ABSTRACT
Antibiotics are widely used in zoo technical and veterinary practices as feed supplementation to ensure wellness of farmed animals and livestock. Several evidences have been suggesting both the toxic role for tetracyclines, particularly for oxytetracycline (OTC). This potential toxicity appears of great relevance for human nutrition and for domestic animals. This study aimed to extend the evaluation of such toxicity. The biologic impact of the drug was assessed by evaluating the proinflammatory effect of OTC and their bone residues on cytokine secretion by in vitro human peripheral blood lymphocytes. Our results showed that both OTC and OTC‐bone residues significantly induced the T lymphocyte and non‐T cell secretion of interferon (IFN)‐γ, as cytokine involved in inflammatory responses in humans as well as in animals. These results may suggest a possible implication for new potential human and animal health risks depending on the entry of tetracyclines in the food‐processing chain.
Keywords: Oxytetracycline, Toxicity, Apoptosis, Inflammatory cytokine, food
INTRODUCTION
The use of antibiotics in the agro‐food industry is a relevant concern 1. They have been still employed as growth promoters in livestock, aquaculture, and pesticides 1, 2, 3. The topic is relevant considering the potential toxic risk derived by the entry/accumulation of antibiotics in animal feed and human food with consequences on health 4.
The use of antibiotics for growth promotion is prohibited in Europe, whereas the United States and Canada still allow use of antibiotics in agriculture for nontherapeutic purposes 5. New regulations from the Food and Drug Administration (FDA) are endeavoring to reduce antibiotic contaminants in foods 6, 7. Although allergic reactions are rarely related to antibiotics, meats and fruit induced by antibiotic residues have been reported in the literature 1, 8. The occurrence of antibiotic toxic effects has negative consequences on the gastrointestinal tract, skin, central nervous system, and even accumulating in calcium‐rich organs such as bones and teeth 9, 10, 11. Food intolerance has described in gym subjects due to intake of meat derived from administration of animals‐fed tetracycline 12. Several models have already been proposed to demonstrate cytotoxic effects of tetracyclines 13, 14, 15. To the best of our knowledge, the possible interplay between tetracyclines, in particular the oxytetracycline (OTC), and immune system is still lacking and not sufficiently addressed to rule out the possible toxicity to human and animal health.
In this regard, the immune system acquires a new pivotal role in modulating toxicological mechanisms that could be triggered by tetracyclines derived by ingested food. Immunity, which has the fundamental role to protect and defend the organism from the disease 16, 17, 18, is also involved in the homeostasis and health maintenance against autoimmunity diseases and tumors 17. The exacerbation of cytotoxic CD8+ T lymphocytes 18 and CD4+ T Helper 1 (TH1) 19 responses were associated with inflammatory diseases and autoimmunity disorders 20. TH1 activity is mainly based on the production of interferon‐gamma (IFN)‐γ, which optimizes the antimicrobial responses and fosters CD8+ T lymphocyte activity, and also appears to play a fundamental role in triggering autoimmune responses 21, 22, 23, 24, 25, 26, 27, 28, 29. Natural killer (NK)‐dependent secretion of IFN‐γ is relevant to autoimmunity 30, 31, 32, 33, allergy 34, and could have a pathogenetic role in gastrointestinal 35 and hematological disorders 36, 37. TH2 response is based on several cytokines including interleukin (IL)‐4 that results in the activation of humoral immunity 21.
It is worth noting that several extrinsic factors, such as drugs and chemicals, can induce the development of autoimmune conditions 38, 39, 40, 41, 42, 43.
Moreover, the use of these drugs to treat inflammatory conditions is still not conclusive and, in some way, controversial as well as the abuse of some veterinary drugs, including tetracyclines, which have a global impact on the environment that could be of great relevance [44–50].
OTC represents the main drug used to control gastrointestinal and respiratory diseases in broiler chickens 51, although its accumulation has been described in chicken edible tissues 52. As a consequence, the European Union established the maximum residue level of OTC in poultry meat 53 to limit drug residues and to preserve health of final consumers that are mainly represented by domestic animals and humans.
Based on a previous study on the toxicity of OTC 54, we investigated the potential toxic effect of OTC in an in vitro human lymphocyte model. In particular, we addressed the potential induction of IFN‐γ production caused by the in vitro exposure of human T and non‐T lymphocytes to OTC or to chicken bone‐derived residues.
