Abstract
The proliferation of cardiac‐related biomarkers and advocacy for their use has often come without adequate discussion of limitations in the interpretation of values and their best use in heart failure (HF) patients to provide a balanced assessment of how cardiac biomarkers are advocated for use in HF and areas where we would argue there are no gaps in knowledge. We include suggestions to address these issues. We have focused on peer‐reviewed publications over the period 2000 to present. Most studies have used samples at one or at most two points in time to define risk. Although biomarkers might add to the magnitude of risk, it is unclear how often they lead to changes in treatment. We suggest that defining the use of serial biomarker testing over time would be more helpful. To do this, it is necessary to take into account the biomarker's analytical and biological variability in addition to its ability to define and monitor therapy. These factors are often overlooked leading to conclusions that may be statistically significant but not clinically or analytically robust. An appreciation of the value and limitations of biomarker use is important to all clinicians who manage HF patients. If the proper studies are done so that biomarkers are used optimally, they will likely be helpful in defining when and how to intervene. If we continue as we have, we will continue to have ambiguity about the use of these valuable probes in the assessment and management of HF.
Keywords: Heart failure, Biomarkers, Serial monitoring, Assessment and management
Introduction—what are biomarkers and how are they used and misused?
Biomarkers are measurable events which function as mediators of events, integrators of a given event with other events, or innocent bystanders that passively change with events. In heart failure (HF) the concept is that biomarkers help to identify and monitor pathophysiologic events and diagnosis and risk stratify individual patients. This approach is not new.1, 2 However, over reliance on values at a single point in time and without understanding the caveats and limitations, and without integration of clinical assessments and judgment can lead to over‐utilization of biomarkers in some situations, and under‐utilization in others. This problem is exacerbated by biases towards publication of positive as opposed to null studies.3, 4 In addition, in the absence of understanding the caveats relative to the proper use of biomarker values, the data can lead to misinterpretation at times. This fosters confusion about the role of biomarkers in patient management. This review attempts to address these issues and provide assistance concerning the proper use and limitations of biomarkers. We emphasize examples from commonly used biomarkers because that will be readily appreciated by clinicians, but that should not obscure the fact that the principles of biomarker interpretation must be improved for all biomarkers. Our advocacy is that biomarkers are helpful diagnostically especially in patients at intermediate risk, interesting for defining risk, but most valuable when/if we harness the potential of serial values to direct therapy and improve outcomes. At present, most efforts focus on the first elements rather than the last. The purpose of this review is not to compare different groups or classes of biomarkers but to present concepts that we feel are often not considered or ignored in published work on biomarkers and convey the point that not taking these concepts into account could have significant impact on the clinical utility of the biomarker(s) involved.
How ‘normal’ biomarker values are determined: pros and cons
What denotes an elevated or abnormal biomarker value and warrants a diagnosis and which values are associated with increased risk are described variously and by different analytical approaches. There is a desire to ‘keep it simple’. Thus, many assume that the value used to define disease is the upper limit of normal. That is true for cardiac troponin but not true for natriuretic peptides (NP).
There is even ambiguity about how to define normal ranges and/or important cut off values. It takes at least 300 subjects of each patient gender, ethnicity, and age grouping5 to define statistically each subset whether to determine normal ranges or elevated values. Doing this for large numbers of subsets is costly. However, if not done, it means ignoring differences that might be present because of age, sex, ethnicity, patient characteristics, medications, subtypes of disease, and comorbidities. An example of this is shown in Figure 1 which are the age and gender adjusted putatively ‘normal’ values for NP.6 It is ‘putative’ because there are substantial issues about how to define a normal population.5 It is rare that true normal subjects are recruited. Most ‘normal range’ studies come from convenience cohorts and involve at most a medical questionnaire. Very few studies include a history or physical examination. It should not be surprising that adding other biomarker measurements such as creatinine, glomerular filtration rate, or an NP value lowers normal values. Even more vigorous screening including imaging decreases values still further. This is not important when testing is used at high values to define disease but when the important metrics impinge on the upper limit of the normal range, they become crucial. For some analytes like cardiac troponin where small differences are important, some suggest that imaging is essential to define normality7 (Figure 2). These considerations are easily lost in small studies and/or in large clinical trials but contribute to confusion in interpretation in individual patients. These considerations are not important for NPs in those with overt HF but are critical to define at risk cohorts where values are low and are compared with ‘normal values’.8, 9, 10, 11
Figure 1.
