Abstract
Defects in multidrug resistance 3 gene (MDR3), which encodes the canalicular phospholipid flippase, cause a wide spectrum of cholangiopathy phenotypes in humans. Mice deficient in Mdr2 (murine ortholog of MDR3) develop liver diseases that closely reproduce the biochemical, histological, and clinical features of human cholangiopathies such as progressive familial intrahepatic cholestasis and primary sclerosing cholangitis. We hypothesized that modulating bile acid metabolism by the gut hormone fibroblast growth factor 19 (FGF19) may represent a novel approach for treating cholangiopathy and comorbidities. We introduced adeno‐associated virus carrying the gene for either the endocrine hormone FGF19 or engineered FGF19 variant M70 to 12‐week old Mdr2‐deficient mice with fully established disease. Effects on serum levels of liver enzymes, liver histology, and bile acid homeostasis were evaluated. FGF19 and M70 rapidly and effectively reversed liver injury, decreased hepatic inflammation, attenuated biliary fibrosis, and reduced cholecystolithiasis in Mdr2‐deficient mice. Mechanistically, FGF19 and M70 significantly inhibited hepatic expression of Cyp7a1 and Cyp27a1, which encode enzymes responsible for the rate‐limiting steps in the classic and alternate bile acid synthetic pathways, thereby reducing the hepatic bile acid pool and blood levels of bile acids. Importantly, prolonged exposure to FGF19, but not M70, led to the formation of hepatocellular carcinomas in the Mdr2‐deficient mice. Furthermore, M70 ameliorated the hepatosplenomegaly and ductular proliferation that are associated with cholangiopathy. Conclusion: These results demonstrate the potential for treating cholangiopathy by safely harnessing FGF19 biology to suppress bile acid synthesis. (Hepatology 2016;63:914–929)
Abbreviations
- AAV
adeno‐associated virus
- ALP
alkaline phosphatase
- ALT
alanine transaminase
- AST
aspartate transaminase
- FGF
fibroblast growth factor
- HCC
hepatocellular carcinoma
- Mdr2/3
multidrug resistance 2/3
- mRNA
messenger RNA
- PFIC
progressive familial intrahepatic cholestasis
- PSC
primary sclerosing cholangitis
- qRT‐PCR
quantitative reverse transcription polymerase chain reaction
- UDCA
ursodeoxycholic acid
Chronic cholangiopathies, a diverse group of genetic and acquired biliary disorders affecting the function and homeostasis of cholangiocytes (biliary epithelial cells), account for the majority of pediatric liver transplantations.1 There are currently no therapeutics approved for cholangiopathies associated with primary sclerosing cholangitis (PSC), Alagille syndrome, biliary atresia, chronic liver graft rejection, or progressive familial intrahepatic cholestasis (PFIC). A wide spectrum of biliary abnormalities arise from defects in the hepatocellular transport system involved in bile formation.2, 3 In particular, lesions in the gene encoding multidrug resistance 3 P‐glycoprotein (MDR3, also known as ABCB4) result in abnormal excretion of biliary phosphatidylcholine and the onset of intrahepatic cholestasis.4, 5 To date, more than 30 mutations in MDR3 have been reported that are causally associated with a variety of biliary diseases, including PFIC type 3 (PFIC3),6, 7 intrahepatic cholestasis of pregnancy,8, 9 low phospholipid‐associated cholelithiasis,10, 11 anicteric cholestasis,12 oral contraceptive‐induced cholestasis,11 and cirrhosis.13
Under physiological conditions, biliary phospholipids are transported into the bile through the canalicular phospholipid flippase MDR3 and subsequently form mixed phospholipid‐bile acid micelles that protect cholangiocytes from bile acid–induced cell injury. In patients with MDR3 deficiency (e.g., PFIC3), biliary accumulation of nonmicellular, free bile salts lead to bile duct injury, fibrosis, and cirrhosis, requiring liver transplant in the first decade of life.6 These histological and biochemical characteristics are reproduced in mice with targeted disruption of the orthologous multidrug resistance 2 gene (Mdr2 −/− mice).14 In addition, Mdr2‐deficient mice develop multifocal strictures and segmental dilatations, “onion skin”‐type periductal fibrosis, and focal fibro‐obliteration of bile ducts, closely resembling biliary abnormalities occurring in primary and secondary sclerosing cholangitis in humans.15, 16 Ursodeoxycholic acid (UDCA), a hydrophilic bile acid thought to function by diluting and displacing the “toxic bile,” improves outcomes for some PFIC3 patients; but even UDCA responders still progress to cirrhosis at age 15‐20.17 Studies of UDCA in PSC patients have produced controversial results, including one report of worsened overall survival.18, 19, 20
Physiological regulation of bile acid synthesis is maintained through a feedback mechanism mediated by the fibroblast growth factor 19 (FGF19) signaling pathway.21, 22, 23, 24 In addition to its role in regulating bile acid homeostasis, FGF19 plays a role in regulating cell proliferation in the liver and has been implicated in the formation of hepatocellular carcinomas (HCCs).25, 26, 27, 28, 29, 30 As a means of harnessing the therapeutic potential of the FGF19 pathway for treating bile acid–related diseases, we have engineered and characterized a nontumorigenic variant of FGF19, M70, that retains activities in regulating bile acid metabolism but does not promote hepatic tumorigenesis.31, 32 M70 differs from wild‐type FGF19 by three amino acid substitutions (A30S, G31S, and H33L) and a five–amino acid deletion at the N terminus. Unlike FGF19, M70 does not cause liver tumors even after prolonged exposure at supraphysiologic levels in db/db or rasH2 mice.31 M70 interacts with the FGFR4 receptor but exhibits the pharmacologic characteristics of a biased ligand that selectively activates certain signaling pathways (e.g., cytochrome P450 7A1, phosphorylated extracellular signal–regulated kinase) to the relative exclusion of others (e.g., tumorigenesis, phosphorylated signal transducer and activator of transcription 3).31 In the current study, we evaluated the effects of ectopic expression of FGF19 and M70 in the Mdr2 −/− mouse model and demonstrate that a one‐time viral delivery of genes encoding these hormones efficiently produces long‐lasting hepatoprotective, anti‐inflammatory, and antifibrotic effects. Importantly, we show that, unlike FGF19, M70 not only fails to trigger hepatic tumor formation even after prolonged exposure but also exhibits antiproliferative effects in the context of sclerosing cholangitis.
