Abstract
Intravenous belimumab is approved for the treatment of systemic lupus erythematosus; subcutaneous self‐administration would enable greater patient access. This study assessed relative bioavailability, tolerability, and safety of 1 subcutaneous dose of self‐administered belimumab by healthy subjects using a single‐use autoinjector or prefilled syringe. Subjects (randomized 1:1:1:1) self‐administered belimumab 200 mg subcutaneously (abdomen or thigh) by prefilled syringe or autoinjector. Pharmacokinetics, adverse events (AEs), injection‐site pain, and administration errors were recorded. Of 81 subjects, 5 experienced administration errors and were excluded from pharmacokinetic analyses. Mean serum belimumab concentration profiles were similar for both devices, with a weak trend toward higher concentrations for thigh injection compared with abdominal injections. Maximum observed serum concentration was slightly higher with the autoinjector (27.0 vs 25.3 µg/mL) and area under the concentration–time curve slightly lower (701 vs 735 day · μg/mL), compared with the prefilled syringe. Incidence of AEs was 51% (41 of 81 subjects; headache was most common), with no serious or severe AEs. Median injection‐site pain scores were low (0 after 1 hour). Device handling was reported as acceptable by ≥95% of autoinjector users and ≥90% of prefilled syringe users for each characteristic assessed. These results support the use of either device for belimumab subcutaneous administration.
Keywords: autoinjector, belimumab, bioavailability, pharmacokinetics, subcutaneous
Systemic lupus erythematosus (SLE) is a multisystem, chronic autoimmune disease with a diverse range of symptoms and presentations, which results in organ damage and increased mortality.1 Belimumab is a recombinant human immunoglobulin (Ig) G1λ monoclonal antibody that binds and antagonizes the biological activity of soluble B‐lymphocyte stimulator (BLyS), a member of the tumor necrosis factor ligand superfamily, which promotes the survival of B lymphocytes.2 Following completion of 2 large, multicenter, randomized, controlled trials, belimumab was approved for the treatment of adults with active, autoantibody‐positive SLE in combination with standard therapy.3, 4
The currently approved dosing regimen of belimumab is 10 mg/kg administered by intravenous infusion every 4 weeks. Patients regularly attend an infusion clinic to receive treatment.5 Weekly subcutaneous administration of a liquid formulation of belimumab 200 mg by prefilled syringe has been shown to achieve plasma levels similar to intravenous infusion6 and yielded pharmacokinetic (PK) data in healthy Japanese subjects that were comparable to non‐Japanese subjects.7 Self‐administration of subcutaneous belimumab at home may be more convenient for patients and potentially more cost effective. To enhance the usability and safety of self‐administration, a single‐use autoinjector for subcutaneous administration has been developed (Figure 1).
Figure 1.
Autoinjector device. Registered Design Protection pending.
This study assessed the relative bioavailability, safety, and device usability and reliability of a single subcutaneous dose of belimumab 200 mg in healthy subjects self‐administered using an autoinjector or a prefilled syringe.
Methods
Study Design
This was a phase 1, randomized, parallel‐group, open‐label, single‐dose study of belimumab in healthy subjects (GSK study BEL117100; NCT01894360), conducted between October 2013 and May 2014 at Quintiles Clinical Research Unit, Overland Park, Kansas. The primary objective was to estimate the relative bioavailability of a single subcutaneous dose of belimumab self‐administered by autoinjector (Figure 1) compared with a prefilled syringe in healthy subjects. The secondary objective was to evaluate the safety and tolerability of the self‐administered dose. The usability and reliability of the devices were also assessed. The study protocol was approved by MidLands Independent Review Board. The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice and the Declaration of Helsinki. Written informed consent was obtained from all subjects prior to study enrollment.
Subjects and Treatments
Men or women 18–55 years of age with a body weight of ≥45 to ≤120 kg and good general health as determined by a physician through medical evaluation (eg, medical history, physical examination, laboratory tests, and cardiac monitoring) were included. Subjects were excluded if they had used any concomitant prescription medications (excluding contraceptives and hormone replacement treatment) or had received a live vaccine within 30 days prior to day 0 or anticipated vaccine administration within the study period.
