Abstract
BACKGROUND
Variation in response to insecticidal proteins is common upon repetition of insect bioassays. Understanding this variation is a prerequisite to detecting biologically important differences. We tracked neonate Spodoptera frugiperda (J.E. Smith) susceptibility to Vip3Aa19 over 17 generations using standardized bioassay methods. Five larval pretreatment conditions and one bioassay condition were tested to determine whether susceptibility was affected. These included: storage time; prefeeding; storage at reduced temperature; storage at reduced humidity; colony introgression of field‐collected individuals. Extremes of photoperiod during the bioassay itself were also examined.
RESULTS
LC50 values for two strains of S. frugiperda varied 6.6‐fold or 8.8‐fold over 17 generations. Storage time and humidity had no impact on Vip3Aa19 susceptibility, whereas prefeeding significantly reduced subsequent mortality (by 27%). Storage at reduced temperature increased mortality for one colony (from 45.6 to 73.0%) but not for the other. Introgression of field‐collected individuals affected susceptibility at the first generation but not for subsequent generations. A 24 h bioassay photophase significantly reduced susceptibility (by 26%) for both colonies.
CONCLUSION
Certain pretreatment and bioassay conditions were identified that can affect S. frugiperda Vip3Aa19 susceptibility, but innate larval heterogeneity was also present. Our observations should help to increase the consistency of insecticidal protein bioassay results. © 2015 Syngenta Crop Protection, LLC. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Vip3A, fall armyworm, bioassay, variation
1. INTRODUCTION
While standardized bioassays provide a reliable method for determining the insecticidal activity of a test material, including those containing insecticidal proteins, the susceptibility of laboratory‐reared insect larvae can vary. This variation in susceptibility can arise from differing geographical sources of the insect, different testing laboratories or even through time for the same laboratory and insect source.1, 2, 3, 4 Furthermore, bioassay variability from different labs using similar methodologies has been observed across different orders of insects and for various proteins tested. An understanding of this natural variation in susceptibility, or that which is inherent to the bioassay methods employed, is a prerequisite to detecting biologically important differences.5
Within a given controlled artificial diet bioassay system, a number of factors have been identified that may influence bioassay response, such as source and type of diet,6 different Bt insecticidal protein preparations and quantification methods7 and selection of different time points after exposure to assess the final mortality.8 An additional factor, the innate heterogeneity (which can be genetically, maternally and/or environmentally influenced) across individual insects tested, is suggested by the fact that bioassay response variation may routinely range from three‐ to sixfold, or even 12‐fold for lab‐reared population comparisons (using the same methodology), and may be greater across field‐derived population comparisons.5, 9 Among different field populations of the same species, the variability in response to the same protein can even be extremely high, of the order of 10–100‐fold or more.10, 11 Other factors that may influence bioassay results, such as larval pretreatment conditions, which are often not well defined or controlled prior to routine artificial diet bioassay, have not been as thoroughly investigated, however. It is likely that these pretreatment conditions might also contribute to subsequent variability in susceptibility determinations for an assay system involving a particular protein and pest insect species.
Understanding the inherent variation and identifying the factors that contribute to insect bioassay variation are therefore critical to obtain accurate, reproducible datasets for measuring insecticidal protein activity against target or non‐target arthropods. These datasets are fundamental to other studies needed in support of risk assessment to consider the likelihood that crops containing the transgenic insecticidal protein might harm the environment or human health.12, 13 Additionally, registrants of Bt plant‐incorporated protectants must provide an insect resistance management plan to the EPA,14, 15 with a component of this plan to include resistance monitoring for targeted pests. This monitoring has an ongoing need for accurate and reproducible measurements of activity using insect bioassay methods. Detecting shifts in target species susceptibility to Bt insecticidal proteins through bioassay‐based monitoring programs is a valuable tool to evaluate the continued effectiveness of Bt traits in the field.2, 4, 16, 17, 18, 19
The present study was designed to examine selected potential sources of laboratory bioassay variation. The objective was to determine whether differential control of certain pretreatment conditions (which may routinely vary after hatching) could influence the susceptibility of Spodoptera frugiperda laboratory populations to Vip3Aa19 insecticidal protein when using a standardized artificial diet bioassay method.
2. MATERIALS AND METHODS
2.1. Strains and insect rearing
In 2011, larvae from a susceptible S. frugiperda colony (originated in 1997 and reared continuously in the absence of selective pressure by any insecticidal agent) were purchased from BioServ (Frenchtown, NJ), and a susceptible colony (identified as ‘SUS’) was established at the UNL toxicology laboratory by randomly selecting 300 larvae and then continuously rearing these as a new colony (methods described in Vélez et al.20). Since 2011, this colony has also been maintained in the absence of selective pressure by any insecticidal agent. In 2013, a second strain (identified as ‘K‐SUS’) was generated from the SUS colony by randomly selecting 300 larvae and then continuously rearing these as a new colony (in isolation from the SUS colony) using the same rearing conditions. Adult rearing techniques for S. frugiperda described by Perkins21 and adapted by Vélez et al.20 were used, with at least 200 adults mating randomly in each generation.
Neonates used in the bioassays were obtained from routine larval collections that consisted of a daily harvest of eggs that were visually homogeneous in color and egg mass size, and collected during the peak of oviposition (3–5 days after initial egg production).22 The collected eggs were stored in petri dishes with moistened filter paper in environmental chambers at 14 °C, 24 h L and 44 ± 2% RH for approximately 3 days. First‐instar S. frugiperda that hatched within a 4 h period were used for all experimental conditions tested. Both strains were bioassayed over 17 generations using standardized artificial diet bioassay methods described below to estimate variation over time.
2.2. Insecticidal protein
The Vip3Aa19 insecticidal protein derived from an Escherichia coli expression system and purified by anion‐exchange chromatography was provided by Syngenta Crop Protection, LLC (Research Triangle Park, NC) as lyophilized protein which was stored at −80 °C. The protein (86.5% purity) was aliquotted and preweighed, so that all Vip3Aa19 protein dilutions could be made on the same day as bioassay initiation. The purified protein was solubilized in 0.25× phosphate‐buffered saline (PBS) by a gentle agitation technique until completely dissolved, then briefly centrifuged at low speed (1677 × g for 5 s). Dilutions were prepared in 0.25× PBS to obtain the desired concentrations prior to bioassay.