Materials and Methods
Animals and Animal Care
Mice were handled and experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee at NGM based on the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. Mdr2 −/− mice on an FVB/N background (stock number 002539) and sex‐matched and age‐matched wild‐type FVB/N mice were obtained from Jackson Laboratory; 12‐week‐old Mdr2 −/− mice (males and females) received a single intravenous dose of 1 × 1011 vector genome of adeno‐associated virus (AAV) containing genes encoding either FGF19, M70, or green fluorescent protein.
Blood Parameters
Blood was collected from the tail vein or after death using microvette tubes (Sarstedt). Alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), triglyceride, and cholesterol levels were measured on a Cobas Integra 400 Clinical Analyzer (Roche Diagnostics). Plasma FGF19 level was determined by FGF19 enzyme‐linked immunosorbent assays (Biovendor). All assays were performed according to the manufacturers' instructions.
Hepatic Bile Acid Pool Size and Serum Bile Acid Concentrations
Livers from female Mdr2 −/− mice were homogenized in 75% ethanol and incubated at 50°C for 2 hours. After centrifugation at 6000g for 10 minutes, the pellets were extracted again with 50% ethanol. Supernatants from the two extraction steps were pooled, evaporated, and reconstituted in 50% ethanol. Concentrations of total bile acids in liver extracts or serum were determined using a 3α‐hydroxysteroid dehydrogenase method (Diazyme).
Hepatic Hydroxyproline Content
Livers from female Mdr2 −/− mice or wild‐type mice were homogenized in water and hydrolyzed in concentrated hydrochloric acid at 120°C for 3 hours. Hydroxyproline concentrations were determined by the reaction of oxidized hydroxyproline with 4‐(dimethylamino)benzaldehyde using a colorimetric kit from Sigma.
For detailed information, please refer to the Supporting Information.
Results
FGF19 and M70 Diminish Liver Injury in Mdr2−/− Mice
Bile duct injury in Mdr2 −/− mice is a progressive process resulting from the detergent‐like properties of nonmicellar‐bound, free biliary bile acids. For the purpose of this study, Mdr2 −/− mice were treated at 12 weeks of age, at which point segmental biliary strictures, dilatations, “onion skin”‐type fibrosis, and severe cholangiocyte injury were already fully developed.15 To assess potential therapeutic efficacy in the context of this disease state, we introduced genes encoding FGF19 or M70 into Mdr2 −/− mice by AAV‐mediated gene delivery (Fig. 1A,B), which allows stable transgene expression for up to 1 year without inflammatory responses.31, 33, 34, 35
Figure 1.
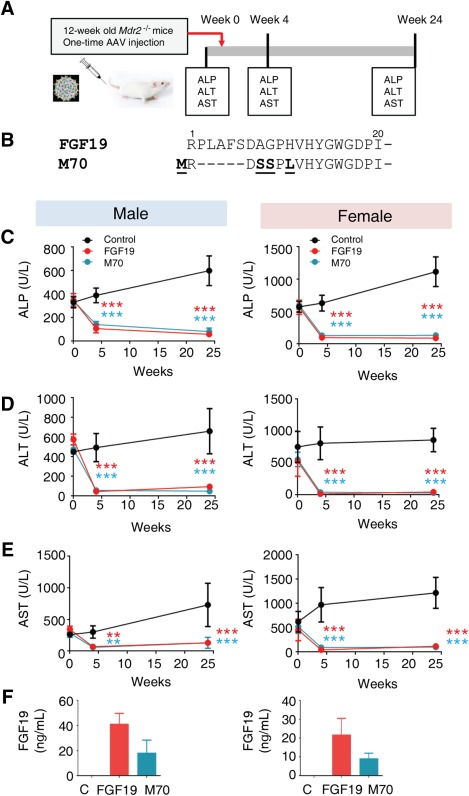
FGF19 and M70 diminish liver injury in Mdr2 −/− mice. (A) Schematics of the experiment. Twelve‐week‐old Mdr2−/− mice were injected with AAV carrying FGF19, M70, or a control gene (the gene for green fluorescent protein; n = 5 per sex per group). Liver enzymes were determined before and 4 and 24 weeks after AAV administration. (B) Sequence alignment of FGF19 and M70. Only N termini of the proteins are shown. (C) Serum levels of ALP over time (n = 5 mice per group). (D) Serum ALT (n = 5). (E) Serum AST (n = 5). (F) Circulating levels of FGF19 and M70 measured by enzyme‐linked immunosorbent assay (n = 5). Values are mean ± standard error of the mean. **P < 0.01, ***P < 0.001 versus control group by two‐way analysis of variance.
Four weeks following gene delivery, significant reductions of serum levels of ALP, a marker of biliary damage, were observed in mice expressing FGF19 (69% reduction from baseline of 349 ± 26 U/L to 107 ± 16 U/L and 83% reduction from baseline of 565 ± 49 U/L to 98 ± 7 U/L in male and female mice, respectively; n = 5, P < 0.001; Fig. 1C; Supporting Fig. S1A). A similarly profound reduction in ALP levels was observed in Mdr2 −/− mice in response to M70 transgene expression (59% reduction from baseline of 343 ± 18 U/L to 142 ± 12 U/L and 78% reduction from baseline of 598 ± 29 U/L to 130 ± 7 U/L in males and female mice, respectively; n = 5, P < 0.001). Notably, these reduced serum levels of ALP were maintained throughout the course of the study period, 24 weeks after gene delivery. The improvement in ALP levels associated with the ectopic expression of FGF19 and M70 in Mdr2 −/− mice was accompanied by marked reductions in the serum levels of ALT and AST (Fig. 1D,E; Supporting Fig. S1B,C). Overall, hepatobiliary damage in female Mdr2 −/− mice appears to be more severe than that in their male counterparts, as manifested by higher serum levels of ALP, ALT and AST. Nonetheless, expression of FGF19 and M70 transgenes restores liver enzymes to normal levels and reverses liver injury in both male and female mice. Mean plasma FGF19 levels were 41 ± 9 ng/mL and 22 ± 9 ng/mL in males and females, respectively, and mean plasma M70 levels were 18 ± 10 ng/mL and 9 ± 3 ng/mL in males and females, respectively, at the end of the study (Fig. 1F). Both FGF19 and M70 reduced body weights in these animals (Supporting Fig. S1D), consistent with a previous report on the regulation of energy metabolism by FGF19.36
Thus, we conclude that ectopic expression of FGF19 or M70 in Mdr2 −/− mice results in rapid, robust, and sustained reduction in serum levels of ALP, ALT, and AST, markers of biliary and hepatocellular damage.