Subjects were randomized 1:1:1:1 to receive belimumab 200 mg administered subcutaneously by prefilled syringe in the abdomen or thigh or belimumab 200 mg administered subcutaneously by autoinjector in the abdomen or thigh. Randomization was stratified by body weight (<70, 70–<80, and ≥80 kg). The prefilled syringe consisted of a USP type I glass syringe with a back‐stop and plunger rod to enable manual administration of liquid belimumab (200 mg in 1.0 mL of solution). The autoinjector was assembled with the same prefilled syringe, and administration was achieved through a spring mechanism. Subjects were trained on study treatment, device handling, and administration techniques by qualified study‐site personnel at screening and on day 0, prior to self‐administration. Under supervision, subjects performed a practice injection into an injection pad. All study treatments were self‐administered under medical supervision at the study site.
Study Endpoints and Assessments
The primary PK study endpoints were the maximum observed serum concentration (Cmax) and area under the serum concentration–time curve (AUC0–t and AUC0– ∞). Secondary endpoints included the incidence of adverse events (AEs), serious AEs, injection‐site pain, and changes in laboratory values and vital signs. All AEs occurring between day 0 and day 70 were recorded. User errors and subjective user feedback were recorded after practice and live injections to assess the usability of the devices. Device malfunctions and subsequent root‐cause investigations were recorded to assess reliability of devices. The investigator or site staff observed each subject's self‐administered injection to determine if the injection was completed and successful, and if any device malfunctions or any user error occurred. Autoinjectors were returned and inspected by technical experts to confirm proper function.
PK blood samples were taken 6 hours postadministration on day 0 and at the following times: days 1–7 (± 4 hours from the day 0 dosing time), days 10 and 14 (± 1 day), and days 21, 28, 42, 56, and 70 (± 2 days). The concentration of belimumab in serum samples was determined using a validated target‐mediated capture sandwich electrochemiluminescence (ECL) assay.8 Serum samples were minimally diluted (1/400) with assay diluent prior to analysis. The assay used biotinylated recombinant BLyS bound to a streptavidin MULTI‐ARRAY 96‐well plate to isolate belimumab from the serum sample. Rabbit anti‐belimumab (fraction variable) was used for detection, followed by SULFO‐TAG (Meso Scale Discovery, Gaithersburg, Maryland) goat anti‐rabbit polyclonal antibody reporter tag. An ECL development solution was used to generate a measurable signal. This analytical method was selective, sensitive, precise, and accurate for the determination of belimumab in serum, with an analytical range of 100–12 800 ng/mL. Vital sign measurements included temperature, systolic and diastolic blood pressure, pulse rate, and respiratory rate and were measured at screening, on day 0 (before and after administration), and on days 1, 2, 3, 4, 7, 14, 21, 28, 42, 56, and 70. Hematology/chemistry, IgG, and urinalysis assessments were carried out at screening and on days 0, 1, 7, 28, 56, and 70. Injection‐site pain was assessed immediately following the injection and 1 and 24 hours (± 4 hours) postinjection. Subjects rated pain using a horizontal 0‐ to 100‐mm visual analog scale (VAS), where 0 is typically “no pain,” and 100 mm is “worst pain imaginable.”9
Statistical Analyses
Sample size was based on the number of subjects needed to estimate relative bioavailability with an acceptable level of precision. A sample size of approximately 72 subjects with evaluable data was calculated to be required for the specified precision. Allowing for a 10% dropout rate, approximately 80 subjects were enrolled. The PK population comprised subjects who were considered to have had complete dosing and for whom a PK sample was obtained and analyzed.