2.3. Bioassays
Artificial diet bioassays were performed, based on the methods described by Marçon et al.,2 in 128‐well bioassay trays (each well 16 mm diameter, 16 mm high; CD International, Pitman, NJ). A quantity of 1 mL of wheat‐germ‐based multispecies lepidopteran diet23 was dispensed into each well and allowed to solidify.
Each well was treated by applying 50 µL of the appropriate concentration of Vip3Aa19 solution. The negative control consisted of wells treated with 50 µL of 0.25× PBS buffer. The treatments were dried onto the diet surface by stacking the trays onto an orbital shaker and using a low rotation speed to ensure uniform coverage of the treatments over the diet.
One S. frugiperda neonate (<4 h after hatching) was transferred into each well using a fine camel hair paint brush. Wells were covered with vented lids (BIO‐CV‐16; CD International), and trays were held in an incubator at 27 °C, 24 h scotophase and 60 ± 10% RH. Mortality was recorded 7 days after infestation, and larvae that were unable to respond to a gentle probe technique were considered to be dead. In each experiment, bioassays were replicated 3–4 times for each strain, with 16 larvae per treatment or control tested.
To establish the variation in LC50 estimates for Vip3Aa19 over multiple generations, diet bioassays were performed using seven concentrations to generate dose responses for both laboratory colonies of S. frugiperda larvae. These analyses were conducted over 17 generations for each colony.
To determine the effect of different larval pretreatment conditions, bioassays were performed with a single concentration of Vip3Aa19 that corresponded to the estimated LC70 (lethal concentration causing 70% mortality) against the laboratory S. frugiperda larvae. This concentration was approximated for both lab colonies, based on the estimate in the bioassay for the first‐generation K‐SUS colony (immediately after isolation from the parental SUS colony).
2.4. Pretreatment conditions
Routine laboratory bioassays to determine larval susceptibility to a given test material involve larval maintenance conditions that may vary in advance of any exposure to insecticidal agents (= ‘pretreatment condition’). To determine the impact of selected pretreatment conditions on the susceptibility to Vip3Aa19 protein, each condition was examined independently with the standardized bioassay methods at a concentration that approximated the LC70 dose as described above. The following five pretreatment conditions were examined: (1) larval storage time prior to exposure; (2) prior feeding on control artificial diet; (3) larval storage at reduced temperature prior to exposure; (4) larval storage at reduced humidity prior to exposure; (5) colony perturbation following introgression with field‐collected individuals. In addition to these pretreatment conditions, one additional bioassay condition (condition 6) was examined that involved extremes of photoperiod settings used during the course of the bioassay itself.
1. Impact of larval storage time prior to exposure. To assess the impact of larval storage time prior to exposure to insecticidal protein, larvae (within 0–4 h after hatch) were distributed among petri dishes containing moistened filter paper and kept for four different time periods in the absence of food. To establish the pretreatment time periods, larvae were then either transferred directly to bioassay trays or held for an additional 2, 6 or 12 h in the petri dishes on moistened filter paper prior to the start of the bioassays. Mortality was determined after 7 days exposure to the estimated LC70 concentration of Vip3Aa19 as described above. The procedure was repeated 3 times for each colony, with a total of 336 insects tested for each treatment.
2. Impact of prior feeding on artificial diet. To determine the impact of prior feeding, larvae (within 0–4 h after hatch) were transferred to individual wells of artificial diet trays (one larva per well) where they were allowed to feed for 2, 6 or 12 h. After the respective pretreatment holding times, the larvae were transferred to bioassay trays for subsequent exposure to the estimated LC70 concentration of Vip3Aa19, and mortality was assessed as described above. Control bioassays consisted of neonates that did not feed on diet prior to being assayed. This procedure was repeated 4 times for each colony, with a total of approximately 448 insects tested in each treatment.
3. Impact of larval storage at reduced temperature. To assess the impact of storage at reduced temperature, larvae (within 0–4 h after hatch) were either transferred directly to bioassay trays (= a control of no storage pretreatment) or stored for 12 h at 14 °C, 24 h L and 44 ± 2% RH without food and then transferred to bioassay trays. Larvae were exposed to the estimated LC70 concentration of Vip3Aa19, and mortality was assessed as described above. The study was repeated 4 times for each colony, with approximately 448 insects tested in each treatment.
4. Impact of storage under reduced humidity. To examine the impact of storage at high humidity (= routine condition with moistened filter paper in a petri dish sealed with Parafilm®) or at reduced humidity, larvae (within 0–4 h after hatch) were exposed to the standard condition or a low‐humidity environment created in a desiccator. For the low‐humidity environment, the larvae were stored in a petri dish covered with an 80‐mesh screen and suspended over a saturated potassium acetate solution24 in the bottom of the desiccator. Relative humidity was measured using data loggers and sensors (model HOBO UX100; Onset Computer Corporation, Bourne, MA). The relative humidity was approximately 90% for the high‐RH environment and 15% for the low‐RH environment. After 3 h of either high‐ or low‐humidity pretreatment condition, the larvae were transferred to bioassay trays for subsequent exposure to the estimated LC70 dose of Vip3Aa19. Mortality was assessed as described above. The study was repeated 4 times for each colony, with a total of approximately 448 insects tested in each treatment.
5. Impact of lab colony introgression with field‐collected individuals. To assess the impact of colony introgression with field‐collected individuals on subsequent Vip3Aa19 susceptibility, a temporary colony of S. frugiperda was established with larvae collected from Winter Beach, Indian River Co., Florida. To establish the colony, 600 larvae collected from fields planted to conventional non‐Bt corn were shipped overnight to the University of Nebraska and reared on artificial diet until pupation. From the field‐collected individuals, a total of 120 male and 140 female pupae were sexed and separated from the field‐collected colony to be crossed with laboratory susceptible strain K‐SUS individuals. The F1 progeny from this cross were reared as described previously, but kept isolated from K‐SUS to obtain F2 and F3 progenies. Mortality was determined after exposure to the estimated LC70 dose of Vip3Aa19.