MATERIALS AND METHODS
Cells
Peripheral blood samples from healthy donor volunteers were collected by vein puncture according to standard procedures and used within the 3 h from the collection. Informed consent was obtained in accordance with the Declaration of Helsinki, as approved within the study protocol by the Institutional Review Board at the Federico II University of Naples. Peripheral blood mononuclear cells (PBMC) were used as mixed population of T (CD3+) and non‐T (CD3–) (the latter are mainly represented by NK cells) lymphocytes 17, 18. Identification of cell subpopulations was performed by immune‐fluorescence and flow cytometry (see paragraph 2.4, Monoclonal Antibodies, Flow Cytometry, Detection of Intracellular IFN‐γ, and IL‐4 Productions). PBMC were isolated by centrifugation on Lymphoprep (Nycomed Pharma) gradients, as described 35.
OTC and the Conditioned Cell Medium
To test the potential toxic role of OTC (Oxytetracycline 20%®, TreI, Reggio Emilia, Italy) and OTC bone residues, two different conditioned cell culture mediums (CCM) were used, as previously described 54. Briefly, to obtain CCM, 10 mL of a RPMI 1640 cell culture medium was incubated and constantly shaken for 48 h at 37°C with 1 g of ground bone (sterilized by autoclaving at 121°C in a steam pressure of 2 atm for 10 min) from chickens reared in the presence (OTC‐CCM) or in the absence (C‐CCM) of treatments with OTC 54. After incubation, the CCMs were recovered and filtered through 0.20 μm syringe filters (Sartorius Stedim Biotech, Goettingen, Germany) to remove any residual ground bone particles and microbial contamination.
Apoptosis Detection
Apoptosis detection was performed as previously described 54. Briefly, OTC‐ CCM and C‐CCM were used at the dilution of 1:4 with an absolute RPMI 1640 growth medium, and the resulting mixtures were incubated with 5 × 105 PBMC/mL for 10 or 48 h at 37°C and 5% CO2 in a cell incubator (Thermo Scientific Heraeus). The effect of OTC alone was evaluated by incubating the drug (1 μg/ mL), as described above.
Apoptosis was assessed by staining of the cell membrane‐exposed phosphatidylserine with fluorescein isothiocyanate‐conjugated (FITC) Annexin V, according to the manufacturer's instructions (Becton Dickinson PharMingen, San Jose, CA) and as previously described 55. Samples were analyzed by means of flow cytometry, using a two laser‐equipped FACSCalibur (Becton Dickinson PharMingen, San Jose, CA), and the CellQuest Analysis Software. The FACS analysis was based on the percentage of Annexin V‐positive cells to have a measurement of the cells undergoing apoptosis.
Monoclonal Antibodies, Flow Cytometry, Detection of Intracellular IFN‐γ, and IL‐4 Productions
FITC, PE, Cychrome, and APC labeled mAbs against CD3, CD8, CD4, IFN‐γ, IL‐4, and isotype‐matched controls (Becton Dickinson PharMingen, San Jose, CA) were used to identify the CD8+ T cytotoxic, CD4+ TH lymphocytes, or CD3– non‐T cells.
To analyze the production of IFN‐γ and IL‐4, purified PBMC were cultured overnight (10–12 h) in the presence of phorbol‐12‐myristate‐13‐acetate (PMA) and ionomycin (Sigma). To avoid extracellular cytokine export, the cultures were performed in the presence of 5 μg/mL of Brefeldin‐A (Sigma‐Aldrich) as described 56.
Intracellular IFN‐γ and IL‐4 production was detected by using a triple staining technique and flow cytometry analysis. Briefly, after the incubation the culture was harvested, the cells were fixed and permeabilized by using a cytokine staining kit, following the manufacturer's instructions (Caltag Laboratories, Burlingame, CA). Samples were analyzed by flow cytometry (see description in the Apoptosis Detection section).
Statistical Analysis
Data were analyzed using GraphPad Prism 6 software (GraphPad Software, La Jolla, CA). All data are presented as the means ± standard error of the mean and were first checked for normality using the D'Agostino‐Pearson normality test. The analysis pertaining to the proinflammatory effect was analyzed using the Kruskal–Wallis test followed by Dunn's multiple comparisons test. p < 0.05 was considered significant. Statistical analysis was specifically performed to evidence differences within the pairs of comparison (OTC vs. ctr, OTC‐CCM vs. ctr, C‐CCM vs. ctr, as indicated in the Figures 1 and 3).
Figure 1.