A PK sensitivity analysis was carried out, including data from all subjects for whom a PK sample was obtained and analyzed. Serum concentration–time data were analyzed by noncompartmental methods using WinNonlin® Pro 6.3 (Pharsight Corporation, Mountain View, California). Calculations were based on the actual sampling times, and from the serum concentration–time data, Cmax, AUC0–t, and AUC0–∞ were calculated. Following loge‐transformation, AUC0–∞, AUC0–t, and Cmax of belimumab were analyzed separately using a fixed‐effects model with fixed‐effect terms for the treatment, location (abdomen or thigh), and baseline weight (<70, 70–<80, ≥80 kg). Point estimates and their associated 2‐sided 90% confidence intervals (CIs) were constructed for the differences, test treatment (autoinjector)‐reference treatment (prefilled syringe). The point estimates and their associated 90%CIs were then back‐transformed to provide point estimates and 2‐sided 90%CIs for the ratios of test/reference, on the original scale. In addition, time of occurrence of Cmax (tmax) and terminal elimination half‐life (t1/2) were determined from the serum concentration–time data. Usability and reliability of the devices were assessed by determining the percentage of successful (complete) injections relative to attempted injections. Further, usability was determined by assessing reported user errors and subjective feedback from subjects regarding the handling and ergonomics of the device. Device malfunctions were reported and investigated for root cause.
Results
Baseline Characteristics and Drug Administration
The baseline characteristics of the 81 healthy volunteers who were enrolled in the study and received an injection were balanced across treatment groups. Approximately half the subjects were male (52%), the mean ± standard deviation (SD) age was 32.6 ± 9.88 years, and the mean ± SD weight was 84.5 ± 16.32 kg. The majority of subjects weighed ≥80 kg (58%), and 22% of subjects weighed <70 kg. Approximately half the subjects were African American/African heritage (51%), and approximately half were white (48%). Two subjects in the autoinjector‐abdomen group chose to withdraw from the study on day 44 and day 50, as they moved away from the study‐site area. Successful (complete) injection was achieved by 94% (76 of 81 of subjects); 5 subjects experienced administration errors (1 autoinjector‐thigh, 2 autoinjector‐abdomen, 2 prefilled syringe‐thigh) and were excluded from the PK analysis. Drug concentration data for the 5 subjects who experienced administration errors indicate that only 1 subject received no dose and that dose reduction for the other four subjects was negligible.
Pharmacokinetics
Cmax was slightly higher with the autoinjector device and AUC slightly lower compared with the prefilled syringe (Table 1), but the mean serum belimumab concentration profile was similar overall for both injection devices throughout the 70‐day period. Peak mean serum concentrations of belimumab occurred approximately 5 days after belimumab was administered and then declined monoexponentially (Table 1 and Figure 2). There was a weak trend toward higher serum concentrations for thigh injection compared with abdomen injection for both devices (Table 1). Subjects with a higher body weight showed a trend toward lower exposure for both devices (Figure 3).
Table 1.
Summary of Serum PK Parameters of Belimumab (PK Population)
| By Injection Device Only | By Injection Device and Injection Site | ||||||
|---|---|---|---|---|---|---|---|
| Parameter (Unit) | Prefilled Syringe (n = 38) | Autoinjector (n = 38) | Prefilled Syringe‐Abdomen (n = 20) | Autoinjector‐Abdomen (n = 18) | Prefilled Syringe‐Thigh (n = 18) | Autoinjector‐Thigh (n = 20) | |
| AUC0–t (day · μg/mL) | Geometric | 676 (34.1) | 652 (32.6) | 631 (35.6) | 619 (23.3) | 729 (31.5) | 684 (39.3) |
| mean (CVb%) | |||||||
| Mean (SD) | 711 (228) | 684 (216) | 667 (227) | 634 (146) | 761 (226) | 729 (259) | |
| AUC0– ∞ (day · μg/mL) | Geometric | 735 (39.9) | 701 (35.7) | 683 (41.8) | 672 (25.1) | 798 (37.1) | 728 (43.8) |
| mean (CVb%) | |||||||
| Mean (SD) | 790 (309) | 743 (261) | 738 (306) | 692 (170) | 847 (311) | 789 (319) | |
| Cmax (μg/mL) | Geometric | 25.3 (33.2) | 27.0 (31.2) | 24.1 (32.6) | 25.3 (25.3) | 26.7 (33.9) | 28.6 (35.3) |
| mean (CVb%) | |||||||
| Mean (SD) | 26.6 (8.07) | 28.2 (8.28) | 25.3 (7.71) | 26.1 (6.35) | 28.1 (8.42) | 30.1 (9.45) | |
| tmax (h) | Median | 141 | 96.8 | 134 | 108 | 141 | 96.8 |
| (min, max) | (47.5, 337) | (47.4, 238) | (68.0, 337) | (47.7, 168) | (47.5, 239) | (47.4, 238) | |
| t1/2 (h) | Geometric | 390 (42.8) | 356 (35.9) | 375 (45.7) | 355 (36.9) | 407 (40.2) | 357 (36.1) |
| mean (CVb%) | |||||||
| Mean (SD) | 424 (184) | 378 (134) | 411 (191) | 378 (144) | 438 (180) | 378 (127) | |
AUC, area under the curve; Cmax, maximum observed serum concentration; CVb, between‐subject coefficient of variation; PK, pharmacokinetic; SD, standard deviation; tmax, time to maximum serum concentration; t1/2, elimination half‐life.