6. Impact of photoperiod during bioassay. To examine the potential impact of differing extremes of photoperiod settings on larval susceptibility during the course of the bioassay, larvae (within 0–4 h after hatch) were transferred to bioassay trays and stored under two different photoperiods, either 24 h continuous scotophase or 24 h continuous photophase for 7 days. Incubators were maintained at the same standard conditions (27 °C and 60 ± 10% RH) for conducting the bioassay. Mortality was recorded after exposure to the estimated LC70 dose of Vip3Aa19 as described above. The study was repeated 3 times for each colony, with a total of 335 insects tested in each treatment.
2.5. Statistical analysis
To estimate the lethal concentrations (LC50 and LC70) and the fiducial limits for the Vip3Aa19 bioassays over multiple generations of the lab colonies, or following introgression of a lab colony with field‐collected individuals (condition 5), the concentration mortality data were analyzed by probit analysis25 using POLO‐PC.26
Data analyses for respective pretreatment conditions 1 to 4 and for the bioassay condition of differential photoperiod (condition 6) were performed as randomized complete blocks, with each block as a temporal replicate for the respective experiments. The distribution of block effects was normally and independently distributed. The percentage mortality was transformed to mean percentage mortality with respective standard errors and analyzed as a binomial distribution arranged in a factorial treatment design (interaction between pretreatment conditions and strains) and performed in PROC GLIMMIX of SAS v.9.4 (SAS Institute, Cary, NC). Values from the interactions and from least‐squares means of the treatments with P‐values less than 0.05 were considered to be statistically significant.
3. RESULTS
Bioassays were conducted to determine the susceptibility of S. frugiperda to Vip3Aa19 insecticidal protein throughout 17 generations of continuous rearing. Even though standardized bioassay methodology was used, considerable variation in the calculated LC50 values for each laboratory colony was found (Table 1 and Fig. 1). The LC50 values (and 95% CL) varied approximately 6.6‐fold for the SUS strain, and ranged from 8.7 (6.9–10.4) ng cm−2 to 54.3 (46.8–60.9) ng cm−2. The LC50 values (and 95% CL) for the K‐SUS strain were similar in magnitude to those of the SUS strain, but varied slightly more (8.8‐fold overall), and ranged from 11.6 (9.8–13.3) ng cm−2 to 102.2 (72.8–129.1) ng cm−2.
Table 1.
LC50 estimates for two laboratory susceptible strains of S. frugiperda larvae exposed to Vip3Aa19 insecticidal protein when tested over multiple generations
| Generation | SUS | K‐SUS | ||||||
|---|---|---|---|---|---|---|---|---|
| Number of insects tested | LC50 (95% CL)a | Slope | SE | Number of insects tested | LC50 (95% CL)a | Slope | SE | |
| 1 | NAb | NA | NA | NA | 380 | 22.5 (17.5–27.9) | 3.55 | 0.42 |
| 2 | 380 | 11.9 (10.0–13.8) | 4.25 | 0.59 | NAb | NA | NA | NA |
| 3 | 767c | 8.7 (6.9–10.4) | 2.7 | 0.35 | 767c | 46.3 (38.2–54.6) | 3.2 | 0.54 |
| 4 | 381 | 12.6 (10.3–15.0) | 3.43 | 0.45 | 381 | 22.4 (19.2–25.8) | 4.3 | 0.54 |
| 5 | 384 | 20.7 (17.5–24.0) | 4.36 | 0.6 | 331 | 19.6 (14.8–24.4) | 4.8 | 0.69 |
| 6 | 508 | 36.2 (27.4–48.2) | 3.32 | 0.3 | 508 | 83.2 (59.3–126.9) | 1.67 | 0.24 |
| 7 | 512 | ∼80d | NC | NC | 508 | 21.7 (17.0–26.0) | 3.2 | 0.44 |
| 8 | 507 | 17.5 (11.1–23.7) | 2.95 | 0.42 | 511 | ∼40d | NC | NC |
| 9 | 512 | 28.8 (18.8–45.5) | 2.59 | 0.2 | 504 | 33.7 (19.0–63.3) | 2.39 | 0.2 |
| 10 | 511 | 38.3 (29.8–47.6) | 3.08 | 0.34 | 511 | 40.4 (35.6–45.1) | 4.98 | 0.64 |
| 11 | 511 | 23.2 (20.5–26.1) | 4.34 | 0.46 | 510 | 36.6 (32.0–41.6) | 3.8 | 0.4 |
| 12 | 510 | 26.7 (20.8–32.6) | 3.31 | 0.37 | 508 | 34.8 (24.1–49.9) | 4.73 | 0.48 |
| 13 | 511 | 23.8 (21.0–26.8) | 3.97 | 0.38 | 511 | 19.2 (16.3–22.2) | 3.16 | 0.33 |
| 14 | 512 | 51.2 (38.8–66.8) | 3.38 | 0.33 | 1017e | 28.1 (22.2–34.6) | 2.5 | 0.18 |
| 15 | 510 | 27.5 (11.2–42.9) | 2.98 | 0.37 | 511 | 11.6 (9.8–13.3) | 4.05 | 0.51 |
| 16 | 500 | 30.8 (22.3–39.3) | 3.4 | 0.42 | 512 | 33.0 (27.8–38.5) | 2.94 | 0.29 |
| 17 | 511 | 54.3 (46.8–60.9) | 5.02 | 0.79 | 507 | 102.2 (72.8–129.1) | 4.4 | 0.87 |
ng Vip3Aa19 cm−2 diet.
Data not available (NA) for this generation, as no bioassay was conducted.
Six replicates used at this generation testing.
The LC50 value was not calculated (NC) by probit analysis and was estimated on the basis of 50% observed mortality.
