_pi.pdf [13 November 2015].
- 3. US Department of Health and Human Services . Title 21‐Food and Drugs. Chapter I‐Food and Drug Administration, Department of Health and Human Services. Subchapter C‐Drugs: General. Part 201‐Labeling. Rockville, MD: FDA, 2013.
- 4. Natoli JL, Manack A, Dean B, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia 2010; 30: 599–609. [DOI] [PubMed] [Google Scholar]
- 5. Lear W, Kessler E, Solish N, Glaser DA. An epidemiological study of hyperhidrosis. Dermatol Surg 2007; 33: S69–75. [DOI] [PubMed] [Google Scholar]
- 6. American Society for Aesthetic Plastic Surgery . 2013 Cosmetic Surgery National Data Bank Statistics. http://www.surgery.org/media/statistics. (accessed 29 April 2015).
- 7. Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990‐2008. Natl Vital Stat Rep 2012; 60: 1–21. [PubMed] [Google Scholar]
- 8. Tang‐Liu DD, Aoki KR, Dolly JO, et al. Intramuscular injection of 125I‐botulinum neurotoxin‐complex versus 125I‐botulinum‐free neurotoxin: time course of tissue distribution. Toxicon 2003; 42: 461–469. [DOI] [PubMed] [Google Scholar]
- 9. Ravichandran E, Gong Y, Al Saleem FH, Ancharski DM, Joshi SG, Simpson LL. An initial assessment of the systemic pharmacokinetics of botulinum toxin. J Pharmacol Exp Ther 2006; 318: 1343–1351. [DOI] [PubMed] [Google Scholar]
- 10. Marsh WA, Monroe DM, Brin MF, Gallagher CJ. Systematic review and meta‐analysis of the duration of clinical effect of onabotulinumtoxinA in cervical dystonia. BMC Neurol 2014; 14: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. FDA Reviewer Guidance: Evaluating the Risks of Drug Exposure in Human Pregnancies USDoHaHS, Food and Drug Administration . http://www.fda.gov/downloads/scienceresearch/specialtopics/womenshealthresearch/ucm133359.pdf April 2005.
- 12. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Research CfBEa . FDA Guidance for Industry: Establishing Pregnancy Exposure Registries. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071639.pdf. (accessed 22 September 2015).
- 13. Bar‐Oz B, Moretti ME, Mareels G, Van Tittelboom T, Koren G. Reporting bias in retrospective ascertainment of drug‐induced embryopathy. Lancet 1999; 354: 1700–1701. [DOI] [PubMed] [Google Scholar]
- 14. Koren G, Nickel S. Sources of bias in signals of pharmaceutical safety in pregnancy. Clin Invest Med 2010; 33: E349–355. [DOI] [PubMed] [Google Scholar]
- 15. The Antiretroviral Pregnancy Registry . Antiretroviral Pregnancy Registry International Interim Report for 1 January 1989–31 January 2015. http://apregistry.com/forms/interim_report.pdf. (accessed 22 September 2015).
- 16. Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ 2000; 320: 1708–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rynn L, Cragan J, Correa A. Update on overall prevalence of major birth defects‐‐Atlanta, Georgia, 1978–2005. MMWR Morb Mortal Wkly Rep 2008; 57: 1–5. [PubMed] [Google Scholar]
- 18. Sokal R, Fleming KM, Tata LJ. Potential of general practice data for congenital anomaly research: Comparison with registry data in the United Kingdom. Birth Defects Res A Clin Mol Teratol 2013; 97: 546–553. [DOI] [PubMed] [Google Scholar]
- 19. Christianson A, Howson CP, Modell B. March of dimes global report on birth defects. White Plains, NY: March of Dimes Birth Defects Foundation, 2006.