All data presented to 3 significant figures.
Figure 2.
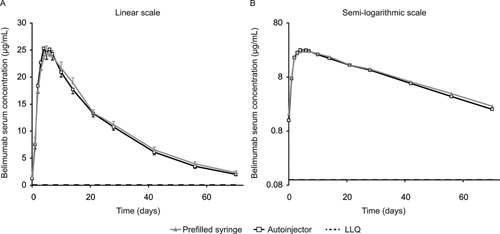
Mean serum belimumab concentration by injection device (PK population). LLQ, 100 ng/mL; below the limit of quantification, 0 (included in calculation of the mean). LLQ, lower limit of quantification; PK, pharmacokinetic.
Figure 3.
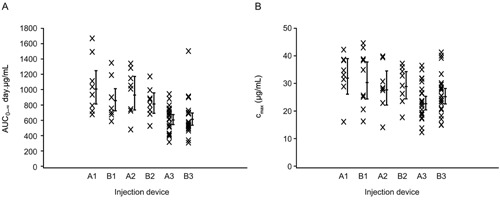
Serum belimumab PK parameters by injection device and baseline weight category: (A) AUC0– ∞ (day · μg/mL); (B) Cmax (µg/mL; PK population). A1, prefilled syringe (<70 kg); B1, autoinjector (<70 kg); A2, prefilled syringe (70–<80 kg); B2, autoinjector (70–<80 kg); A3, prefilled syringe (≥80 kg); B3, autoinjector (≥80 kg). AUC, area under the curve; Cmax, maximum observed serum concentration; PK, pharmacokinetic.
In the PK population, the overall relative bioavailability for the autoinjector was less than 7% different compared with the prefilled syringe for all 3 PK parameters (Table 2). In the sensitivity analysis, which included all subjects with a PK sample, the relative bioavailability (90%CI) of belimumab was 89.10 (78.13–101.61) for AUC0–∞ and 86.90 (65.84–114.70) for Cmax. One subject (autoinjector group) in the sensitivity analysis had no detectable serum belimumab concentrations; AUC0–∞ was not calculated for this subject, and Cmax was imputed as half the lower limit of quantification.
Table 2.
Relative Bioavailability of Belimumab (PK Population)
| Prefilled Syringe | Autoinjector | ||||
|---|---|---|---|---|---|
| Parameter | N | Geometric Meana | N | Geometric Mean a | Relative Bioavailability (90%CI) |
| AUC(0‐∞) (day · μg/mL) | 38 | 812 | 38 | 761 | 93.6 (83.2–105.4) |
| AUC(0‐t) (day · μg/mL) | 38 | 734 | 38 | 698 | 95.0 (85.4–105.7) |
| Cmax (μg/mL) | 38 | 26.8 | 38 | 28.2 | 105.2 (94.0–117.7) |
AUC, area under the curve; CI, confidence interval; Cmax, maximum observed serum concentration; PK, pharmacokinetic. PK population excludes the following subjects with incomplete dosing; 2, autoinjector‐abdomen; 1, autoinjector‐thigh; 2, prefilled syringe‐thigh.
An analysis of variance was performed on natural logarithms of PK parameters. Estimated geometric means are presented; the model included covariates of injection location and baseline body weight and has made adjustments for the geometric means for any imbalance of these variables between the 2 injection device groups.