Eight replicates used at this generation testing.
Figure 1.
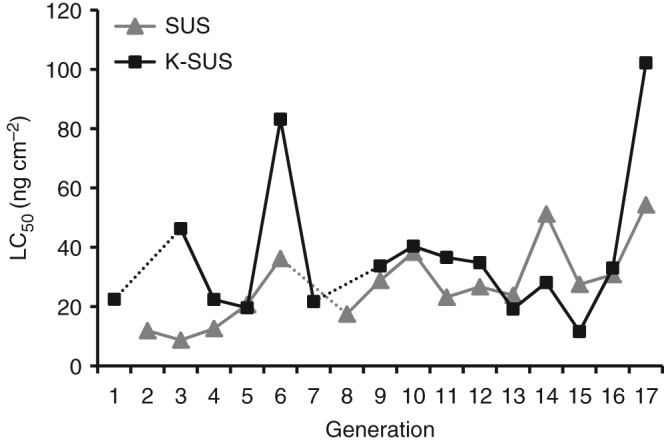
Variation in susceptibility of S. frugiperda larvae to Vip3Aa19 insecticidal protein for two laboratory colonies over multiple generations. Dashed line between points indicates LC50 not available.
The estimated LC70 value for the first generation of K‐SUS was 31.5 (25.4–41.4) ng cm−2, and this concentration was used to test the five pretreatment conditions.
3.1. Condition 1: impact of storage time prior to Vip3A exposure
The larval storage time (without feeding) before Vip3Aa19 exposure did not significantly affect subsequent mortality at the tested concentration (P > 0.05) (Fig. 2). The control with no additional holding time had a similar mean percentage mortality of 67 ± 11.2% or 60.3 ± 12.0% for the SUS or K‐SUS colony respectively. Although the mean percentage mortality showed some variation for each colony across the different time points up to 12 h, no significant trends were found for either, and therefore this condition also did not cause significant interaction between factors (h and colony, P > 0.05).
Figure 2.
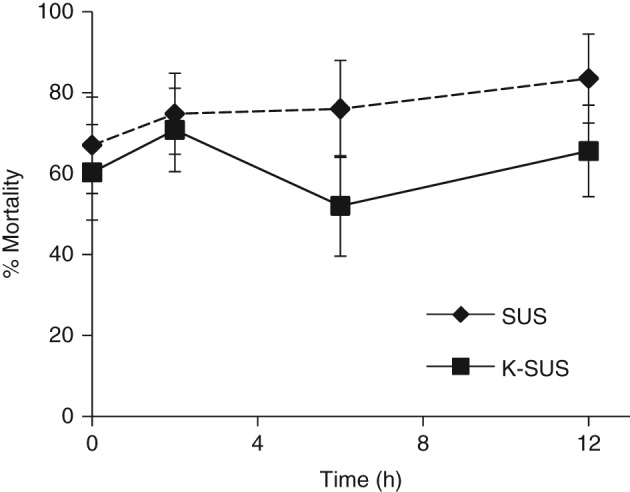
Susceptibility of S. frugiperda larvae to Vip3Aa19 insecticidal protein after extended holding time pretreatment.
3.2. Condition 2: impact of prior feeding
Prior feeding of larvae significantly reduced the subsequent mortality resulting after Vip3Aa19 exposure. The mean percentage mortality was lower for each treatment where prior feeding on artificial diet had occurred compared with control larvae which did not experience prior feeding (Fig. 3). A similar overall trend was observed for SUS and K‐SUS colonies, where longer periods of pretreatment feeding significantly reduced the susceptibility of S. frugiperda (P < 0.05) to Vip3Aa19. The net decrease in mortality over the 12 h was similar for each colony, with a reduction of about 27% (45.2 ± 8.8% to 17.4 ± 5.4% or 51.6 ± 8.9% to 25.3 ± 6.8% for the SUS or K‐SUS colony respectively). No statistically significant colony by treatment interactions were observed (P > 0.05).
Figure 3.
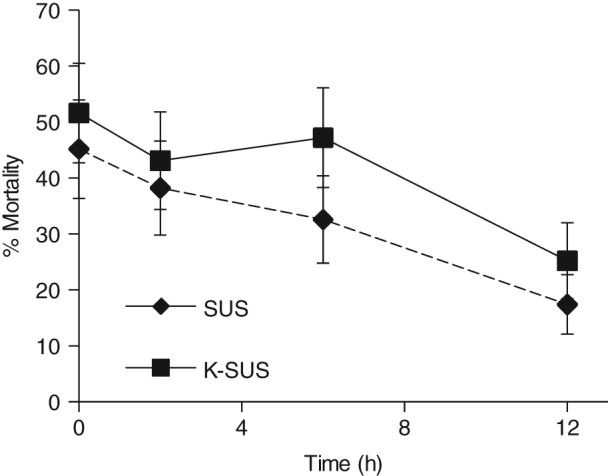
Susceptibility of S. frugiperda larvae to Vip3Aa19 insecticidal protein after prior feeding on artificial diet.
3.3. Condition 3: impact of larval storage at reduced temperature
Storage of S. frugiperda larvae overnight at reduced temperature (14 °C) showed different results for each colony, somewhat complicating the interpretation of the impact of this pretreatment. While the SUS colony demonstrated similar mortality for both conditions (i.e. larvae used within 0–4 h after hatch as compared with those that had experienced the additional 12 h pretreatment), the K‐SUS colony exhibited significantly increased mortality with the 12 h pretreatment (Fig. 4). The mean percentage mortality for the K‐SUS colony increased from 45.6 ± 3.6% to 73.0 ± 3.0%. This change was significant for the K‐SUS colony (P < 0.05), and there was a significant interaction between factors (time and colonies, P < 0.05), confirming the observation that one colony was affected by the pretreatment condition, while the other was unaffected.
Figure 4.