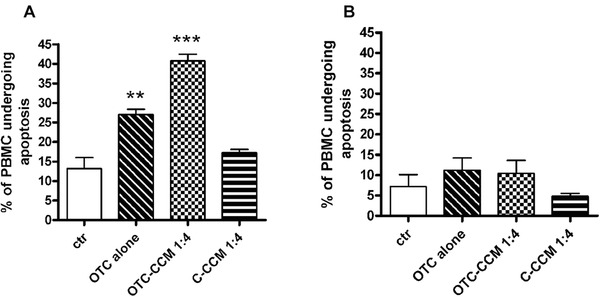
Apoptosis induction measured as a percentage of PBMC positive for the FITC‐Annexin binding. The graph bar columns represent the mean values of the percentage of PBMC undergoing apoptosis in the performed experiments (n = 4). The different cell incubations and conditioned cell culture medium dilutions are indicated on the x‐axis. The abbreviations indicate the growth medium with the addition of a conditioned cell culture medium (CCM) obtained from the ground bone of chickens reared in the presence (OTC‐CCM) or in the absence (C‐CCM) of a treatment with OTC, a growth medium with the addition of 1 μg/ mL of OTC alone. The bar column depicted as “ctr” indicates the incubation in the growth medium with Annexin V staining, which has been used as a control of the apoptosis that occurs in the cells when in a culture without any other incubation is maintained. The statistical significance is indicated with asterisk(s): *p < 0.05, **p < 0.01, and ***p < 0.001.
Figure 3.
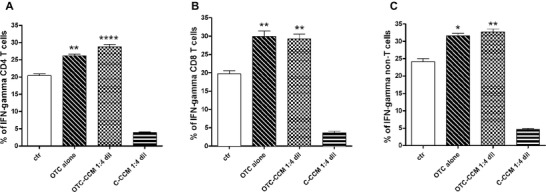
Statistical analysis of the all experiments (n = 10) showing the IFN‐γ production in CD4+ and CD8+ T lymphocytes and in non‐T cells. Cytokine production was evaluated as the percentage of IFN‐γ producing cells. The bar column graphs represent the mean values of the percentage of IFN‐γ producing cells. The different cell incubations and conditioned cell culture medium dilutions are indicated on the x axis. The abbreviations indicate the growth medium with the addition of a conditioned cell culture medium (CCM) obtained from the ground bone of chickens reared in the presence (OTC‐CCM) or in the absence (C‐CCM) of an OTC treatment, a growth medium with the addition of 1 μg/ mL of OTC alone. The condition indicates as “ctr” refers to basal IFN‐γ production. All the cell cultures (ctr, OTC alone, OTC‐CCM and C‐CCM) were maintained in a growth medium added with PMA and Ionomycin to induce cytokine production (see materials and methods). Panels A, B, and C show IFN‐γ production in CD4+ T lymphocytes, CD8+ T lymphocytes and in non‐T cells, respectively. The statistical significance is indicated with asterisk(s): *p < 0.05, **p < 0.01, and ***p < 0.001.
RESULTS
The Toxic Effect of OTC and Their Residues from Bone as Induction of Apoptotic Phenomenon in PBMC
According to previous data 56, the OTC was able to induce the apoptosis in PBMC after 48 h of incubation (Figure 1, panel A). A similar effect was obtained using the OTC‐CCM, whereas no results were obtained with C‐CCM (Figure 1).
Note that the incubation with OTC or with OTC‐CCM did not exert significant apoptosis phenomenon in PBMC after an incubation of approximately 10–12 h (Figure 2).
Figure 2.
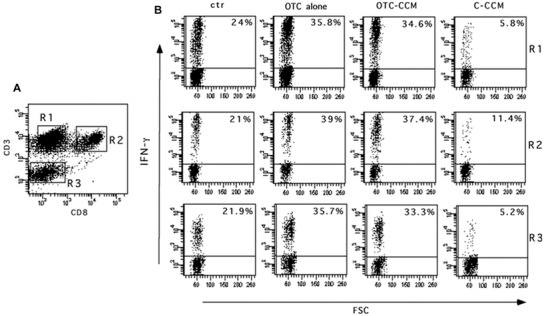
One representative experiment showing the IFN‐γ production in CD4+ and CD8+ T lymphocytes and in non‐T cells. Cytokine production was evaluated as the percentage of IFN‐γ producing cells. Panel A refers to fluorescence gating strategy to identify the CD4+ T lymphocytes (CD3+ CD8–, cells in R1), CD8+ T lymphocytes (CD3+ CD8+ cells in R2) and the non‐T cells (CD3– cells in R3). B panels represent the percentage of IFN‐γ producing CD4 T (R1), CD8 T (R2), and non‐T (R3) cells. The different cell incubations and conditioned cell culture medium dilutions are indicated on the top. The abbreviations indicate the growth medium with the addition of a conditioned cell culture medium (CCM) obtained from the ground bone of chickens reared in the presence (OTC‐CCM) or in the absence (C‐CCM) of an OTC treatment, a growth medium with the addition of 1 μg/ mL of OTC alone. The condition indicates as “ctr” refers to basal IFN‐γ production. All the cell cultures (ctr, OTC alone, OTC‐CCM and C‐CCM) were maintained in a growth medium added with PMA and ionomycin to induce cytokine production (see the Materials and Methods section).