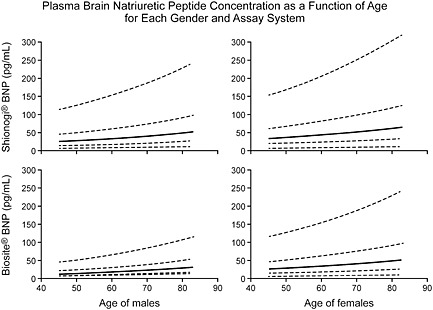
Plasma B‐type natriuretic peptide concentrations shown as a function of age, gender, and assay system. Shown for the 5th, 25th, 50th, 75th, and 95th percentiles of B‐type natriuretic peptide. (Reproduced with permission, ref 6).
Figure 2.
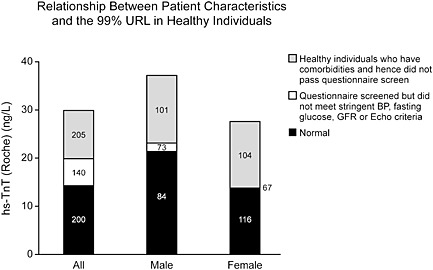
Relationship between patient characteristics and the 99th percentile upper range limit of high‐sensitivity troponin T in healthy individuals. Values of the 99th percentile go down with more rigorous screening. Questionnaire screened = no history of vascular disease or diabetes, and not taking cardio‐active drugs. Normal = no history of vascular or cardiovascular disease, diabetes, hypertension, or heavy alcohol intake and who were receiving no cardiac medications and had blood pressure ≤140/90 mm Hg, fasting glucose <110 mg/dL, epidermal growth factor receptor >60 mL/min, left ventricular ejection fraction >50%, normal lung function, and no significant valvular heart disease, left ventricular hypertrophy, diastolic heart failure, or regional wall‐motion abnormalities on echocardiography. (Reproduced with permission, ref 7).
These considerations are less important when the values are markedly elevated as with NPs and HF. However, the concepts associated with the differences are critically important in interpreting values in subsets of patients such as older women and in the obese. Some might argue that the significant difference in the diagnosis of HF seen in the ‘Breathing Not Properly Trial’ was because of the use of a cut‐off of 100 ng/mL when that value was substantially less than upper limit of normal values one might see in elderly women who often have diastolic dysfunction or HFPEF and are notoriously difficult to diagnose. In the obese, the suggestion has been made that the cut‐off values for HF should be reduced but even this recommendation varies. In addition, these influences will have more important effects in models that attempt to predict events in groups at risk such as diabetics where the optimal cut‐off values have not been defined.12
For better or worse, near normal values are often not the critical cut‐off values for clinical use. That level is often defined based upon values derived by the area under an receiver operating characteristic (ROC) curve (C‐statistic) which suggests the best sensitivity and specificity. This approach equates the importance clinically of sensitivity and specificity and often there is a tension between the two13 as shown in Figure 3.14 These sorts of issues reflect different clinical concerns. Emergency physicians do not want to miss disease and thus focus on sensitivity, whereas hospitalists are concerned about what to do when it is difficult to explain biomarker results and thus would prefer high specificity.
Figure 3.
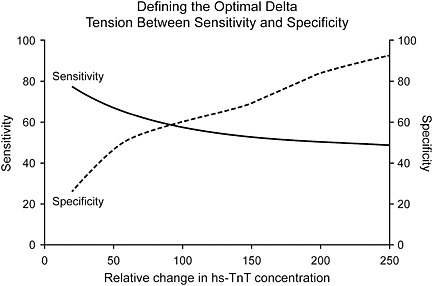
Defining the optimal delta: tension between sensitivity and specificity. (Reproduced with permission, ref 14).