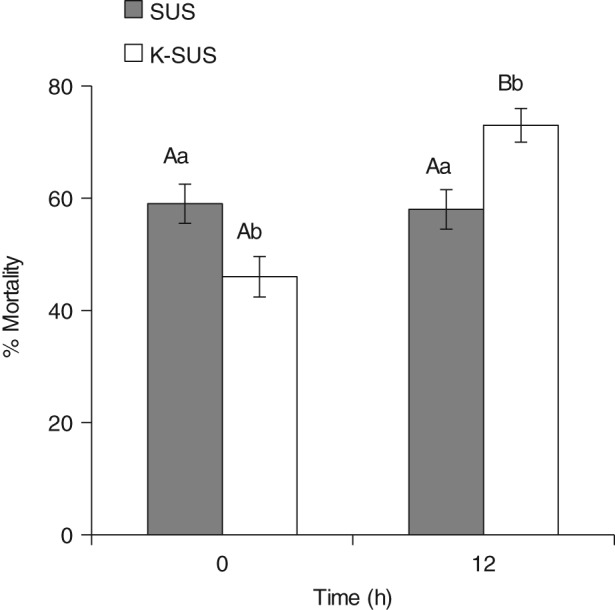
Susceptibility of S. frugiperda larvae to Vip3Aa19 insecticidal protein following overnight storage at 14 °C. Means with different letters are significantly different (LS means, P < 0.05) over time (A or B) or between strains (a or b).
3.4. Condition 4: impact of storage under reduced humidity
The differential exposure of larvae to different pretreatment conditions of humidity (Fig. 5) did not have a significant impact on resultant mortality, and there was no interaction between colonies (P > 0.05) for this pretreatment. Overall mean percentage mortalities were similar within each colony tested, irrespective of the high or low RH pretreatment, at 49.6 ± 6.8% and 47.5 ± 6.8% for SUS respectively, and at 45.2 ± 6.7% and 38.5 ± 6.5% for K‐SUS respectively.
Figure 5.
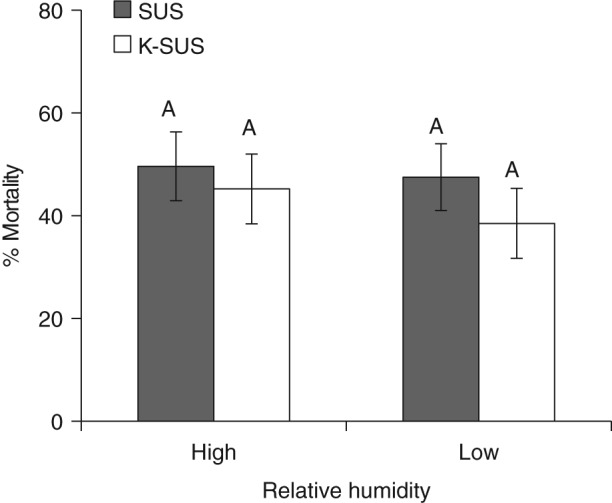
Susceptibility of S. frugiperda larvae to Vip3Aa19 insecticidal protein following extreme differences in relative humidity pretreatment. Means with the same letters are not significantly different (LS means, P > 0.05).
3.5. Condition 5: impact of lab colony introgression with field‐collected individuals
The LC50 values for the field‐collected colony were similar to those for the K‐SUS laboratory susceptible colony, with estimates (and 95% fiducial limits) of 24.3 (14.1–33.8) ng cm−2 and 28.1 (22.2–34.6) ng cm−2 for the field and laboratory colonies respectively (Table 2). The introgressed colony exhibited increased tolerance to Vip3Aa19 relative to the two parental colonies at the first generation after crossing, with an estimated LC50 value (and 95% fiducial limits) of 117.2 (98.3–146.4) ng cm−2 (Table 2). Bioassay of the second and third generations of the introgressed colony, however, showed an increase in susceptibility to Vip3Aa19 compared with the first generation tested, with LC50 values (and 95% fiducial limits) of 15.6 (13–18.2) ng cm−2 and 32.9 (22.6–44.6) ng cm−2 for the F2 and F3 progeny respectively. The second and third generations of the introgressed colony also demonstrated LC50 values similar to those of the K‐SUS parental strain (Table 2).
Table 2.
Effect of introgression on S. frugiperda laboratory colony susceptibility to Vip3Aa19 insecticidal protein
| Strain | Generation | Number of insects tested | LC50 (95%CL)a |
|---|---|---|---|
| Winter Beach | 1 | 448 | 24.3 (14.1–33.8) |
| K‐SUS | 14 | 1017 | 28.1 (22.2–34.6) |
| Infused | 1 | 1016 | 117.2 (98.3–146.4) |
| K‐SUS | 15 | 448 | 11.6 (9.8–13.3) |
| Infused | 2 | 441 | 15.6 (13.0–18.2) |
| K‐SUS | 16 | 448 | 33.0 (27.8–38.5) |
| Infused | 3 | 448 | 32.9 (22.6–44.6) |
ng Vip3Aa19 cm−2 diet.
3.6. Condition 6: impact of photoperiod during bioassay
The presence or absence of light during bioassay of Vip3Aa19 significantly affected S. frugiperda larval mortality (Fig. 6). Mean percentage mortality for the 24 h scotophase treatment was 83.6 ± 2.8%, compared with 56.3 ± 4.3% for 24 h photophase in the K‐SUS colony. Similar results were seen with the SUS colony, where mean percentage mortality decreased from 46.8 ± 4.4% to 21.3 ± 3.2% for 24 h scotophase compared with 24 h photophase respectively. Although both strains showed similar response to the presence or absence of light (with a net decrease of approximately 26% under 24 h photophase), there were significant differences in susceptibility between strains for this treatment (P < 0.05).
Figure 6.