These results significantly confirmed the toxicity of OTC and of their residues in bone (OTC‐CCM) and also evidenced the difference between the early (10‐12 h) and late (48 h) exposure to this drug. Such data allowed to identify the range of time (10‐12 h), in which the cells do not undergo OTC‐dependent apoptosis, to perform experiments of cytokine secretion (see the Materials and Methods section).
The Proinflammatory Toxic Effect of OTC and Their Residues from Bone as Significantly Increasing IFN‐γ Production in T Lymphocytes as well as in Non‐T Cells
In the light of the described observation (see the preceding paragraph), we evaluated the other possible alterations caused in human lymphocytes after the exposure to OTC 54. With this aim, we focused on the IFN‐γ production, as the main proinflammatory cytokine is able to foster the TH1 and T cytotoxicity immune‐responses as well as to be involved in several etiopathogenetic mechanisms on the basis of inflammatory‐mediated disease 19.
Since the evaluation in vitro of IFN‐γ production by T and non‐T lymphocytes is usually performed in short time (8–18 h) to obtain an optimal functional cytokine secretion 56 and we demonstrated that after 10 h the apoptosis was not induced (Figure 1, panel B), we incubated human PBMC with OTC and OTC‐CCM for 10 h to avoid this phenomenon. Indeed, the good viability of lymphocytes is crucial for the induction of cytokine production functions.
As shown, the incubation with OTC and OTC‐CCM was able to significantly increase the IFN‐γ production in CD4+ TH cells (R1 in Figure 2 and panel A in Figure 3) and CD8+ lymphocytes (R2 in Figure 2 and panel B in Figure 3) as well as in non‐T cells (R3 in Figure 2 and panel C in Figure 3). The cytokine was slightly detectable at the dilution 1:8 and 1:16 of OTC‐CCM (data not shown), whereas the dilution of 1:2 appeared to induce high level of apoptosis 56.
In the same cytokine production test, the incubation of PBMC from 12 to 18–24 h resulted in a very poor cell viability (data not shown) likely dependent on the here described proapoptotic effect of OTC and on Brefeldin A exposure used to allow the cytokine intracellular retention for the measurement (see the Materials and Methods section)
It is worth noting that C‐CCM incubation did not produce a cytokine increase, while appeared to reduce IFN‐γ production. Although we did not investigated this phenomenon, we do not exclude that some substances present in the bone (i.e., cytokines derived from osteoblasts or fibroblasts) may have an inhibitory role on the cytokine secretion. However, the difference in effects between OTC‐CCM and C‐CCM highlights the specificity of OTC action since the used chickens were of the same type and the only difference was the OTC administration 54.
The basal IL‐4 production was only slightly detectable in T and non‐T lymphocytes, as expected in PBMC from healthy donors after exposure to PMA and ionomycin 56 and was not modulated after 10 h of OTC or CCM incubations (data not shown).
DISCUSSION
In this article, we suggest that an antibiotic, the OTC, is able to determine the in vitro toxic effects. Data acquire great relevance especially in light of the wide use of OTC in animal breeding.
The OTC or the OTC‐conditioned culture medium, obtained with the incubation of ground bone from OTC‐treated chickens, appeared to generate the in vitro toxic effect of cell death by apoptosis in human cells 56.
Here, we suggest that the toxicity of OTC may also be extended to the induction of a proinflammatory microenvironment potentially responsible for initiation of tissue inflammatory spreading 21 or of autoimmune diseases 59.
In this regard, besides the ability to induce mortality of both the T lymphocytes and non‐T cells in an in vitro system for incubation with OTC for 48 h, the drug potently promotes the production of proinflammatory cytokines in the first 10–12 h of cell exposure. Specifically, human lymphocytes increase their production of IFN‐γ when exposed to the OTC or to the conditioned culture media with the bone of chickens treated with such drug as usually happens in livestock common breeding 51. In our in vitro model, both the innate (non‐T cells that are mainly represented by NK lymphocytes) and acquired (CD8+ and CD4+ lymphocytes) immunity 17, 18 appeared to be involved in this process and to suffer the OTC‐dependent toxicity.
In this context, it is known that IFN‐γ represents the main cytokine involved in the immune response 19, as well as a crucial element in the onset of impaired tissue homeostasis conditions, typically related to autoimmunity or chronic inflammation 23, 24, 25, 26, 27, 28, 29, 30, 31.
A number of workers 46, 47, 48, 49, 50, 51, 52 have suggested that OTC would likely to represent a toxic compound and could be harmful to human health and animals that can eat meat derived from chikens by intensive livestock.
In addition, the induction of cell mortality could generate an altered tissue condition, as well as a relevant impact on tissue homeostasis 57, 58 and the emergence of autoimmune reactions 59, 60, 61, 62, 63.