FGF19 and M70 Ameliorate Hepatic Inflammation and Fibrosis in Mdr2−/− Mice
Most cholangiopathies are associated with portal inflammation in proximity to the biliary epithelium, and the initiation of inflammation plays a critical role in the pathogenesis of sclerosing cholangitis. We determined the expression profiles for a panel of genes encoding cytokines and chemokines in livers isolated from Mdr2 −/− mice (Fig. 2A‐C). Quantitative reverse‐transcription polymerase chain reaction (qRT‐PCR) analysis showed marked reductions in the expression of Tnf‐α, Il‐6, Ccl2, Cxcl2, and Icam‐1 in mice expressing either FGF19 or M70. Histologically, Mdr2 −/− mice develop pronounced hepatic inflammation as the disease progresses, characterized by extensive mixed inflammatory infiltrates in the portal tracts and the parenchyma (Fig. 2D). In contrast, FGF19 and M70 clearly decreased the numbers of infiltrating leukocytes. Both FGF19 and M70 reduced hepatic Cd11b (Itgam)–positive myeloid cells, F4/80 (Emr1)‐positive macrophages, and Cd4‐positive T cells as shown by qRT‐PCR analysis (Fig. 2E).
Figure 2.
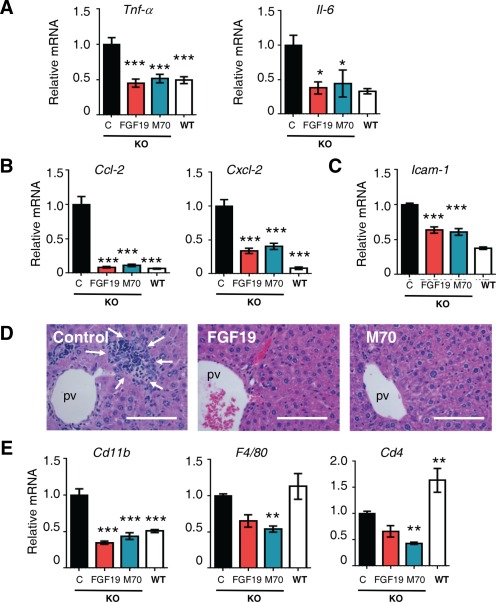
FGF19 and M70 ameliorate hepatic inflammation in Mdr2 −/− mice. Hepatic gene expression and liver histology were determined 24 weeks after tail vein injections of AAV carrying FGF19, M70, or a control gene (n = 5 per group; Mdr2−/− mice were 12 weeks old at study initiation). Mdr2+/+ mice (wild type) were included as comparators for Mdr2−/− (knockout) mice in gene expression analysis. (A) mRNA expression of proinflammatory cytokines (n = 5). (B) mRNA levels of proinflammatory chemokines (n = 5). (C) Icam‐1 levels (n = 5). (D) Representative hematoxylin and eosin staining showing FGF19 and M70 reduce inflammatory infiltrates in the liver. Arrows outline the area of infiltrating leukocytes. Scale bars = 100 μm. (E) qRT‐PCR analysis of immune cell subsets (n = 5). Values are mean ± standard error of the mean. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group by one‐way analysis of variance. Abbreviations: KO, knockout; pv, portal vein; WT, wild type.
Whereas pronounced “onion skin”‐like fibrotic rings were evident in Mdr2 −/− mice injected with the control virus, as revealed by staining with hematoxylin and eosin, sirius red, and trichrome, evidence of periductal fibrosis was not observed in mice treated with AAV‐FGF19 or AAV‐M70 (Fig. 3A). In addition to the effects on periductal fibrosis, ectopic expression of either FGF19 or M70 dramatically reduced hepatocellular fibrosis in the parenchyme (Supporting Fig. S2). In accordance with these histological observations, qRT‐PCR analysis revealed that AAV‐FGF19‐treated or M70‐treated Mdr2 −/− mice had markedly reduced levels of hepatic collagen messenger RNAs (mRNAs; Col1a1, Col1a2, and Col3a1 in Fig. 3B). Moreover, the expression of profibrogenic cytokines (Tgf‐b1, Galectin‐3, and Ctgf; Fig. 3C), as well as metalloproteases and inhibitors critical for extracellular matrix remodeling (Mmp2, Mmp12, Timp1; Fig. 3D), was also reduced following AAV‐mediated delivery of the FGF19 and M70 transgenes. The pronounced liver fibrosis in Mdr2 −/− mice was accompanied by an increased number of α‐smooth muscle actin‐positive periductal myofibroblasts and significant increases in the hepatic levels of α‐smooth muscle actin mRNA. In contrast, mRNA levels of α‐smooth muscle actin and Vimentin, markers of activated myofibroblasts, were reduced or normalized in Mdr2 −/− mice expressing either FGF19 or M70 (Supporting Fig. S3). These results were further confirmed by the reduced hepatic hydroxyproline content in the livers of FGF19‐treated or M70‐treated animals (Fig. 3E).
Figure 3.
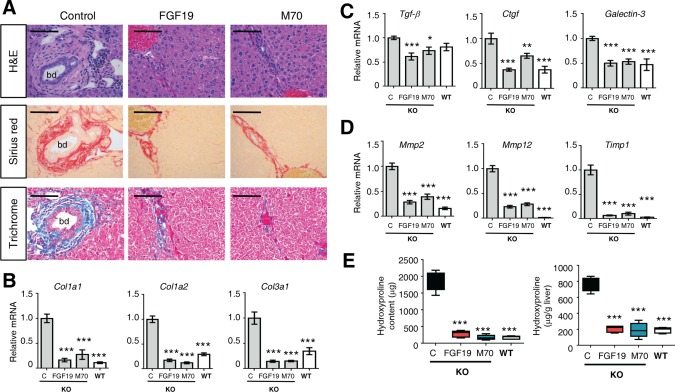
FGF19 and M70 exhibit antifibrotic effects in Mdr2 −/− mice. Twelve‐week old Mdr2−/− mice were injected with AAV carrying FGF19, M70, or a control gene (n = 5 per group). Hepatic fibrosis was assessed 24 weeks after AAV administration. Mdr2+/+ mice (wild type) were included as comparators for Mdr2−/− mice (knockout) in gene expression analysis. (A) Representative images of liver stained with hematoxylin and eosin, sirius red, or trichrome. Livers from Mdr2 −/− mice treated with control virus show characteristic “onion skin” morphology with a pronounced periductal fibrotic ring. Periductal collagen fibers appear red in sirius red staining and blue in trichrome staining. Scale bars = 100 μm. (B) mRNA levels of collagens (n = 5). (C) mRNA levels of profibrotic genes (n = 5). (D) mRNA levels of genes in tissue remodeling (n = 5). (E) Hepatic hydroxyproline content (n = 5). Values are mean ± standard error of the mean. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group by one‐way analysis of variance. Abbreviations: bd, bile duct; C, control; H&E, hematoxylin and eosin; KO, knockout; WT, wild type.