In addition, a particular cut‐off value may not apply to every patient cohort with different clinical characteristics (different case mixes). Also, it is valuable to keep in mind that the concept of positive or negative cut‐point of biomarkers is an artifact of the need for clinical convenience. Most biomarkers are continuous variables of risk, and cut‐points should be viewed with that limitation in mind. Additionally, optimal biomarker cut‐points will vary in HF patients according to age, gender, ethnicity, specific assay employed, acuity and duration of HF, severity of HF, ongoing treatment (presence and absence of established drug therapy), left ventricular ejection fraction (interpretation of biomarker levels, cut‐points, and associated risk differ between HFpEF and HFrEF), renal function, and co‐morbidities (e.g. diabetes). Thus, biomarkers must be interpreted in the ‘proper clinical context’ and not per arbitrary cut‐off values. The concept of keeping it simple is reasonable for many situations, but the concept often means one does not know when to make exceptions. These issues are critical but often ignored in reviews and especially by advocates for extensive use.
For some biomarkers the lowest detectable level different from zero is used to identify the cut‐point associated with increased risk. This is often the case when the assay for a given analyte is not particularly sensitive. A practical and more universally applicable approach is the use of the upper reference value of normal values to provide a cut‐point that can be applied to different patient cohorts with a common basis for comparison.15, 16 Often a more complex schema is needed as provided by the ICON group for NPs.17
Single‐sample values vs. serial measurements over time and the capacity to predict outcomes, stratify risk, or guide therapy
Disease activity changes over time and is variable between patients and within the same patient. Thus, serial measurements over time rather than single point‐in‐time measurements are needed to optimally manage patients. However, most studies focus on the predictive value of an initial sample. It is no surprise that the more abnormal the value, the sicker the patient. How does that help? Conventional wisdom is that clinicians will be more aggressive with those patients in applying conventional therapies. Recent data concerning the ordering of mineralocorticoid antagonists suggest such a strategy is frequently not pursued.18 Not only that, but in a clinically stable patient does an elevated biomarker value trump other clinical information? This is why serial values over time should be much more valuable and we have championed that approach.16, 19, 20, 21 An example is shown in Figure 4.20 It is likely that biomarker levels that decrease over time should reflect clinical improvement, while those that increase reflect disease progression. Serial measurements thus provide information on increasing risk but also indicate which patients are responding to a given therapy or need a change in treatment. One size does not fit all.
Figure 4.
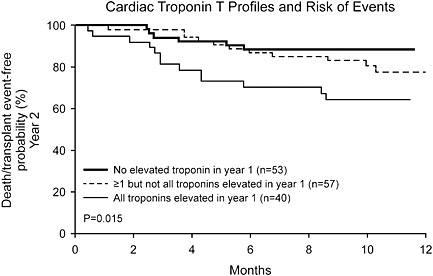
Cardiac troponin T profiles and risk of events. Kaplan–Meier analysis of the risk of death or cardiac transplantation in year two of follow‐up based upon cardiac troponin T patterns from year 1 of clinical follow‐up. (Reproduced with permission, ref 20).