Both pets and humans could take this antibiotic as a residue from meat or in meat‐derived products and might likely suffer the OTC‐dependent toxicity. In this respect, it is interesting that, over the past 20 years, there has been an exacerbation of the emergence of immune‐mediated diseases (such as allergies, autoimmune reactions, and disorders of the gastrointestinal tract and the skin) in domestic animals 64, 65, 66, 67 and humans 68, 69. Moreover, it is surprising that the drastic increase of antibiotics resistance phenomena is partly due to the widespread and uncontrolled use of drugs in breeding 7, 70, 71, 72, 73, 74. We previously correlated the use of specific meats to the occurrence of these pathologies in humans 12.
This unusual increase is probably dependent on a complex set of multifactor events related to new life habits of humans and pets, as well as to the increasingly introduction of industrialized diets. Hence, the needing to change the approach to livestock by promoting sustainable breeding avoiding overcrowding and by reducing antibiotics favoring the use of alternative treatments. Unfortunately, the use of several drugs can promote development of autoimmunity 38, 39, 40, 41, 42, 43.
In conclusion, a special attention is probably needed on nutrition for large mass since it might expose humans and pets to increased risk of disease.
STUDY LIMITATIONS
Notably, this research has some study limitations. In this regard, the absence of in vivo experiments, able to verify the in vitro observed OTC toxicity, represents the main relevant limitation. Therefore, clinical studies are required to ascertain the in vivo effect of the drug in inducing the inflammatory status in animals and/or in humans.
In addition, the use of CCM obtained by incubation with bones from chickens reared in the presence of OTC did not directly demonstrate that the cell toxicity is due to bone's drug residues and did not ruled out that other substances could have a role.
CONFLICT OF INTERESTS
None of authors have financial or personal relationships with other people or organizations that could inappropriately influence or bias the content of the paper. This research was performed in collaboration with some scientists from the Division of Research and Development of Sanypet SpA and of GRAF S.p.A (as indicated in the author's affiliation) according to scientific and ethical principles of the scientific community. None financial funding was obtained from Sanypet Industry nor from GRAF Lab for this research study
References
- 1. Graham F, Paradis L, Begin P, Paradis J, Babin Y, Des Roches A. Risk of allergic reaction and sensitization to antibiotics in foods. Ann Allergy Asthma Immunol 2014;113(3):329–330. [DOI] [PubMed] [Google Scholar]
- 2. Bruning A, Brem GJ, Vogel M, Mylonas I. Tetracyclines cause cell stress‐dependent ATF4 activation and mTOR inhibition. Exp Cell Res 2014;320(2):281–289. [DOI] [PubMed] [Google Scholar]
- 3. Di Cerbo A, Pezzuto F, Canello S, Guidetti G, Palmieri B. Therapeutic effectiveness of a dietary supplement for management of halitosis in dogs. J Vis Exp 2015;101:e52717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palmieri B, Di Cerbo A, Laurino C. Antibiotic treatments in zootechnology and effects induced on the food chain of domestic species and, comparatively, the human species. Nutr Hosp 2014;29(6):1427–1433. [DOI] [PubMed] [Google Scholar]
- 5. (FAO) FaAO . Maximum residue limits for veterinary drugs in foods. Rome, Italy: Codex Alimentarius Commission; 35th Session; 2012. [Google Scholar]
- 6. Agency UEP . Electronic Code of Federal Regulations (eCFR). Title 21: Food and drugs. PART 556d Tolerances for residues of new animal drugs in food. Subpart Bd‐specific tolerances for residues of new animal drugs; 2014.