Taken together, these results indicate that the expression of FGF19 and M70 in Mdr2 −/− mice markedly reduces the hepatic infiltration of inflammatory cells, the induction of proinflammatory cytokines, and the activation of periductal myofibroblasts, leading to the effective resolution of hepatic fibrosis and sclerosing cholangitis observed in this model.
FGF19 and M70 Inhibit Bile Acid Synthesis and Regulate the Expression of Key Genes Implicated in Bile Acid Homeostasis in Mdr2 −/− Mice
To gain further insights into mechanisms leading to the reversal of chronic liver injury and cholangiopathy by FGF19 and M70 in Mdr2 −/− mice, we examined the effects of these hormones on the hepatic expression of genes encoding key enzymes in the bile acid synthetic pathways. The expression of Cyp7a1, which catalyzes the first and rate‐limiting step in the classic bile acid synthetic pathway,24 is highly regulated through a negative feedback mechanism mediated by FGF19.37, 38 The mRNA levels of Cyp7a1 were markedly suppressed by FGF19 and M70 in Mdr2 −/− mice (87% and 70% reduction by FGF19 and M70, respectively; n = 5, P < 0.001; Fig. 4A). In contrast, mRNA levels of Cyp8b1, a key enzyme controlling the synthesis of cholic acid, were not significantly different in Mdr2 −/− mice expressing the FGF19, M70, or control transgene. Hepatic expression of Cyp27a1, which catalyzes the initial step of the alternative bile acid biosynthetic pathway, was also significantly suppressed by FGF19 and M70 (41% and 40% reduction by FGF19 and M70, respectively; n = 5, P < 0.001, Fig. 4B), whereas mRNA levels of Cyp7b1 were not affected.
Figure 4.
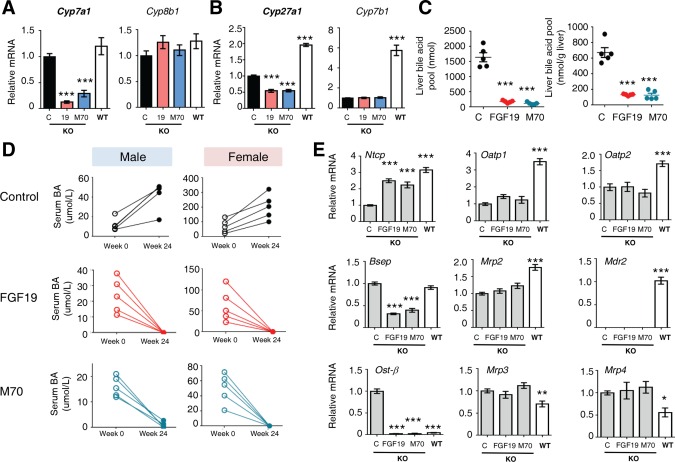
FGF19 and M70 inhibit bile acid synthesis and regulate key genes in bile acid homeostasis in Mdr2 −/− mice. Twelve‐week‐old Mdr2−/− mice were injected with AAV carrying FGF19, M70, or a control gene (n = 5 per group). Hepatic gene expression was determined 24 weeks after AAV administration. Mdr2+/+ mice (wild type) were included as comparators for Mdr2−/− mice (knockout) in gene expression analysis. (A) mRNA levels of bile acid synthetic enzymes in the classic pathway (n = 5). (B) mRNA levels of bile acid synthetic enzymes in the alternate pathway (n = 5). (C) Hepatic bile acid pool (n = 5). (D) Serum levels of total bile acids. (E) Expression profiles of bile acid uptake, canalicular efflux, and basolateral efflux transporters as measured by qRT‐PCR (n = 5). Values are mean ± standard error of the mean. ***P < 0.001 versus control group by one‐way analysis of variance. Abbreviations: BA, bile acid; C, control; KO, knockout; WT, wild type.
Importantly, expression of either FGF19 or M70 dramatically reduced the hepatic pool of bile acids in these mice compared with the severely elevated levels measured in livers isolated from Mdr2 −/− mice expressing a control transgene (Fig. 4C). The dysregulated bile acid metabolism resulting from Mdr2 deficiency leads to elevated levels of bile acids in the serum of these mice, which continue to rise as the animals age and the disease progresses (Fig. 4D). Consistent with the observations regarding the increased serum levels of liver enzymes, female Mdr2 −/− mice appear to manifest more severe disease symptoms, as evidenced by higher baseline serum levels of total bile acids and more pronounced progression over time compared with their male counterparts in the study. Strikingly, the elevated serum levels of total bile acids associated with the Mdr2 deficiency in these mice were completely normalized in both male and female mice expressing FGF19 or M70 (Fig. 4D).
In addition to the regulated synthesis of bile acids, a variety of hepatic membrane transporters play critical roles in preserving bile acid homeostasis.39 Disruption of the Mdr2 transporter in Mdr2 −/− mice markedly disturbs bile acid homeostasis in these animals, leading to severe conditions of cholestasis and exacerbated bile acid–related liver damage.40 To determine whether changes in bile acid transport contribute to the beneficial effects of ectopic FGF19 and M70 expression in Mdr2 −/− mice, we examined the mRNA levels of key bile acid transporters. Neither FGF19 nor M70 inhibited expression of the hepatocellular uptake transporters (Ntcp, Oatp1, and Oatp2) or induced expression of the canalicular (Bsep, Mrp2, and Mdr2; note the lack of Mdr2 expression in these mice) or basolateral (Ost‐β, Mrp3, and Mrp4) efflux pumps (Fig. 4E). Based on these observations, adaptive changes in the expression of hepatic bile acid transporters do not account for the restoration of bile acid homeostasis and the normalization of hepatic and serum bile acid levels in response to FGF19 or M70.
Collectively, these data strongly suggest that inhibition of de novo bile acid synthesis by FGF19 and M70, rather than induction of adaptive responses, limits hepatic accumulation of toxic bile and leads to improvement in liver health in Mdr2 −/− mice.