The specific subset of patients is also important. The response of biomarkers such as NPs in acute HF patients is related to acute hemodynamics and may respond rapidly22, 23 whereas those found in more chronic HF where biomarker pathways have been stimulated longer do not correlate as well and require more time to change.24 Thus, changes are clinical subset dependent. For example, we have shown in chronic HF that very large differences in NPs are necessary to see a change in risk,21 yet lesser changes have been reported to be prognostic.24 It may be that both observations are correct and that the changes which identified with only two samples24, 25 would achieve greater reductions if the analysis was done over a more prolonged time period and/or that the group that is going down is enriched by acute elevations that are declining rapidly. Also, because in chronic HF, deterioration occurs slowly, changes in biomarkers over time could potentially be used to provide early indications of disease progression and allow for earlier therapy. Thus, biomarkers that have low levels of biological and analytical variability should have an advantage. The high biological variation in NPs may be in part why trials using NP values to direct therapy have shown mixed results because most have not resulted in marked changes. If changes in biomarkers can be used to demonstrate that risk is modified, then such an approach might be used to test specific interventions. We have shown that the ability to monitor changes in serial biomarker measurements, most notably troponin T, adds significantly to risk assessment in chronic HF.16, 19, 20 Increases measured serially any time during clinical follow‐up independently predicted increased mortality, the need for cardiac transplantation, or HF‐related hospitalizations. Therefore, serial changes maybe the means to identify risk prospectively in individual patients and to help determine which therapy might be most effective. However, it is likely that criteria needed may change over time. There are data to suggest that at the end‐stage of HF, NP values may diminish. 26 Thus, in such patients, one should not be reassured by lower values.13, 27, 28
Analytical and biological variability, and magnitude of change in biomarker value needed to be clinically meaningful
The clinical significance of biomarker levels over time is in part dependent on its analytical and biological variability. These factors are often overlooked and conclusions drawn based on changes in a biomarker concentration that may be statistically significant but not clinically or analytically significant. To be sure values are different they must be further apart than three standard deviations of the variation around the measures. This is called the reference change value (RCV).11, 29 For biomarkers, it contains both analytical and biological variability. Biological variation can only be measured in normal subjects since pathologies will influence it in diseased patients. This caveat is often ignored. Analytical variability identifies the imprecision of assay results and by issues related to sample quality. If the quality of the sample is problematic then reliable values cannot be obtained. For example, hemolysis is common and can raise some troponin values and lower others.30 For NPs, sample degradation is common.31 These issues provide the reasons why most groups urge that precision be very high (CV <10%), especially near critical values. However, contrary to common belief, imprecision does not cause false positives.32 The importance of taking variation into account is critical to interpretation of serial biomarker measurements. Some identified changes, although statistically significant, may be because of variability alone. The RCV which reflects this combined variability and indicates the minimal percent change required to be sure that a meaningful change has occurred. It varies from a low of 30% (e.g. ST2) to 138% (e.g. BNP) for month to month measurements.33 Troponin T has very low variability.29 Thus, analytes with low RCVs are likely to be better at detecting change than those with high RCVs. This is what we found in our study assessing changes in BNP in patients with chronic HF, where increases in BNP of over 80% were necessary to indicate an increased risk of events and only >80% decreases were associated with reduced risk.21 Because ST2 has a low RCV, it may be potentially valuable for assessing change over time.33
Multi‐biomarker paradigm
There is interest in combining biomarkers that differ in their assessment of pathophysiology. However, there is much less interest in comparing biomarkers. In the long term, the best combinations will only be found by comparisons.16, 19, 34 Part of this reflects concern that the approaches used because different biomarkers may be optimal with one design and less effective with another. This means that some biomarkers are put at a disadvantage by a given study design approach taken but this is rarely acknowledged.
Ideally, one would like to see major changes in the ability of multi‐biomarker approaches to predict events such that targeted interventions can be employed. Often the impact of the ROC curve thought statistical is minimal leading investigators to seek other measures such as the ability to reclassify events which is more sensitive.35 However, some have argued that this approach is statistically flawed and may be too sensitive.36 It should be clear that the more robust the findings, the more likely they are to be clinically relevant. If one combines too many biomarkers, one may improve prediction but the details of the scoring system may be too complex for clinicians to identify where gaps exist. Therefore, understanding the pathophysiology of each biomarker can be useful, if not essential, not only in developing diagnostic and therapeutic interventions, but in assisting interpretation of calculated scores. These issues become particularly important when using scores to titrate treatment. Given the dynamic nature of HF progression it would not necessarily be expected that multiple distinct pathways of disease progression would be stimulated or activated to the same degree at any one time, and, therefore, a multi‐marker profile may identify disease activity missed by adherence to the monitoring of a single selected biomarker. This concept needs further development and evaluation and includes the thorny issue of which biomarkers best contribute to a multi‐biomarker panel. However, it is also more likely that it will take different strategies at different times during the process of HF and that a ‘one size fits all’ multi‐marker approach may not be optimal.