- 7. Authority EFS, Control ECfDPa. EU summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2013. EFSA J 2015;13:4036. [Google Scholar]
- 8. Empedrad R, Darter AL, Earl HS, Gruchalla RS. Nonirritating intradermal skin test concentrations for commonly prescribed antibiotics. J Allergy Clin Immunol 2003;112(3):629–630. [DOI] [PubMed] [Google Scholar]
- 9. Macy E, Poon KYT. Self‐reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med 2009;122(8):778 e1–e7. [DOI] [PubMed] [Google Scholar]
- 10. Smith K, Leyden JJ. Safety of doxycycline and minocycline: a systematic review. Clin Ther 2005;27(9):1329–1342. [DOI] [PubMed] [Google Scholar]
- 11. Saikali Z, Singh G. Doxycycline and other tetracyclines in the treatment of bone metastasis. Anticancer Drugs 2003;14(10):773–778. [DOI] [PubMed] [Google Scholar]
- 12. Di Cerbo A, Canello S, Guidetti G, Laurino C, Palmieri B. Unusual antibiotic presence in gym trained subjects with food intolerance; a case report. Nutr Hosp 2014;30(2):395–398. [DOI] [PubMed] [Google Scholar]
- 13. Fife RS, Sledge GW, Jr . Effects of doxycycline on cancer cells in vitro and in vivo. Adv Dent Res 1998;12(2):94–96. [DOI] [PubMed] [Google Scholar]
- 14. Fife RS, Sledge GW, Jr. , Roth BJ, Proctor C. Effects of doxycycline on human prostate cancer cells in vitro. Cancer Lett 1998;127(1–2):37–41. [DOI] [PubMed] [Google Scholar]
- 15. Çelik A, Dilek E. The assessment of cytotoxicity and genotoxicity of tetracycline antibiotic in human blood lymphocytes using CBMN and SCE analysis, in vitro. Int J Hum Genet 2011;11(1):23–29. [Google Scholar]
- 16. Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature 2007;449(7164):819–826. [DOI] [PubMed] [Google Scholar]
- 17. Delves PJ, Roitt IM. The immune system. First of two parts. N Engl J Med 2000;343(1):37–49. [DOI] [PubMed] [Google Scholar]
- 18. Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science 2010;327(5963):291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol 1996;80(3, Pt 1):225–235. [DOI] [PubMed] [Google Scholar]
- 20. Crane IJ, Forrester JV. Th1 and Th2 lymphocytes in autoimmune disease. Crit Rev Immunol 2005;25(2):75–102. [DOI] [PubMed] [Google Scholar]
- 21. Pollard KM, Cauvi DM, Toomey CB, Morris KV, Kono DH. Interferon‐gamma and systemic autoimmunity. Discov Med 2013;16(87):123–131. [PMC free article] [PubMed] [Google Scholar]
- 22. Baccala R, Kono DH, Theofilopoulos AN. Interferons as pathogenic effectors in autoimmunity. Immunol Rev 2005;204:9–26. [DOI] [PubMed] [Google Scholar]
- 23. Funauchi M, Sugishima H, Minoda M, Horiuchi A. Serum level of interferon‐gamma in autoimmune diseases. Tohoku J Exp Med 1991;164(4):259–267. [DOI] [PubMed] [Google Scholar]
- 24. Hertzog P, Forster S, Samarajiwa S. Systems biology of interferon responses. J Interferon Cytokine Res 2011;31(1):5–11. [DOI] [PubMed] [Google Scholar]
- 25. Hu X, Ivashkiv LB. Cross‐regulation of signaling pathways by interferon‐gamma: implications for immune responses and autoimmune diseases. Immunity 2009;31(4):539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ronnblom L, Eloranta ML. The interferon signature in autoimmune diseases. Curr Opin Rheumatol 2013;25(2):248–253. [DOI] [PubMed] [Google Scholar]
- 27. Tang H, Sharp GC, Peterson KP, Braley‐Mullen H. IFN‐gamma‐deficient mice develop severe granulomatous experimental autoimmune thyroiditis with eosinophil infiltration in thyroids. J Immunol 1998;160(10):5105–5112. [PubMed] [Google Scholar]
- 28. Yu S, Sharp GC, Braley‐Mullen H. Dual roles for IFN‐gamma, but not for IL‐4, in spontaneous autoimmune thyroiditis in NOD.H‐2h4 mice. J Immunol 2002;169(7):3999–4007. [DOI] [PubMed] [Google Scholar]
- 29. Baechler EC, Gregersen PK, Behrens TW. The emerging role of interferon in human systemic lupus erythematosus. Curr Opin Immunol 2004;16(6):801–807. [DOI] [PubMed] [Google Scholar]
- 30. Moretta L, Montaldo E, Vacca P, Del Zotto G, Moretta F, Merli P, Locatelli F, Mingari MC. Human natural killer cells: origin, receptors, function, and clinical applications. Int Arch Allergy Immunol 2014;164(4):253–264. [DOI] [PubMed] [Google Scholar]