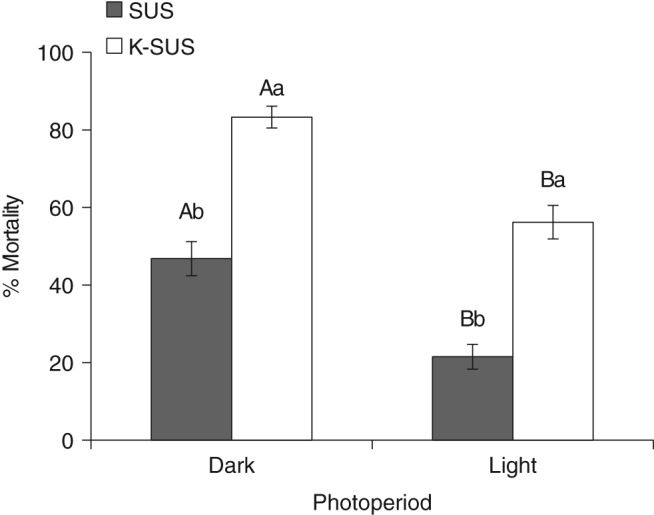
Susceptibility of S. frugiperda larvae to Vip3Aa19 insecticidal protein with light present or absent during the bioassay. Means with different letters are significantly different (LS means, P < 0.05) over treatment (A or B) or between strains (a or b).
4. DISCUSSION
Establishing the bioactivity via laboratory bioassay methods is critically important for discovery efforts to uncover new candidate insecticidal agents, but is also vital to support product development needs. The latter needs routinely include: (1) a description of efficacy and degree of activity toward the potential spectrum of target arthropods; (2) establishing the activity of a representative insecticidal protein test substance that may be used for expanded toxicological and environmental safety testing,27 including an assessment of any activity toward representative non‐target arthropods;28 (3) support for a resistance management plan, which often requires extensive laboratory bioassay testing over time. High confidence in bioassay results is very important for decision‐making during product development and registration, but can be challenging to achieve in the face of bioassay system variability. Intrapopulation variation in response to chemical and microbial insecticides is clearly a common phenomenon when any bioassay is repeated.1, 29
A number of studies have reported the insecticidal activity of Vip3A against S. frugiperda and have documented its utility as a novel Bt technology, and as a stacking protein with other Cry proteins to delay the development of resistance.8, 19, 30, 31, 32 The present study identified pretreatment conditions that can significantly affect susceptibility of S. frugiperda larvae to Vip3Aa19, as well as other conditions that have no apparent effect. In addition, one condition was differentially controlled during the course of the bioassay and demonstrated to have a significant impact on resultant mortality.
Bioassays conducted throughout 17 generations of continuous rearing showed considerable variation in calculated LC50 values for Vip3Aa19 for both laboratory colonies, with an overall range of approximately 6.6‐fold difference for the SUS strain, and 8.8‐fold difference for the K‐SUS strain. Such variation is not uncommon, as noted previously, and reinforces the need to conduct treatment comparisons side by side in a given standardized bioassay system to draw the best conclusions about any real differences that may exist. Making comparisons across experiments over time or among laboratories would not be recommended, except for the purpose of establishing an overall expected range that one might encounter for a given insecticidal protein: larval test organism bioassay system.33 It remains plausible that inherent variability in larval susceptibility can arise from the innate heterogeneity of the individuals that are selected and tested, even from a laboratory colony that is tested in a standardized way. An example of this inherent variation (which can be genetically, maternally and/or environmentally influenced) was observed by Vélez et al.,22 where S. frugiperda eggs laid during the peak of oviposition exhibited increased larval fitness. Similarly, variation in susceptibility to Bt proteins has been hypothesized as being due to differences in genotype and nutritional status of the egg, for both Lymantria dispar dispar and Ostrinia nubilalis.2, 34
Data from the present study indicate that there is no significant difference in susceptibility to Vip3Aa19 between S. frugiperda larvae that are held as much as an additional 12 h (plus the 0–4 h collection time after hatching) before being exposed to Vip3Aa19 and larvae that are exposed within 0–4 h of hatching. These results confirm that there can be some flexibility in conducting bioassays with insects that hatch asynchronously, without affecting the outcome. Similarly, exposure to Vip3Aa19 following different pretreatment extremes of relative humidity indicated no significant effect on S. frugiperda larval susceptibility. As larval pretreatment holding time during bioassay preparation or larval transfer may be extended, neonates may be exposed to more extreme environmental conditions such as reduced relative humidity. Our data suggest that exposure to a change in relative humidity (for at least up to 3 h) may not affect subsequent larval susceptibility.
In contrast to larval pretreatment storage time and differential relative humidity status, other pretreatment conditions do significantly affect larval susceptibility to insecticidal protein. In particular, prior feeding on artificial diet and, potentially, overnight storage at a reduced temperature can have a significant impact on response to Vip3Aa19. Larvae that were previously fed with artificial diet for up to 12 h were significantly less susceptible to Vip3Aa19 than those that were not fed prior to the bioassay. This result likely reflects that even a short period of growth on the control diet can alter the actual status of the larvae that then go into the bioassay. Change in susceptibility to insecticidal protein, based on the stage of larval development, has been previously reported,35 but our data suggest that this could be manifested even before approaching a larval molt. Therefore, it is advisable to avoid this pretreatment condition of differential prefeeding in the interest of reduced bioassay variability. Additionally, our data suggest that maintaining hatched neonates at a reduced temperature overnight could potentially affect the subsequent susceptibility of larvae to insecticidal protein. Overall results therefore indicate that maintaining hatched larvae in a hydrated condition (for up to 12 h, if necessary, but in the absence of artificial diet), and without overnight storage at reduced temperatures, should increase the consistency of bioassay results.
Infusion of wild‐type individuals into an established lab colony population is a common practice to increase genetic diversity, which can be lost compared with field populations.36, 37 This may be essential to have the laboratory colony more accurately reflect an anticipated response for the field population (e.g. to facilitate discovery efforts, or to test and project what might happen to a control agent in the field environment). This practice, however, may introduce further variation into the bioassay system, which can be detrimental from the standpoint of using insect bioassay as a reproducible test organism system (e.g. in support of product development needs). Our data suggest that such infusion of field‐collected individuals into the laboratory colony could be an important factor to consider when seeking to reduce bioassay variability. Early in the introgression of field individuals with the laboratory colony, large differences in susceptibility to Vip3Aa19 resulted. This could possibly have been due to hybrid vigor, as the original two parental colony susceptibilities to Vip3Aa19 were not that different. Within two generations, however, the introgressed colony was not different from the parental colony in susceptibility to Vip3Aa19, suggesting that if colony introgression is practiced routinely, bioassays should be delayed for at least 2–3 generations of random mating. After this delay, the susceptibility may then be expected to fall within the range of variability that was previously established for the bioassay system.