FGF19 and M70 Restore Lipid Homeostasis in Mdr2 −/− Mice
Mdr2 −/− mice display multiple derangements of lipid homeostasis,41 including alterations in cholesterol and triglyceride metabolism, that closely resemble clinical observations in PFIC3 patients.42, 43 For example, Mdr2 −/− mice exhibit lower serum levels of total cholesterol compared with wild‐type mice. Strikingly, treatment with AAV‐FGF19 and AAV‐M70 restored serum cholesterol to normal levels 24 weeks after injection (Fig. 5A). Furthermore, gene expression analysis by qRT‐PCR revealed that mRNA levels of Srebf2, the master regulator of cholesterol synthesis, and Hmgcr, the rate‐limiting cholesterol biosynthetic enzyme, were significantly reduced following FGF19 or M70 treatment (Fig. 5B). On the other hand, hepatic expression of Ldlr was modestly reduced in mice treated with FGF19 or M70, which may at least partially contribute to the increase in serum cholesterol in these study groups. Interestingly, the mRNAs for Abcg5 and Abcg8, efflux pumps responsible for transporting cholesterol out of the liver, were increased in animals expressing the FGF19 or M70 transgene (Fig. 5C). No significant differences in Scarb1 mRNA levels were observed in livers isolated from mice in the various treatment groups. Collectively, these data indicate that multiple genes involved in cholesterol metabolism and transport are regulated in response to FGF19 and M70 to reduce hepatic cholesterol synthesis and enhance cholesterol efflux.
Figure 5.
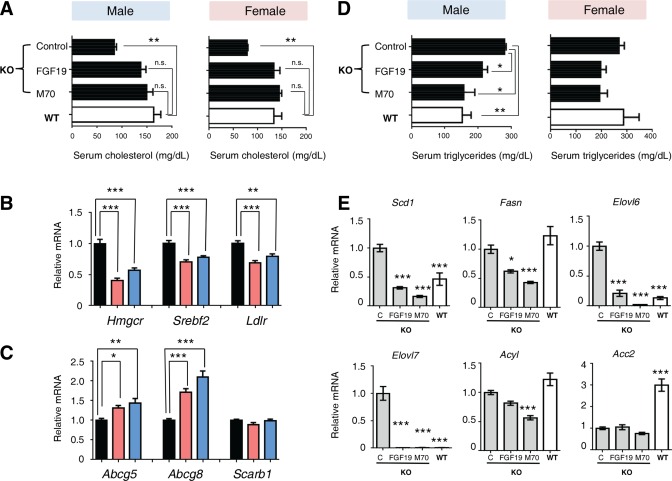
FGF19 and M70 restore lipid homeostasis in Mdr2 −/− mice. Twelve‐week‐old Mdr2−/− mice were injected with AAV carrying FGF19, M70, or a control gene (n = 5 per sex per group). Serum concentrations of triglycerides and cholesterol and hepatic gene expression were determined 24 weeks after AAV administration. Mdr2+/+ mice (wild type) were included as comparators for Mdr2−/− mice (knockout) in gene expression analysis. (A) Serum levels of total cholesterol (n = 5). (B) Expression of key genes involved in cholesterol synthesis and clearance (n = 5). (C) Expression of cholesterol efflux pumps (n = 5). (D) Serum levels of triglycerides (n = 5). (E) Hepatic mRNA levels of genes involved in lipogenesis (n = 5). Values are mean ± standard error of the mean. *P < 0.05, **P < 0.01, ***P < 0.001 versus control group by one‐way analysis of variance. Unpaired, two‐tailed t test was used to compare two groups. Abbreviations: C, control; KO, knockout; WT, wild type.
Contrary to the effects on circulating cholesterol levels, serum triglycerides were reduced by administration of AAV‐FGF19 or AAV‐M70 in Mdr2 −/− mice (Fig. 5D). Consistent with reductions in hepatic triglyceride synthesis, we observed a dramatic decrease in hepatic expression of key regulators of lipogenesis, including Scd1, Fasn, Elovl6, Elovl7, Acly, and Acacb, in Mdr2 −/− mice expressing either the FGF19 or M70 transgenes (Fig. 5E).
These data suggest that the endocrine hormones FGF19 and M70 act to restore lipid homeostasis in Mdr2 −/− mice by mediating the transcriptional regulation of genes implicated in the efflux of cholesterol from the liver, as well as genes involved in the hepatic synthesis of cholesterol and triglycerides.
FGF19 and M70 Reduce Cholecystolithiasis in Mdr2 −/− Mice
To determine whether FGF19 and M70 affect bile composition, we performed a cholecystectomy on Mdr2−/− mice 12 weeks after injection of AAV‐encoded transgenes and examined gallbladder bile (Fig. 6A,B). Both FGF19 and M70 markedly reduced gallbladder bile volume in Mdr2−/− mice (Fig. 6C). Gallbladder bile in Mdr2−/− mice contains only trace amounts of phospholipids due to disruption of the Mdr2 phospholipid flippase, and this low level of phospholipids was not affected by FGF19 or M70 expression (Fig. 6D). In contrast, concentrations of total bile acids in gallbladder bile were significantly reduced by either FGF19 or M70 treatment (Fig. 6D).
Figure 6.
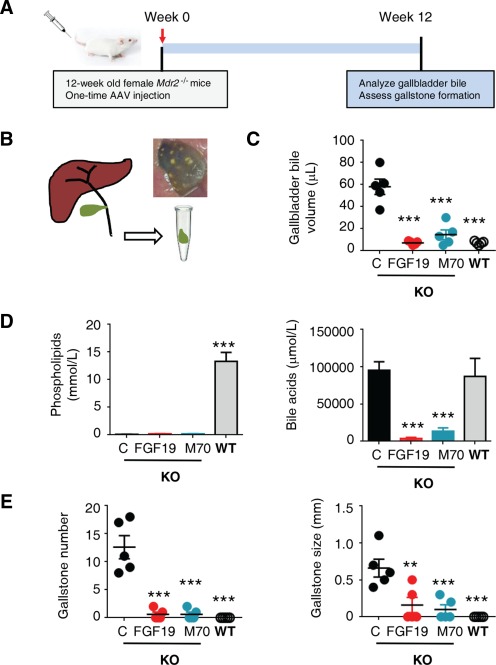
FGF19 and M70 reduce cholecystolithiasis in Mdr2 −/− mice. (A) Study design. Twelve‐week‐old female Mdr2−/− mice were injected with AAV carrying FGF19, M70, or a control gene (n = 5 per group). Gallbladders were harvested 12 weeks after AAV administration after overnight fast. (B) Illustration of cholecystectomy and a representative photo of gallbladder from a 24‐week‐old female Mdr2−/− mouse with gallstones. (C) Gallbladder bile volume (n = 5). Mdr2+/+ mice (wild type) were included as comparators for Mdr2−/− mice (knockout). (D) Concentrations of phospholipids and total bile acids in gallbladder bile (n = 5). (E) Gallstone numbers and maximum diameters (n = 5). Values are mean ± standard error of the mean. **P < 0.01, ***P < 0.001 versus control group by one‐way analysis of variance. Abbreviations: C, control; KO, knockout; WT, wild type.