- 20. Eser O, Yaman M, Cosar E, et al. Does Botox effect neural tube development in early chick embryos? Eur Spine J 2007; 16: 235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. St Clair EH, DiLiberti JH, O'Brien ML. Letter: observations of an infant born to a mother with botulism. J Pediatr 1975; 87: 658. [DOI] [PubMed] [Google Scholar]
- 22. Polo JM, Martin J, Berciano J. Botulism and pregnancy. Lancet 1996; 348: 195. [DOI] [PubMed] [Google Scholar]
- 23. Robin L, Herman D, Redett R. Botulism in a pregnant women. N Engl J Med 1996; 335: 823–824. [DOI] [PubMed] [Google Scholar]
- 24. Morrison GA, Lang C, Huda S. Botulism in a pregnant intravenous drug abuser. Anaesthesia 2006; 61: 57–60. [DOI] [PubMed] [Google Scholar]
- 25. Magri K, Bresson V, Barbier C. [Botulism and pregnancy] [Article in French. J Gynecol Obstet Biol Reprod (Paris) 2006; 35: 624–626. [DOI] [PubMed] [Google Scholar]
- 26. Leclair D, Fung J, Isaac‐Renton JL, et al. Foodborne botulism in Canada, 1985–2005. Emerg Infect Dis 2013; 19: 961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hildebrand GJ, Lamana C, Heckly RJ. Distribution and particle size of type A botulinum toxin in body fluids of intravenously injected rabbits. Proc Soc Exp Biol Med 1961; 107: 284–289. [Google Scholar]
- 28. Bodkin CL, Maurer KB, Wszolek ZK. Botulinum toxin type A therapy during pregnancy. Mov Disord 2005; 20: 1081–1082; author reply 1082. [DOI] [PubMed] [Google Scholar]
- 29. Newman WJ, Davis TL, Padaliya BB, et al. Botulinum toxin type A therapy during pregnancy. Mov Disord 2004; 19: 1384–1385. [DOI] [PubMed] [Google Scholar]
- 30. Morgan JC, Iyer SS, Moser ET, Singer C, Sethi KD. Botulinum toxin A during pregnancy: a survey of treating physicians. J Neurol Neurosurg Psychiatry 2006; 77: 117–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Oliveira Monteiro E. Botulinum toxin and pregnancy. Skinmed 2006; 5: 308. [DOI] [PubMed] [Google Scholar]
- 32. Wataganara T, Leelakusolvong S, Sunsaneevithayakul P, Vantanasiri C. Treatment of severe achalasia during pregnancy with esophagoscopic injection of botulinum toxin A: a case report. J Perinatol 2009; 29: 637–639. [DOI] [PubMed] [Google Scholar]
- 33. Li Yim JF, Weir CR. Botulinum toxin and pregnancy‐a cautionary tale. Strabismus 2010; 18: 65–66. [DOI] [PubMed] [Google Scholar]
- 34. Aranda MA, Herranz A, del Val J, Bellido S, García‐Ruiz P. Botulinum toxin A during pregnancy, still a debate. Eur J Neurol 2012; 19: e81–82. [DOI] [PubMed] [Google Scholar]
- 35. Greenberg AE, Mohney BG, Diehl NN. Clinical characteristics of childhood strabismus from a population‐based cohort. J AAPOS 2007; 11: 89–90. [Google Scholar]
- 36. Peckham EL, Lopez G, Shamim EA, et al. Clinical features of patients with blepharospasm: a report of 240 patients. Eur J Neurol 2011; 18: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Epidemiological Study of Dystonia in Europe (ESDE) Collaborative Group . A prevalence study of primary dystonia in eight European countries. J Neurol 2000; 247: 787–792. [DOI] [PubMed] [Google Scholar]
- 38. Defazio G, Jankovic J, Giel JL, Papapetropoulos S. Descriptive epidemiology of cervical dystonia. Tremor Other Hyperkinet Mov 2013; 3 http://tremorjournal.org/article/view/193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov Disord 1991; 6: 119–126. [DOI] [PubMed] [Google Scholar]
- 40. Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology 1991; 41: 1088–1091. [DOI] [PubMed] [Google Scholar]
- 41. Carruthers A, Sadick N, Brandt F, et al. Evolution of facial aesthetic treatment over five or more years: a retrospective cross‐sectional analysis of continuous onabotulinumtoxinA treatment. Dermatol Surg 2015; 41: 693–701. [DOI] [PubMed] [Google Scholar]
- 42. Strutton DR, Kowalski JW, Glaser DA, Stang PE. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. J Am Acad Dermatol 2004; 51: 241–248. [DOI] [PubMed] [Google Scholar]
- 43. Di Carlo A, Launer LJ, Breteler MM, et al. Frequency of stroke in Europe: a collaborative study of population‐based cohorts. ILSA Working Group and the Neurologic Diseases in the Elderly Research Group. Italian Longitudinal Study on Aging. Neurology 2000; 54(11 Suppl 5): S28–S33. [PubMed] [Google Scholar]
- 44. Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Migraine Prevalence and Prevention Study. Headache 2012; 52: 1456–1470. [DOI] [PubMed] [Google Scholar]
- 45. Manack A, Motsko SP, Haag‐Molkenteller C, et al. Epidemiology and healthcare utilization of neurogenic bladder patients in a US claims database. Neurourol Urodyn 2011; 30: 395–401. [DOI] [PubMed] [Google Scholar]
- 46. Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol 2003; 20: 327–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