Incremental value of biomarkers—use for diagnosis, prognosis, and guiding treatment
The most common measure used to evaluate biomarkers is the area under the ROC curve or c‐statistic which reports the probability of identifying risk from non‐risk. The integrated discrimination improvement provides another way to quantify the incremental value of biomarkers, share the limitations of patient mix, and the impact of unequal contribution of risk factors to model prediction. Novel risk reclassification measures are now also being introduced in the literature to evaluate new biomarkers. One commonly reported is net reclassification improvement which attempts to identify the net change in clinical risk when a new biomarker is added to previously established risk factors. While these tests are intended to identify ‘real value’ in new biomarkers, it is not clear that these methods appropriately reclassify patients based upon relevant clinical events and the variability of changes in risk factor categories. More work is needed to improve the quantification of risk prediction and the performance of patient reclassification based upon the incremental value of a new or old biomarker.
Conclusions
The goals for biomarker development should include consideration of the characteristics of an ‘ideal’ biomarker and emphasize actionable patterns. But today, the preponderance of current analyses relate largely to diagnostic and prognostic impact. While it is important to predict which patients are at increased risk, knowing that a patient who is at risk without biomarker data is at even more risk as indicated by the biomarker is not clinically helpful. Does it matter whether a given patient is at a four‐fold compared with a 10‐fold risk? We suggest that unless we know what to do, this may not be a cost‐effective use of the biomarker. On the other hand, when one can identify individuals at risk where it was not anticipated clinically, it is more important. Perhaps recent data concerning the importance of minor increases in hs cTnT and NT‐proBNP in patients with left ventricular hypertrophy (LVH) might be an example of the latter.37 It was only with the use of biomarkers that the subset of individuals who were at risk was identified among those with LVH by MRI or ECG. What is most important is to provide information to guide therapeutic modifications based upon biomarker values or changes in biomarker values—this would be the ‘Holy Grail’ of HF management. Trials using NP have started (PONTIAC and STOP CHF) and are important. An example from the STOP CHF38 is shown in Figure 5. Pursuing such trials is expensive and often what is advocated, based on observations from other situations, is the use of biomarkers to modify therapy, but absent data. Perhaps, the recent enthusiasm for biomarkers of fibrosis exemplifies this situation. We know that mineralocorticoid antagonists retard fibrosis;39 thus, elevations of biomarkers that are part of the pathway of fibrosis should lead to the effective use of these agents. This is a reasonable extrapolation and there are experimental and a few observational data to suggest that this may work,40 but more studies are needed. It will take RCTs and/or well done registries and pathophysiological trials before we can be sure enough to advocate such an option. One approach that may be useful is to probe multiple biomarkers when testing one specific biomarker to guide therapy. Unfortunately, this was done only sparsely in the recent trials aimed at using NP levels to guide therapy. Hopefully, the latest trial in progress, ‘GUIDE‐IT’, will bring some clarity to this issue and correct this oversight.
A trip of a thousand miles begins with the first step. It is time to start taking the first steps.
Figure 5.
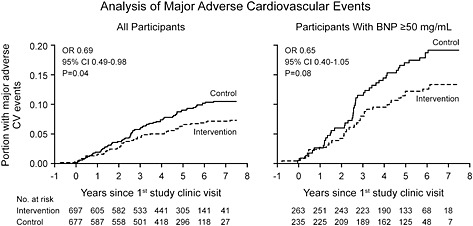
Kaplan–Meier analysis of major adverse cardiovascular events in the full study sample and in participants with B‐type natriuretic peptide ≥50 pg/mL. (Reproduced with permission, ref 39).
Conflict of interest
Dr. Jaffe reports consulting honorarium from Alere, Abbott, Roche, Radiometer, Siemens, Diadexus, Beckman‐Coulter, Trinity, ET Healthcare, Novartis, the heart.org. Dr Miller has no conflicts of interest to report.