Similar to human low phospholipid‐associated cholelithiasis patients with MDR3 defects, Mdr2−/− mice develop gallbladder stones over time, with female mice displaying a markedly higher susceptibility to gallstone formation.44 Multiple gallstones were observed in the gallbladders from 24‐week‐old female Mdr2−/− mice (Fig. 6E), whereas treatment with FGF19 or M70 reduced both the number and the size of gallstones in these mice (Fig. 6E).
These data suggest that expression of FGF19 and M70 decreases biliary bile acids without altering levels of phospholipids in the bile and reduces the formation of gallstones associated with Mdr2 deficiency.
FGF19, but not M70, Induces Hepatocellular Carcinogenesis in Mdr2−/− Mice
Despite impressive hepatoprotective effects in the weeks immediately following gene delivery, prolonged ectopic overexpression of FGF19 also drives the development of liver tumors in Mdr2 −/− mice (Fig. 7A,B). In contrast, mice injected with AAV‐M70 remained tumor‐free for the duration of the experiment. Importantly, whereas all liver tumors in FGF19‐expressing mice stained positive for glutamine synthetase, a marker of pericentral hepatocytes (Fig. 7C), increased staining of glutamine synthetase was not observed in Mdr2 −/− mice expressing M70. The FGF19‐induced liver tumors displayed macroscopic and microscopic characteristics typical of HCC. HCC tumor area occupied 69%, 0%, and 0% of the entire liver area in Mdr2 −/− mice injected with recombinant AAV encoding FGF19, M70, or the control gene, respectively (Fig. 7D). Increased hepatic expression of α‐fetoprotein (Afp) and glypican‐3 (Gpc3), markers often increased in human HCC, was induced by AAV‐FGF19 but not AAV‐M70 (Fig. 7E). Moreover, mRNA levels of cyclins involved in mitosis and cell cycle progression, including cyclin a2 (Ccna2), Ccnb1, and Ccnb2, were significantly elevated in the livers expressing FGF19 but not M70 (Fig. 7F).
Figure 7.
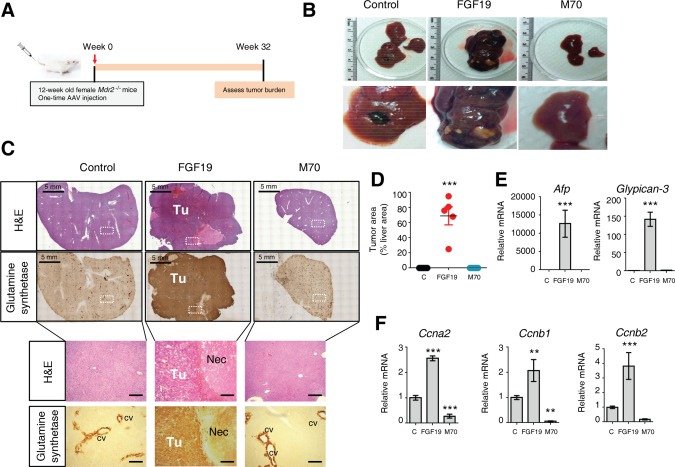
FGF19, but not M70, induces hepatocellular carcinoma in Mdr2−/− mice. (A) Study design. Twelve‐week‐old Mdr2−/− mice were injected with AAV carrying FGF19, M70, or a control gene (n = 5 per group). Liver histology and gene expression were determined 32 weeks after AAV administration. (B) Macroscopic appearance of livers. (C) Representative hematoxylin and eosin and anti‐glutamine synthetase staining of livers. White boxes denote area showing at higher magnifications below. 3,3′‐Diaminobenzidine substrates were used for anti‐glutamine synthetase immunostaining (brown color). The necrotic area in liver tumors induced by AAV‐FGF19 is indicated. Scale bars = 5 mm (top panels) and 100 μm (bottom panels). (D) Morphometric quantification of glutamine synthetase‐positive tumor areas (n = 5). (E) mRNA levels of key genes implicated in hepatocarcinogenesis (n = 5). (F) mRNA levels of Cyclins (n = 5). Values are mean ± standard error of the mean. **P < 0.01, ***P < 0.001 versus control group by one‐way analysis of variance. Abbreviations: C, control; cv, central vein; H&E, hematoxylin and eosin; Nec, necrotic area; Tu, tumor.
In summary, we used intravenous delivery of AAV‐FGF19 in Mdr2 −/− mice to generate a mouse model of HCC in the context of severe cholangiopathy. The resulting tumors display macroscopic, histopathologic, and molecular features characteristic of HCC. Notably, unlike FGF19, expression of M70 does not promote the formation of hepatic tumors in this model.
M70 Exhibits Antiproliferative Effects in Mdr2−/− Mice
Closely resembling the histopathological aspects of the disease observed in patients with PSC, PFIC3, and other cholangiopathies,6 ductular proliferation in Mdr2 −/− mice resulted in extensive portal expansion as the disease progressed. We evaluated cholangiocyte proliferation by Ki‐67 staining. Strikingly, Mdr2 −/− mice expressing M70 displayed significantly decreased numbers of proliferating cholangiocytes compared with mice receiving a control virus (Fig. 8A). Morphometric analysis of the bile duct area, as revealed by Dolichos biflorus agglutinin‐fluorescein and hematoxylin and eosin staining, showed a marked reduction in bile duct mass in M70‐treated mice (Fig. 8B; Supporting Fig. S4A). Furthermore, mRNA levels of a ductal marker, cytokeratin‐19, were also reduced in mice expressing M70.
Figure 8.