Safety
At least 1 AE was reported by 41 subjects (51%; Table 3). Five subjects (25%) reported an AE in the prefilled syringe‐abdomen group compared with 11–13 subjects (52%–65%) across the other 3 study groups. All AEs were of mild intensity except 1 incident of lower back pain spasms on day 13, which was of moderate intensity but not considered by the investigator to be drug‐related. The most common AEs were headache (3 subjects [15%], autoinjector‐abdomen; 5 subjects [25%], prefilled syringe‐thigh) and upper respiratory tract infection (3 subjects [15%], prefilled syringe‐abdomen). No serious or severe AEs were reported. Nine subjects (11%) reported at least 1 drug‐related AE (Table 3). All drug‐related AEs were reported by subjects injecting the thigh. The median VAS scores of injection‐site pain at dosing were low (4.5–12.0 mm across study groups); the median score was 0 for all groups 1 and 24 hours postdose.
Table 3.
Summary of AEs (All Subjects)
| Prefilled Syringe‐Abdomen(n = 20) | Autoinjector‐Abdomen(n = 20) | Prefilled Syringe‐Thigh(n = 20) | Autoinjector‐Thigh(n = 21) | Total (n = 81) | |
|---|---|---|---|---|---|
| Any a AE, n (%) | 5 (25) | 12 (60) | 13 (65) | 11 (52) | 41 (51) |
| Headache | 2 (10) | 3 (15) | 5 (25) | 0 | 10 (12) |
| Upper respiratory tract infection | 0 | 1 (5) | 3 (15) | 1 (5) | 5 (6) |
| Viral upper respiratory tract infection | 2 (10) | 1 (5) | 1 (5) | 1 (5) | 5 (6) |
| Pruritis | 0 | 1 (5) | 2 (10) | 1 (5) | 4 (5) |
| Abdominal pain | 0 | 0 | 0 | 2 (10) | 2 (2) |
| Gastroenteritis | 0 | 2 (10) | 0 | 0 | 2 (2) |
| Injection‐site pruritus | 0 | 0 | 2 (10) | 0 | 2 (2) |
| Drug‐related b AE, n (%) | 0 | 0 | 5 (25) | 4 (19) | 9 (11) |
| Injection‐site erythema | 0 | 0 | 1 (5) | 1 (5) | 2 (2) |
| Injection‐site pruritus | 0 | 0 | 2 (10) | 0 | 2 (2) |
| Nausea | 0 | 0 | 1 (5) | 1 (5) | 2 (2) |
| Abdominal pain | 0 | 0 | 0 | 1 (5) | 1 (1) |
| Contusion | 0 | 0 | 0 | 1 (5) | 1 (1) |
| Decreased appetite | 0 | 0 | 1 (5) | 0 | 1 (1) |
| Injection‐site urticaria | 0 | 0 | 1 (5) | 0 | 1 (1) |
| Nasal congestion | 0 | 0 | 1 (5) | 0 | 1 (1) |
| Urticaria | 0 | 0 | 1 (5) | 0 | 1 (1) |
AE, adverse event.
AEs occurring in ≥10% of subjects in any study group are listed.
All related AEs are listed.
Four subjects (1 per treatment group) had a vital sign value of potential clinical significance including elevations of diastolic blood pressure and heart rate, although none were reported as an AE. Analysis of the laboratory data showed that a worsening in neutrophils from grade 0 at baseline to grade 2 postbaseline was observed in a total of 3 subjects (2 in the prefilled syringe‐thigh group, 1 in the autoinjector‐abdomen group). One subject in the prefilled syringe‐abdomen group had a worsening in phosphate from grade 0 at baseline to grade 2 postbaseline, and 1 other subject in the autoinjector‐abdomen group had a worsening in glucose from grade 0 at baseline to grade 2 postbaseline. One subject in the prefilled syringe‐abdomen group had a worsening in urinalysis from grade 0 at baseline to grade 2 postbaseline. Mean IgG concentration was similar between groups at all times (data not shown). A slight increase in mean IgG concentrations was observed for all study groups 1 day postdose; however, concentrations subsequently decreased to baseline levels for all groups by day 28. No subject had a grade 2, 3, or 4 IgG level during the study.