An additional factor that significantly affected susceptibility of S. frugiperda to Vip3Aa19 was the presence or absence of light during the course of the bioassays. This observation was true for both lab colonies tested, with a net decrease of approximately 26% mortality under 24 h photophase compared with the 24 h scotophase condition. It is possible that the presence or absence of light affects larval feeding behavior, resulting in a different ingestion of insecticidal protein from the treated artificial diets. However, even if this occurred, this interpretation is somewhat complicated as insecticidal proteins also commonly have a feeding cessation and gut paralysis effect. It would also be interesting to see how a more balanced light/dark cycle, mimicking the natural setting, would compare in terms of resultant impact on susceptibility. These results indicate that maintaining a standardized condition of lighting during a bioassay is important to obtain consistent results.
Our findings regarding the pretreatment conditions tested in this study substantiated the hypothesis that control of such conditions can affect the outcome of the bioassay. While these findings are specific for the conditions tested, they are likely to extend to other insect bioassay systems.
Finally, from a practical standpoint, understanding the inherent variability in a given bioassay system and which pretreatment factors may (or may not) affect the variability can be of great benefit on a day‐to‐day basis in the bioassay lab. For example, our data indicate that the time of selecting S. frugiperda larvae for setting up a bioassay with Vip3Aa19 can be more loosely controlled up to 12 h after hatch, as long as the other standardized bioassay factors are observed, and the selected larvae have not fed on artificial diet.
ACKNOWLEDGEMENTS
We thank the protein production team at the Syngenta Jealott's Hill research station for the purified Vip3Aa19 protein. We acknowledge Clark Lovelady and Elijah Meck from the insect control team at Syngenta's research center in Vero Beach, Florida, for collecting a fall armyworm field population. We also thank Dr Walter Stroup (Department of Statistics, UNL) for statistical analysis assistance, and Marilyn Weidner for assistance with manuscript preparation.
REFERENCES
- 1. Robertson JL, Preisler HK, Ng SS, Hickle LA and Gelernter WD, Natural variation: a complicating factor in bioassays with chemical and microbial pesticides. J Econ Entomol 88:1–10 (1995). [Google Scholar]
- 2. Marçon PC, Young LJ, Steffey KL and Siegfried BD, Baseline susceptibility of European corn borer (Lepidoptera: Crambidae) to Bacillus thuringiensis toxins. J Econ Entomol 92:279–285 (1999). [Google Scholar]
- 3. Gaspers C, Siegfried BD, Spencer T, Alves AP, Storer NP, Schuphan I et al, Susceptibility of European and North American populations of the European corn borer to the Cry1F insecticidal protein. J Appl Entomol 135:7–16 (2011). [Google Scholar]
- 4. Bernardi O, Amado D, Sousa RS, Segatti F, Fatoretto J, Burd AD et al, Baseline susceptibility and monitoring of Brazilian populations of Spodoptera frugiperda (Lepidoptera: Noctuidae) and Diatraea saccharalis (Lepidoptera: Crambidae) to Vip3Aa20 insecticidal protein. J Econ Entomol 107:781–790 (2014). [DOI] [PubMed] [Google Scholar]
- 5. Siegfried BD, Spencer T, Crespo AL, Storer NP, Head GP, Owens ED et al, Ten years of monitoring for Bt resistance in the European corn borer: what we know, what we don't know and what we can do better. Am Entomol 53:208–214 (2007). [Google Scholar]
- 6. Blanco CA, Gould F, Vega‐Aquino P, Jurat‐Fuentes JL, Perera OP and Abel CA, Response of Heliothis virescens (Lepidoptera: Noctuidae) strains to Bacillus thuringiensis Cry1Ac incorporated into different insect artificial diets. J Econ Entomol 102:1599–1606 (2009). [DOI] [PubMed] [Google Scholar]
- 7. Crespo AL, Spencer TA, Nekl E, Pusztai‐Carey M, Moar WJ and Siegfried BD, Comparison and validation of methods to quantify Cry1Ab toxin from Bacillus thuringiensis for standardization of insect bioassays. Appl Environ Microbiol 74:130–135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chakroun M, Bel Y, Caccia S, Abdelkefi‐Mesrati L, Escriche B and Ferré J, Susceptibility of Spodoptera frugiperda and S. exigua to Bacillus thuringiensis Vip3Aa insecticidal protein. J Invertebr Pathol 110:334–339 (2012). [DOI] [PubMed] [Google Scholar]
- 9. Bird LJ and Ackhurst RJ, Variation in susceptibility of Helicoverpa armigera (Hübner) and Helicoverpa punctigera (Wallengren) (Lepidoptera: Noctuidae) in Australia to two Bacillus thuringiensis toxins. J Invertebr Pathol 94:84–94 (2007). [DOI] [PubMed] [Google Scholar]
- 10. Stone TB and Sims SR, Geographic susceptibility of Heliothis virescens and Helicoverpa zea (Lepidoptera: Noctuidae) to Bacillus thuringiensis . J Econ Entomol 86:989–994 (1993). [Google Scholar]
- 11. Ali MI and Luttrell RG, Susceptibility of Helicoverpa zea and Heliothis virescens (Lepidoptera: Noctuidae) to Vip3A insecticidal protein expressed in VipCot cotton. J Invertebr Pathol 108:76–84 (2011). [DOI] [PubMed] [Google Scholar]
- 12. Craig W, Tepfer M, Degrass G and Ripandelli D, An overview of general features of risk assessments of genetically modified crops. Euphytica 164:853–880 (2008). [Google Scholar]
- 13. Romeis J, Bartsch D, Bigler F, Candolfi MP, Gielkens MMC, Hartley SE et al, Assessment of risk of insect‐resistant transgenic crops to nontarget arthropods. Nat Biotechnol 26:203–208 (2008). [DOI] [PubMed] [Google Scholar]
- 14. FIFRA Scientific Advisory Panel , Subpanel on Bacillus thuringiensis (Bt) Plant‐Pesticides and Resistance Management, 9–10 February 1998, Docket No. EPA‐HQ‐OPP‐00231, US Environmental Protection Agency, Washington, DC (1998).