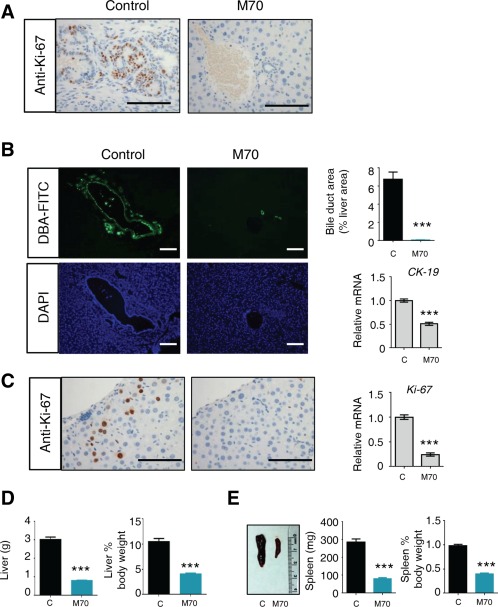
M70 reduces ductular and hepatocellular proliferation in Mdr2−/− mice. Twelve‐week‐old Mdr2−/− mice received a single dose of AAV carrying M70 or a control gene (n = 5 per group). Liver histology and gene expression were determined 24 weeks after AAV administration. (A) Representative Ki67 immunostaining of bile ducts, quantification of bile duct mass (n = 5), and qRT‐PCR analysis of ductal marker cytokeratin‐19 (n = 5). 3,3′‐Diaminobenzidine substrates were used with anti‐Ki67 staining (brown color). Scale bars = 100 μm. (B) Representative images of the bile ducts. Bile ducts were stained with fluorescein‐labeled Dolichos biflorus agglutinin. Green (fluorescein) and blue (4′,6‐diamidino‐2‐phenylindole for nuclear counterstain) channel images are shown. The pronounced bile duct proliferation and biliary tract expansion in Mdr2 −/− mice were reversed by M70 treatment. Scale bars = 100 μm. (C) M70 inhibits proliferation of hepatocytes as shown by reduced Ki‐67 staining (brown color) and Ki‐67 mRNA levels (n = 5). Scale bars = 100 μm. (D) Liver weight and ratios of liver‐to‐body weight (n = 5). (E) Spleen photo, spleen weight, and ratios of spleen‐to‐body weight (n = 5). Values are mean ± standard error of the mean. ***P < 0.001 versus control group by unpaired, two‐tailed t test. Abbreviations: C, control; DAPI, 4′,6‐diamidino‐2‐phenylindole; DBA, Dolichos biflorus agglutinin.
Moreover, Mdr2 −/− mice treated with AAV‐M70 showed a clear reduction of proliferating (Ki‐67‐positive) hepatocytes compared with those receiving a control virus (Fig. 8C). Evidence of advanced liver disease commonly associated with Mdr2 deficiency, including cytological atypia and dysplasia, architectural disorganization, and aberrant mitotic figures, were markedly improved in the livers of mice expressing M70 (Supporting Fig. S4B). Consistent with reductions in both proliferating cholangiocytes and hepatocytes, M70‐treated Mdr2 −/− mice exhibited normal liver weight, as well as liver‐to‐body weight ratios (Fig. 8D), suggesting the reversal of the hepatomegaly commonly observed in Mdr2 −/− mice as well as in patients with cholangiopathy.45 In conjunction with reductions in liver weight, Mdr2 −/− mice treated with M70 also showed improvement in splenomegaly (Fig. 8E), another feature clinically associated with portal hypertension and cirrhosis.
In summary, M70 exerts antiproliferative effects in the context of sclerosing cholangitis and appears to reverse hepatosplenomegaly in Mdr2 −/− mice. These results indicate that M70 treatment may confer additional protective benefits, in addition to anti‐inflammatory and antifibrotic effects.
Discussion
We report here that, in a mouse model of chronic cholangiopathy AAV‐mediated delivery of FGF19, or engineered analogue M70, provides effective, long‐term improvement of liver injury, hepatic inflammation, and fibrosis. Both FGF19 and M70 profoundly inhibit de novo bile acid synthesis, resulting in significant reduction in liver damage without induction of adaptive responses in membrane transporters. Furthermore, we show that FGF19 and M70 restore hepatic and systemic lipid homeostasis and reduce cholecystolithiasis. Importantly, in striking contrast to FGF19, M70 does not induce HCC formation, even after prolonged exposure, and further corrects ductular proliferation and hepatosplenomegaly in these mice. Thus, we have established an in vivo system for evaluating the tumorigenic effect of FGF19 in the context of chronic cholangiopathy and demonstrate that the engineered, nontumorigenic FGF19 variant M70 reverses sclerosing cholangitis in this mouse model with genetic, clinical, and phenotypic features that closely resemble human diseases.
As an endocrine hormone, FGF19 plays a key role in the regulation of hepatic bile acid metabolism under physiological conditions. Predominantly expressed in the ileum, FGF19 is released into portal circulation as part of the feedback mechanism to suppress bile acid synthesis in the liver. Hepatocytes are the only cell type in the liver that expresses the FGF19 receptor complex FGFR4‐βKlotho.46 Therefore, the anti‐inflammatory and antifibrosis effects of FGF19 and M70 likely result from direct targeting of hepatocytes. Concentrations of FGF19 increase under cholestatic and cirrhotic conditions,47 suggesting that FGF19 itself represents a component of the adaptive hepatic response to these pathophysiological circumstances. Seemingly paradoxically, we show here that, while providing important hepatoprotective benefits, prolonged exposure to FGF19 at circulating levels as low as 20 ng/mL strongly promotes HCC formation in the Mdr2 −/− model. Thus, FGF19 acts as a “double‐edged sword” that, on the one hand, serves as part of an adaptive response to limit liver injury and, on the other hand, may play a causal role in tumor promotion and contribute to the increased HCC risk in patients with chronic liver diseases. Nontumorigenic FGF19 variants, such as M70, provide a novel approach to capitalize on our knowledge of this pathway for potential therapeutic use.