Device Usability and Reliability
Five of 81 subjects received incomplete or no dosing; thus, 94% of the injections were classified as successful for both devices. There were 3 user errors (1 autoinjector‐thigh and 2 prefilled syringe‐thigh), all involving positioning and maintenance of the needle in the injection site. There was 1 report of a device malfunction (autoinjector failed to activate) for a subject in the autoinjector‐abdomen group. The device was investigated for root cause, and possible causes were identified, including drying of the needle tip with drug product (resulting in blockage) and/or higher than usual activation force potentially because of a minor component defect observed by x‐ray. One administration error was not classified (autoinjector‐abdomen), and it was noted that the drug leaked out as the autoinjector was removed; however, the source of the leakage could not be confirmed.
Ergonomic and handling characteristics of both devices were reported to be acceptable by the subjects; handheld comfort and effort to remove cap were both reported as acceptable by 39 of 40 prefilled syringe users (98%) and all autoinjector users. The effort required to activate the device was reported as acceptable for 36 of 40 prefilled syringe users (90%) and 40 of 41 autoinjector users (98%), and the time to inject was acceptable for all prefilled syringe users and 38 of 40 autoinjector users (95%).
Discussion
In this randomized, parallel‐group, open‐label, single‐dose study, the relative bioavailability of belimumab administered subcutaneously was similar for both the autoinjector device and the prefilled syringe across 2 injection sites. Safety data were consistent with the known safety profile of belimumab,3, 4 and the majority of subjects (94%) had successful self‐injections, indicating a high level of usability and reliability for the devices.
No statistically significant difference was observed in the total and peak exposures of belimumab between the prefilled syringe and the autoinjector for a single dose of belimumab 200 mg subcutaneously. The 90%CIs of relative bioavailability between the autoinjector and the prefilled syringe overlapped with 100% and were completely within the standard bioequivalence criterion interval of 80% to 125%; this study was not designed as a bioequivalence study, the bioequivalence criterion was met. Although comparison of injection sites was not an objective of the present study, both abdomen and thigh injection sites were included; in clinical practice, with repeat dosing, patients would most likely wish to alternate between injection sites. Injections into the thigh resulted in slightly higher serum concentrations compared with injections into the abdomen, regardless of device. In a previous subcutaneous study by Cai et al in healthy US subjects, exposure was evaluated after subcutaneous administration of 2 × 120 mg (n = 19), 1 × 240 mg (n = 18), and 1 × 200 mg (n = 18) belimumab with a prefilled syringe.6 In that study, the half‐life and Cmax values were similar to those in the present study, though the AUC0–∞ was slightly lower (geometric mean of 612.1 day · µg/mL for the single‐dose 200‐mg group) in the study by Cai et al. In contrast to the Cai et al study, we observed a consistent trend toward higher exposure for injections into the thigh, and this finding has also been demonstrated for other monoclonal antibodies following subcutaneous administration.10 Belimumab exposure has also been examined in healthy Japanese volunteers following a single subcutaneous administration of belimumab 200 mg with a prefilled syringe.7 The results of the present study are generally consistent with the Japanese study in terms of Cmax and AUC0–∞ values, although there was a trend toward higher AUC0–∞ in Japanese subjects. This may be because of the smaller size of Japanese subjects (mean body weight, 63.6 kg, compared with 84.5 kg in the present study). In addition, in the Japanese study, the thigh was the only injection site. Peak mean serum concentrations of belimumab occurred approximately 5 days after dosing and declined monoexponentially in the present study. This is in contrast to the biexponential decline observed for intravenous administration of belimumab and other monoclonal antibodies.8, 11 This difference is likely because of slower absorption from the subcutaneous compartment compared with intravenous administration, which masks the faster distribution from central circulation to peripheral tissues.12
A single 200‐mg dose of belimumab administered subcutaneously by either prefilled syringe or autoinjector was generally safe and well tolerated in the present study. The incidence of AEs was 25%–65% across all groups, with most AEs being reported by fewer than 2 subjects per group, demonstrating consistency with the known safety profile of belimumab. No serious or severe AEs were reported, and all drug‐related AEs were mild. A lower percentage of subjects reported an AE in the prefilled syringe‐abdomen group compared with the other 3 study groups. However, this was not the case for drug‐related AEs, which were reported for no subjects in both abdomen groups compared with 5 subjects (25%) and 4 subjects (19%) in the prefilled syringe‐thigh and autoinjector‐thigh groups, respectively. Along with the low incidence of AEs, injection‐site pain scores were low, with median scores of 4.5–12.00 mm across the study groups. Pain did not persist, as median postdose scores at 1 and 24 hours were 0.