Miller, W. L. , and Jaffe, A. S. (2016) Biomarkers in heart failure: the importance of inconvenient details. ESC Heart Failure, 3: 3–10. doi: 10.1002/ehf2.12071.
References
- 1. Elster SK, Braunwald E, Wood HF. A study of C‐reactive protein in the serum of patients with congestive heart failure. Am Heart J 1956; 51: 533–541. [DOI] [PubMed] [Google Scholar]
- 2. Vasan RS. Biomarkers of cardiovascular disease. Molecular basis and practical considerations. Circulation 2006; 113: 2335–2362. [DOI] [PubMed] [Google Scholar]
- 3. Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA 1990; 263: 1385–1389. [PubMed] [Google Scholar]
- 4. Johnson RT, Dickersin K. Publication bias against negative results from clinical trials: three of the seven deadly sins. Nat Clin Pract Neurol 2007; 3: 590–591. [DOI] [PubMed] [Google Scholar]
- 5. Apple FS, Collinson PO, IFCC Task Force on Clinical Applications of Cardiac Biomarkers . Analytical characteristics of high‐sensitivity cardiac troponin assays. Clin Chem 2012; 58: 54–61. [DOI] [PubMed] [Google Scholar]
- 6. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40: 976–982. [DOI] [PubMed] [Google Scholar]
- 7. Collinson PO, Heung YM, Gaze D, Boa F, Senior F, Christenson R, Apple FS. Influence of population selection on the 99th percentile reference value of cardiac troponin assays. Clin Chem 2012; 58: 219–225. [DOI] [PubMed] [Google Scholar]
- 8. Christ M, Laule‐Kilian K, Hochholzer W, Klima T, Breidthardt T, Perruchoud AP, Mueller C. Gender‐specific risk stratification with B‐type natriuretic peptide levels in patients with acute dyspnea (BASAL study). J Am Coll Cardiol 2006; 48: 1808–1812. [DOI] [PubMed] [Google Scholar]
- 9. Christenson RH, Azzazy HME, Duh S‐H, Maynard S, Seliger SL, deFilippi CR. Impact of increased body mass index on accuracy of B‐type natriuretic peptide (BNP) and N‐terminal proBNP for diagnosis of decompensated heart failure and prediction of all‐cause mortality. Clin Chem 2010; 56: 633–641. [DOI] [PubMed] [Google Scholar]
- 10. Das SR, Abdullah SM, Leonard D, Drazner MH, Khera A, McGuidre DK, deLemos JA. Association between renal function and circulating levels of natriuretic peptides (Dallas Heart Study). Am J Cardiol 2008; 102: 1394–1398. [DOI] [PubMed] [Google Scholar]
- 11. Wu AHB. Serial testing of B‐type natriuretic peptide and NT‐proBNP for monitoring therapy of heart failure: the role of biologic variation in the interpretation of results. Clin Chem 2006; 152: 828–834. [DOI] [PubMed] [Google Scholar]
- 12. Huelsmann M, Neubold S, Resl M, Strunk G, Brath H, Francesconi C, Adlbrecht C, Prage R, Luger A, Pacher R, Clodi M. PONTIAC (NT‐proBNP selection prevention of cardiac events in a population of diabetic patients without a history of cardiac disease). J Am Coll Cardiol 2013; 62: 1365–1372. [DOI] [PubMed] [Google Scholar]
- 13. Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N‐BNP) concentrations. Lancet 2000; 355: 1126–1130. [DOI] [PubMed] [Google Scholar]
- 14. Korley FK, Jaffe AS. Preparing the United States for high‐sensitivity cardiac troponin assays. J Am Coll Cardiol 2013; 61: 1753–1758. [DOI] [PubMed] [Google Scholar]