- 15. Bt Plant‐Incorporated Protectants; Biopesticides Registration Action Document. [Online]. US Environmental Protection Agency, Washington, DC: (2001). Available: http://www.epa.gov/oppbppd1/biopesticides/pips/bt_brad.htm [7 July 2015]. [Google Scholar]
- 16. Luttrell RG, Wan L and Knighten K, Variation in susceptibility of noctuid (Lepidoptera) larvae attacking cotton and soybean to purified endotoxin proteins and commercial formulations of Bacillus thuringiensis . J Econ Entomol 92:21–32 (1999). [Google Scholar]
- 17. Marçon PC, Siegfried BD, Spencer T and Hutchison WD, Development of diagnostic concentrations for monitoring Bacillus thuringiensis resistance in European corn borer (Lepidoptera: Crambidae). J Econ Entomol 93:925–930 (2000). [DOI] [PubMed] [Google Scholar]
- 18. Ali MI, Luttrell RG, Young SY, III , Allen KC and Luttrell LT, Monitoring insect resistance in Arkansas to chemical insecticides and Bt‐endotoxins Proc Beltwide Cotton Conf, National Cotton Council of America, Memphis, TN, pp. 1138–1149. (2003). [Google Scholar]
- 19. Farias JR, Andow DA, Horikoshi RJ, Sorgatto RJ, Fresia P, dos Santos AC et al, Field‐evolved resistance to Cry1F maize by Spodoptera frugiperda (Lepidoptera: Noctuidae) in Brazil. Crop Prot 64:150–158 (2014). [Google Scholar]
- 20. Vélez AM, Spencer TA, Alves AP, Moellenbeck D, Meagher RL, Chirakkal H et al, Inheritance of Cry1F resistance, cross‐resistance and frequency of resistant alleles in Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Bull Entomol Res 103:700–713 (2013). [DOI] [PubMed] [Google Scholar]
- 21. Perkins WD, Laboratory rearing of the fall armyworm. Fla Entomol 62:87–90 (1979). [Google Scholar]
- 22. Vélez AM, Spencer TA, Alves AP, Crespo ALB and Siegfried BD, Fitness costs of Cry1F resistance in fall armyworm, Spodoptera frugiperda . J Appl Entomol 138:315–325 (2014). [Google Scholar]
- 23. Lewis LC and Lynch RE, Rearing the European corn borer on corn leaf and wheat germ diets. Iowa State J Sci 44:9–14 (1969). [Google Scholar]
- 24. Greenspan L, Humidity fixed points of binary saturated aqueous solutions. J Res Nat Bur Stand 81:89–96 (1977). [Google Scholar]
- 25. Finney DJ, Probit Analysis, 3rd edition Cambridge University Press, Cambridge, UK, 350 pp. (1971). [Google Scholar]
- 26. POLO‐PC: a User's Guide to Probit and Logit Analysis. LeOra Software, Berkeley, CA: (1987). [Google Scholar]
- 27. Raybould A, Kilby P and Graser G, Characterizing microbial protein test substances and establishing their equivalence with plant‐produced proteins for use in risk assessments of transgenic crops. Transgen Res 22:445–460 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burns A and Raybould A, Nontarget organism effects tests on eCry3.1Ab and their application to the ecological risk assessment for cultivation of Event 5307 maize. Transgen Res 23:985–994 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Siegfried BD and Spencer T, Bt resistance monitoring in European corn borers and western corn rootworms, in Plant Gene Containment, ed. by Oliver MJ. and Li Y. Blackwell Publishing Ltd, Oxford, UK, pp. 43–55 (2012). [Google Scholar]
- 30. Lee MK, Walters FS, Hart H, Palekar N and Chen JS, The mode of action of the Bacillus thuringiensis vegetative insecticidal protein Vip3A differs from that of Cry1Ab delta‐endotoxin. Appl Environ Microbiol 69:4648–4657 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kurtz RW, McCaffery A and O'Reilly D, Insect resistance management for Syngenta's VipCot™ transgenic cotton. J Invertebr Pathol 95:227–230 (2007). [DOI] [PubMed] [Google Scholar]
- 32. Burkness EC, Dively G, Patton T, Morey AC and Hutchison WD, Novel Vip3A Bacillus thuringiensis (Bt) maize approaches high‐dose efficacy against Helicoverpa zea (Lepidoptera: Noctuidae) under field conditions: implications for resistance management. GM Crops 1:337–343 (2010). [DOI] [PubMed] [Google Scholar]
- 33. González‐Cabrera J, Herrero S, Sayyed AH, Escriche B, Liu YB, Meyer SK et al, Variation in susceptibility to Bacillus thuringiensis toxins among unselected strains of Plutella xylostella . Appl Environ Microbiol 67:4610–4613 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rossiter M, Yendol WG and Dubois NR, Resistance to Bacillus thuringiensis in gypsy moth (Lepidoptera: Lymantriidae): genetic and environmental causes. J Econ Entomol 83:2211–2218 (1990). [Google Scholar]
- 35. Huang FL, Buschman LL and Higgins RA, Susceptibility of different instars of European corn borer (Lepidoptera: Crambidae) to diet containing Bacillus thuringiensis . J Econ Entomol 92:547–550 (1999). [Google Scholar]
- 36. Chambers DL, Quality control in mass rearing. Annu Rev Entomol 22:289–308 (1977). [Google Scholar]
- 37. Leppla NC and Ashley TR, Quality control in insect mass production: a review and model. Bull Entomol Soc Am 70:33–44 (1989). [Google Scholar]