For both the autoinjector and prefilled syringe devices, the majority of attempted injections were classified as complete and successful, taking into account both usability and reliability. The user errors were low frequency, did not pose a safety risk, and will be correctable through additional training and experience. This demonstrates a good level of usability and reliability.
One limitation of this study was that subjects received only 1 dose; therefore, exposure/accumulation following repeat dosing is not known. Furthermore, this single injection did not allow for the likely correction and mitigation of the observed user errors. Further studies will explore the effects of repeat dosing. The population studied here comprised healthy subjects; further studies will also assess whether patients with SLE can use the autoinjector equally as well. In addition, this was a parallel‐group study; a crossover study design was not possible because of the long half‐life of the compound.
The results of this study support the use of the autoinjector and prefilled syringe for belimumab subcutaneous self‐administration. The results of ongoing studies will provide further insight into the use of subcutaneous belimumab for the treatment of SLE and of the autoinjector device for subcutaneous administration.
Declaration of Conflicting Interests
M.E.B., N.L.F., J.Gr., J.F., D.G., and D.R. are all employees of and hold stock in GSK. J.Gi. is a contractor for GSK. H.S. is an employee of PAREXEL; H.S. was an employee of GSK at the time of the study and holds GSK stock. T.M. is an employee of Quintiles, which was contracted by GSK to conduct this study.
Funding
This study was funded and conducted by GSK.
Acknowledgments
Medical writing assistance was provided by Louisa Pettinger, of Fishawack Indicia Ltd, and funded by GSK.
References
- 1. Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008; 358:929–939. [DOI] [PubMed] [Google Scholar]
- 2. Baker KP, Edwards BM, Main SH, et al. Generation and characterization of LymphoStat‐B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003; 48:3253–3565. [DOI] [PubMed] [Google Scholar]
- 3. Furie R, Petri M, Zamani O, et al. A phase III, randomized, placebo‐controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011; 63:3918–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Navarra SV, Guzmán RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo‐controlled, phase 3 trial. Lancet. 2011; 377:721–731. [DOI] [PubMed] [Google Scholar]
- 5.Benlysta (belimumab) prescribing information. GlaxoSmithKline. 2014. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125370s016lbl.pdf
- 6. Cai WW, Fiscella M, Chen C, Zhong ZJ, Freimuth WW, Subich DC. Bioavailability, pharmacokinetics, and safety of belimumab administered subcutaneously in healthy subjects. Clin Pharmacol Drug Develop. 2013; 2:349–357. [DOI] [PubMed] [Google Scholar]
- 7. Shida Y, Takahashi N, Sakamoto T, Ino H, Endo A, Hirama T. The pharmacokinetics and safety profiles of belimumab after single subcutaneous and intravenous doses in healthy Japanese volunteers. J Clin Pharm Ther. 2014; 39:97–101. [DOI] [PubMed] [Google Scholar]
- 8. Struemper H, Chen C, Cai W. Population pharmacokinetics of belimumab following intravenous administration in patients with systemic lupus erythematosus. J Clin Pharmacol. 2013; 53:711–720. [DOI] [PubMed] [Google Scholar]
- 9. Williamson A, Hoggart B. Pain: a review of three commonly used pain rating scales. J Clin Nurs. 2005; 14:798–804 [DOI] [PubMed] [Google Scholar]
- 10. Xu Z, Wang Q, Zhuang Y, et al. Subcutaneous bioavailability of golimumab at 3 different injection sites in healthy subjects. J Clin Pharmacol. 2010; 50:276–284. [DOI] [PubMed] [Google Scholar]
- 11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008; 84:548–558. [DOI] [PubMed] [Google Scholar]
- 12. Richter WF, Jacobsen B. Subcutaneous absorption of biotherapeutics: knowns and unknowns. Drug Metab Dispos. 2014; 42:1881–1889. [DOI] [PubMed] [Google Scholar]