- 31. Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8(+) T cells. Nat Rev Immunol 2011;11(10):645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Terrazzano G, Carbone E. NK cells blur the frontier between innate and acquired immunity. Front Immunol 2012;3:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Poggi A, Zocchi MR. NK cell autoreactivity and autoimmune diseases. Front Immunol 2014;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Deniz G, van de Veen W, Akdis M. Natural killer cells in patients with allergic diseases. J Allergy Clin Immunol 2013;132(3):527–535. [DOI] [PubMed] [Google Scholar]
- 35. Terrazzano G, Sica M, Gianfrani C, Mazzarella G, Maurano F, De Giulio B, de Saint‐Mezard S, Zanzi D, Maiuri L, Londei M, Jabri B, Troncone R, Auricchio S, Zappacosta S, Carbone E. Gliadin regulates the NK‐dendritic cell cross‐talk by HLA‐E surface stabilization. J Immunol 2007;179(1):372–381. [DOI] [PubMed] [Google Scholar]
- 36. Ruggiero G, Sica M, Luciano L, Savoia F, Cosentini E, Alfinito F, Terrazzano G. A case of myelodysplastic syndrome associated with CD14(+)CD56(+) monocytosis, expansion of NK lymphocytes and defect of HLA‐E expression. Leuk Res 2009;33(1):181–185. [DOI] [PubMed] [Google Scholar]
- 37. Terrazzano G, Rubino V, Palatucci AT, Giovazzino A, Annunziatella M, Vitagliano O, Alfinito F, Ruggiero G. Natural killer expansion, human leukocyte antigens‐E expression and CD14(+) CD56(+) monocytes in a myelodysplastic syndrome patient. Eur J Haematol 2013;91(3):265–269. [DOI] [PubMed] [Google Scholar]
- 38. Pollard KM, Hultman P, Kono DH. Toxicology of autoimmune diseases. Chem Res Toxicol 2010;23(3):455–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dedeoglu F. Drug‐induced autoimmunity. Curr Opin Rheumatol 2009;21(5):547–551. [DOI] [PubMed] [Google Scholar]
- 40. Vedove CD, Del Giglio M, Schena D, Girolomoni G. Drug‐induced lupus erythematosus. Arch Dermatol Res 2009;301(1):99–105. [DOI] [PubMed] [Google Scholar]
- 41. Rubin RL. Drug‐induced lupus. Toxicology 2005;209(2):135–147. [DOI] [PubMed] [Google Scholar]
- 42. Patterson R, Germolec D. Review article toxic oil syndrome: review of immune aspects of the disease. J Immunotoxicol 2005;2(1):51–58. [DOI] [PubMed] [Google Scholar]
- 43. Pollard KM, Hultman P, Kono DH. Immunology and genetics of induced systemic autoimmunity. Autoimmun Rev 2005;4(5):282–288. [DOI] [PubMed] [Google Scholar]
- 44. Nelson ML, Levy SB. The history of the tetracyclines. Ann N Y Acad Sci 2011;1241:17–32. [DOI] [PubMed] [Google Scholar]
- 45. Marshall TG, Marshall FE. Sarcoidosis succumbs to antibiotics–implications for autoimmune disease. Autoimmun Rev 2004;3(4):295–300. [DOI] [PubMed] [Google Scholar]
- 46. Lenert P, Icardi M, Dahmoush L. ANA (+) ANCA (+) systemic vasculitis associated with the use of minocycline: case‐based review. Clin Rheumatol 2013;32(7):1099–1106. [DOI] [PubMed] [Google Scholar]
- 47. Christen U, von Herrath MG. Transgenic animal models for type 1 diabetes: linking a tetracycline‐inducible promoter with a virus‐inducible mouse model. Transgenic Res 2002;11(6):587–595. [DOI] [PubMed] [Google Scholar]
- 48. Attar SM. Tetracyclines: what a rheumatologist needs to know? Int J Rheum Dis 2009;12(2):84–89. [DOI] [PubMed] [Google Scholar]
- 49. Sarmah AK, Meyer MT, Boxall AB. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006;65(5):725–759. [DOI] [PubMed] [Google Scholar]
- 50. Halling‐Sorensen B, Sengelov G, Tjornelund J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline‐resistant bacteria. Arch Environ Contam Toxicol 2002;42(3):263–271. [DOI] [PubMed] [Google Scholar]
- 51. Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 2001;65(2):232–260; second page, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Black WD. A study in the pharmacodynamics of oxytetracycline in the chicken. Poult Sci 1977;56(5):1430–1434. [DOI] [PubMed] [Google Scholar]
- 53. Union E. Commission Regulation EU/37/2010 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. J Eur Union 2010;15:1–72. [Google Scholar]