- 15. Gore MO, Seliger SL, deFilippi CR, Namabi V, Christenson RH, Hashim IA, Hoogeveen RC, Ayers CR, Sun W, McGuire DK, Ballantyne CM, de Lemos JA. Age and sex‐dependent upper reference limits for the high‐sensitivity cardiac troponin T assay. J Am Coll Cardiol 2014; 63: 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller WL, Hartman KA, Burritt MF, Grill DE, Rodeheffer RJ, Burnett JC Jr, Jaffe AS. Serial biomarker measurements in ambulatory patients with chronic heart failure—the importance of change over time. Circulation 2007; 116: 249–257. [DOI] [PubMed] [Google Scholar]
- 17. Januzzi JL, van Kimmenade R, Lainchbury J, Bayes‐Genis A, Ordonez‐Llanos J, Santalo‐Bel M, Pinto YM, Richards M. NT‐proBNP testing for diagnosis and short‐term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients—the international collaborative of NT‐proBNP study. Eur Heart J 2006; 27: 330–337. [DOI] [PubMed] [Google Scholar]
- 18. Maron BA, Leopold JA. Aldosterone receptor antagonists. Effective but often forgotten. Circulation 2010; 121: 934–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller WL, Hartman KA, Grill DE, Struck J, Bergmann A, Jaffe AS. Serial measurements of mid‐region‐pro‐ANP and C‐terminal‐pro‐vasopressin (Copeptin) in relation to B‐type natriuretic peptide and troponin T in ambulatory chronic heart failure patients—incremental prognostic value. Heart (BHJ) 2012; 98: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller WL, Hartman KA, Burritt MF, Grill DE, Jaffe AS. Profiles of serial changes in cardiac troponin T concentrations and outcomes in ambulatory patients with chronic heart failure. J Am Coll Cardiol‐Heart Failure 2009; 54: 1715–1721. [DOI] [PubMed] [Google Scholar]
- 21. Miller WL, Hartman KA, Grill DE, Burnett JC, Jaffe AS. Only large reductions in natriuretic peptide concentrations (BNP and NT‐proBNP) are associated with improved outcomes in ambulatory patients with chronic heart failure. Clin Chem 2009; 55: 78–84. [DOI] [PubMed] [Google Scholar]
- 22. Forfia PR, Watkins SP, Rame E, Stewart KJ, Shapiro EP. Relationship between B‐type natriuretic peptides and pulmonary capillary wedge pressure in the intensive care unit. J Am Coll Cardiol 2005; 45: 1667–1671. [DOI] [PubMed] [Google Scholar]
- 23. Cioffi G, Tarantini L, Stefenelli C, Azzetti G, Marco R, Carlucci S, Furlanello F. Changes in plasma N‐terminal proBNP levels and ventricular filling pressures during intensive unloading therapy in elderly with decompensated congestive heart failure and preserved left ventricular systolic function. J Card Fail 2006; 12: 608–615. [DOI] [PubMed] [Google Scholar]
- 24. Latini R, Masson S, Wong M, Barlera S, Carretta E, Staszewsky L, Vago T, Maggioni AP, Anand IS, Tan LB, Tognoni G, Cohn JN, Val‐HeFT Investigators . Incremental prognostic value of changes in B‐type natriuretic peptide in heart failure. Am J Med 2006; 119: 70e23–70e30. [DOI] [PubMed] [Google Scholar]
- 25. Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, Angelici L, Barlera S, Parrinello G, Maggioni AP, Tognoni G, Cohn JN, for the Val‐HeFT Investigators . Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007; 116: 1242–1249. [DOI] [PubMed] [Google Scholar]
- 26. Miller WL, Burnett JC Jr, Hartman KA, Henle MP, Burritt MF, Jaffe AS. Lower rather than higher levels of b‐type natriuretic peptides (NT‐proBNP and BNP) predict short‐term mortality in end‐stage heart failure patients treated with nesiritide. Am J Cardiol 2005; 96: 837–841. [DOI] [PubMed] [Google Scholar]
- 27. Richards AM, Troughton RW. Use of natriuretic peptides to guide and monitor heart failure therapy. Clin Chem 2012; 58: 62–71. [DOI] [PubMed] [Google Scholar]