- 54. Odore R, De Marco M, Gasco L, Rotolo L, Meucci V, Palatucci AT, Rubino V, Ruggiero G, Canello S, Guidetti G, Centenaro S, Quarantelli A, Terrazzano G, Schiavone A. Cytotoxic effects of oxytetracycline residues in the bones of broiler chickens following therapeutic oral administration of a water formulation. Poult Sci 2015; 94(8):1979–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. De Vitis S, Sonia Treglia A, Ulianich L, Turco S, Terrazzano G, Lombardi A, Miele C, Garbi C, Beguinot F, Di Jeso B. Tyr phosphatase‐mediated P‐ERK inhibition suppresses senescence in EIA + v‐raf transformed cells, which, paradoxically, are apoptosis‐protected in a MEK‐dependent manner. Neoplasia 2011;13(2):120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alfinito F, Ruggiero G, Sica M, Udhayachandran A, Rubino V, Pepa RD, Palatucci AT, Annunziatella M, Notaro R, Risitano AM and others. Eculizumab treatment modifies the immune profile of PNH patients. Immunobiology 2012;217(7):698–703. [DOI] [PubMed] [Google Scholar]
- 57. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 2004;116(2):205–219. [DOI] [PubMed] [Google Scholar]
- 58. Buchakjian MR, Kornbluth S. The engine driving the ship: metabolic steering of cell proliferation and death. Nat Rev Mol Cell Biol 2010;11(10):715–727. [DOI] [PubMed] [Google Scholar]
- 59. Emlen W, Niebur J, Kadera R. Accelerated in vitro apoptosis of lymphocytes from patients with systemic lupus erythematosus. J Immunol 1994;152(7):3685–3692. [PubMed] [Google Scholar]
- 60. Cohen PL. Apoptotic cell death and lupus. Springer Semin Immunopathol 2006;28(2):145–152. [DOI] [PubMed] [Google Scholar]
- 61. Turbyville JC, Rao VK. The autoimmune lymphoproliferative syndrome: A rare disorder providing clues about normal tolerance. Autoimmun Rev 2010;9(7):488–493. [DOI] [PubMed] [Google Scholar]
- 62. Stuart L, Hughes J. Apoptosis and autoimmunity. Nephrol Dial Transplant 2002;17(5):697–700. [DOI] [PubMed] [Google Scholar]
- 63. Rovere P, Vallinoto C, Bondanza A, Crosti MC, Rescigno M, Ricciardi‐Castagnoli P, Rugarli C, Manfredi AA. Bystander apoptosis triggers dendritic cell maturation and antigen‐presenting function. J Immunol 1998;161(9):4467–4471. [PubMed] [Google Scholar]
- 64. Olivry T, Bizikova P. A systematic review of randomized controlled trials for prevention or treatment of atopic dermatitis in dogs: 2008–2011 update. Vet Dermatol 2013;24(1):97–117 e25–e26. [DOI] [PubMed] [Google Scholar]
- 65. Olivry T. A review of autoimmune skin diseases in domestic animals: I ‐ superficial pemphigus. Vet Dermatol 2006;17(5):291–305. [DOI] [PubMed] [Google Scholar]
- 66. Scott DW, Paradis M. A survey of canine and feline skin disorders seen in a university practice: Small Animal Clinic, University of Montreal, Saint‐Hyacinthe, Quebec (1987‐1988). Can Vet J 1990;31(12):830–835. [PMC free article] [PubMed] [Google Scholar]
- 67. Jergens AE, Moore FM, Haynes JS, Miles KG. Idiopathic inflammatory bowel disease in dogs and cats: 84 cases (1987‐1990). J Am Vet Med Assoc 1992;201(10):1603–1608. [PubMed] [Google Scholar]
- 68. El‐Gabalawy H, Guenther LC, Bernstein CN. Epidemiology of immune‐mediated inflammatory diseases: incidence, prevalence, natural history, and comorbidities. J Rheumatol Suppl 2010;85:2–10. [DOI] [PubMed] [Google Scholar]
- 69. Shurin MR, Smolkin YS. Immune‐mediated diseases: where do we stand? Adv Exp Med Biol 2007;601:3–12. [PubMed] [Google Scholar]
- 70. Adesokan HK, Akanbi IO, Akanbi IM, Obaweda RA. Pattern of antimicrobial usage in livestock animals in south‐western Nigeria: The need for alternative plans. Onderstepoort J Vet Res 2015;82(1):E1–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jones PJ, Marier EA, Tranter RB, Wu G, Watson E, Teale CJ. Factors affecting dairy farmers' attitudes towards antimicrobial medicine usage in cattle in England and Wales. Prev Vet Med 2015. [DOI] [PubMed] [Google Scholar]
- 72. Kuang X, Hao H, Dai M, Wang Y, Ahmad I, Liu Z, Zonghui Y. Serotypes and antimicrobial susceptibility of Salmonella spp. isolated from farm animals in China. Front Microbiol 2015;6:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Piras C, Soggiu A, Greco V, Martino PA, Del Chierico F, Putignani L, Urbani A, Nally JE, Bonizzi L, Roncada P. Mechanisms of antibiotic resistance to enrofloxacin in uropathogenic Escherichia coli in dog. J Proteomics 2015. [DOI] [PubMed] [Google Scholar]
- 74. Authority EFS . EFSA's assistance for the 2015 Codex Committee on Residues of Veterinary Drugs in Food (CCRVDF) in relation to rBST;2015. pp 1–89.