- 28. Januzzi JL, Rehman SU, Mohammed AA, Bhardwaji A, Barajas L, Barajas J, Kim H‐N, Baggish AL, Weiner RB, Chen‐Tournoux A, Marshall JE, Moore SA, Carlson WD, Lewis GD, Shin J, Sullivan D, Parks K, Wang TJ, Gregory SA, Uthamalingam S, Semigran MJ. Use of amino‐terminal pro‐B‐type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction (PROTECT). J Am Coll Cardiol 2011; 58: 1881–1889. [DOI] [PubMed] [Google Scholar]
- 29. Vasile VC, Saenger AK, Kroning JM, Jaffe AS. Biological and analytical variability of a novel high‐sensitivity cardiac troponin T assay. Clin Chem 2010; 56: 1086–1090. [DOI] [PubMed] [Google Scholar]
- 30. Bais R. The effect of sample hemolysis on cardiac troponin I and T assays. Clin Chem 2010; 56: 1357–1359. [DOI] [PubMed] [Google Scholar]
- 31. Belenky A, Smith A, Zhang B, Lin S, Despres N, Wu AHB, Bluestein BI. The effect of class‐specific protease inhibitors on the stabilization of B‐type natriuretic peptide in human plasma. Clin Chim Acta 2004; 340: 163–172. [DOI] [PubMed] [Google Scholar]
- 32. Panteghini M, Pagani F, Yeo KT, Apple FS, Christenson RH, Dati F, Mair J, Ravkilde J, Wu AHB, on behalf of the Committee on Standardization of Markers of Cardiac Damage of the IFCC . Evaluation of the imprecision at low‐range concentrations of the assays for cardiac troponin determination. Clin Chem 2004; 50: 327–332. [DOI] [PubMed] [Google Scholar]
- 33. Wu AHB, Wians F, Jaffe AS. Biological variation of galectin‐3 and soluble ST2 for chronic heart failure: implications on interpretation of test results. Am Heart J 2013; 665: 995–999. [DOI] [PubMed] [Google Scholar]
- 34. Miller WL, Grill DE, Struck J, Jaffe AS. Association of elevated Copeptin and hyponatremia with enhanced risk in patients with chronic ambulatory heart failure. Am J Card 2013; 111: 880–85. [DOI] [PubMed] [Google Scholar]
- 35. Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med 2009; 150: 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hilden J, Gerds TA. A note on the evaluation of novel biomarkers: do not rely on IDI and NRI. Stat Med 2014; 33: 3405–3414. [DOI] [PubMed] [Google Scholar]
- 37. Neeland IJ, Dranzer MH, Berry JD, Ayers CR, de Filippi C, Seliger SL, Nambi V, McGuire DK, Omland T, de Lemos JA. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol 2013; 61: 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ledwidge M, Gallagher J, Conlon C, Talon E, O'Connell E, Dawkins I, Watson C, O'Hanlon R, Bermingham M, Patle A, Badabhagni MR, Murtagh G, Boon V, Tilson L, Barry M, McDonald L, Maurer B, McDonald K. Natriuretic peptide‐based screening and collaborative care of heart failure—the STOP‐HF randomized trial. JAMA 2013; 310: 66–74. [DOI] [PubMed] [Google Scholar]
- 39. Li X, Qi Y, Li Y, Zhang S, Guo S, Chu S, Gao P, Zhu D, Wu Z, Lu L, Shen W, Jai N, Niu W. Impact of mineralocorticoid receptor antagonists on changes in cardiac structure and function of left ventricular dysfunction. Circ Heart Fail 2013; 6: 156–165. [DOI] [PubMed] [Google Scholar]
- 40. Weir RAP, Miller AM, Murphy GEJ, Clements S, Steedman T, Connell JMC, McInnes IB, Dargie HJ, McMurray JJV. Serum soluble ST2: a potential novel mediator in left ventricular and infarct remodeling after acute myocardial infarction. J Am Coll Cardiol 2010; 55: 243–250. [DOI] [PubMed] [Google Scholar]