Enhanced transcription of MDR3 by experimental drugs such as farnesoid X receptor and peroxisome proliferator‐activated receptor α agonists contributes, at least in part, to the proposed mechanism of action of such compounds.48, 49 An understanding of the allelic variation and polymorphism of MDR3, as well as how MDR3 interacts with other genes to affect individual susceptibility to adult‐onset liver disease, is only just beginning to emerge. Despite tremendous progress in understanding the molecular basis of cholangiopathy in MDR3‐related genetic disorders, effective treatments are still lacking. To date, there are no drugs approved by the US Food and Drug Administration, or other regulators, for treating cholangiopathies, including PSC and PFIC3. UDCA, a hydrophilic bile acid, is thought to reduce liver injury by promoting choleresis, diluting and displacing toxic bile acids, and inducing the bile acid detoxification and export systems. Previous studies have shown that when administered in the diet for 4 weeks, UDCA only slightly decreases periductal fibrosis but does not repair liver injury in Mdr2 −/− mice.50 Administration of farnesoid X receptor agonist INT‐747 aggravated liver damage in this model, presumably resulting from the induction of bile acid efflux transporters in the context of biliary strictures.51, 52 None of these studies examined the effects on cholecystolithiasis in Mdr2−/− mice. In contrast, M70, acting through a novel mechanism by suppression of bile acid synthesis without promoting bile acid efflux, effectively resolves periductal fibrosis and sclerosing cholangitis in Mdr2−/− mice and appears significantly more effective than UDCA or farnesoid X receptor agonists in this setting. Previous studies describing therapeutic agents in the Mdr2 −/− mouse model often start treatment in 8‐week‐old male mice, and with studies using female mice (which develop much more severe liver disease) mostly nonexistent, the efficacy of M70 in this setting is particularly notable. Additionally, the therapeutic approach described in this report requires only a single intravenous injection of AAV carrying M70 to achieve the sustained efficacy, a property especially attractive for treating genetic disorders where lifelong intervention is required.
The increase in serum cholesterol by FGF19 and M70 in the Mdr2 −/− mouse model is of particular interest because low serum levels of cholesterol have been observed in patients with MDR3 mutations and in Mdr2 −/− mice.41, 42, 43 Both FGF19 and M70 act to reduce hepatic synthesis of cholesterol and triglyceride and promote cholesterol efflux from the liver. These lipid regulatory mechanisms may further contribute to the beneficial effects ascribed to FGF19 and M70 expression.
In humans, high concentrations of FGF19 are detected in the gallbladder bile (>20 ng/mL, comparing with ∼0.2 ng/mL in plasma).53 High levels of FGF19 mRNA are found in the gallbladders of mice expressing an FGF19 transgene, including regulatory sequences, from a human genomic BAC clone.54 Notably, the induction of FGF19 transcripts in human liver during cholestasis was shown to originate in the nonparenchymal cells rather than hepatocytes.54 Our current study does not exclude the intriguing possibility that FGF19 in the bile could have a direct, cytochrome P450 7A1‐independent, protective effect on cholangiocytes. Previous studies also suggested a direct action of FGF19 on gallbladder motility.55 The mechanism underlying improvement in cholecystolithiasis by FGF19 and M70 in Mdr2−/− mice, particularly in the context of reducing biliary bile acids without altering biliary phospholipids, should be an area of future investigation.
In summary, we find compelling evidence that M70, an engineered analogue of the gut hormone FGF19, possesses profound anti‐inflammatory, antifibrotic, and hepatoprotective activities in mice exhibiting chronic cholangiopathy. Together with previous work, our studies support a unifying model that the suppression of de novo bile acid synthesis by FGF19 and analogues protects not only hepatocytes but also cholangiocytes from toxic bile‐induced injury. These findings have broadened concepts regarding the role of bile acids in the initiation and progression of cholangiopathy and expanded our ability to potentially devise precision medicines for patients with MDR3 and/or phospholipid deficiency.
Author names in bold denote shared co‐first authorship.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.28257/suppinfo.
Supporting Information
Acknowledgment
We thank Dr. J.L. Chen for advice and insightful discussions. We thank H. Yang, M. Chen, M. Humphrey, and H. Galon‐Tilleman for technical assistance and the NGM vivarium staff for the care of animals used in the studies.
Potential conflict of interest: Dr. Zhou is employed by and owns stock in NGM. Dr. Learned is employed by and owns stock in NGM. Dr. Rossi is employed by and owns stock in NGM. Dr. DePaoli is employed by and owns stock in NGM. Dr. Tian is employed by and owns stock in NGM. Dr. Ling is employed by and owns stock in NGM.
Supported by NGM Biopharmaceuticals, Inc.
References
- 1. Lazaridis KN, LaRusso NF. The cholangiopathies. Mayo Clin Proc 2015;90:791‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, et al. A gene encoding a liver‐specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet 1998;20:233‐238. [DOI] [PubMed] [Google Scholar]
- 3. Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, et al. A gene encoding a P‐type ATPase mutated in two forms of hereditary cholestasis. Nat Genet 1998;18:219‐224. [DOI] [PubMed] [Google Scholar]
- 4. Oude Elferink RP, Ottenhoff R, van Wijland M, Smit JJ, Schinkel AH, Groen AK. Regulation of biliary lipid secretion by mdr2 P‐glycoprotein in the mouse. J Clin Invest 1995;95:31‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davit‐Spraul A, Gonzales E, Baussan C, Jacquemin E. The spectrum of liver diseases related to ABCB4 gene mutations: pathophysiology and clinical aspects. Semin Liver Dis 2010;30:134‐146. [DOI] [PubMed] [Google Scholar]
- 6. de Vree JM, Jacquemin E, Sturm E, Cresteil D, Bosma PJ, Aten J, et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci USA 1998;95:282‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Degiorgio D, Colombo C, Seia M, Porcaro L, Costantino L, Zazzeron L, et al. Molecular characterization and structural implications of 25 new ABCB4 mutations in progressive familial intrahepatic cholestasis type 3 (PFIC3). Eur J Hum Genet 2007;15:1230‐1238. [DOI] [PubMed] [Google Scholar]
- 8. Jacquemin E, Cresteil D, Manouvrier S, Boute O, Hadchouel M. Heterozygous non‐sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet 1999;353:210‐211. [DOI] [PubMed] [Google Scholar]
- 9. Dixon PH, Weerasekera N, Linton KJ, Donaldson O, Chambers J, Egginton E, et al. Heterozygous MDR3 missense mutation associated with intrahepatic cholestasis of pregnancy: evidence for a defect in protein trafficking. Hum Mol Genet 2000;9:1209‐1217. [DOI] [PubMed] [Google Scholar]
- 10. Rosmorduc O, Hermelin B, Poupon R. MDR3 gene defect in adults with symptomatic intrahepatic and gallbladder cholesterol cholelithiasis. Gastroenterology 2001;120:1459‐1467. [DOI] [PubMed] [Google Scholar]
- 11. Pasmant E, Goussard P, Baranes L, Laurendeau I, Quentin S, Ponsot P, et al. First description of ABCB4 gene deletions in familial low phospholipid‐associated cholelithiasis and oral contraceptives‐induced cholestasis. Eur J Hum Genet 2012;20:277‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ziol M, Barbu V, Rosmorduc O, Frassati‐Biaggi A, Barget N, Hermelin B, et al. ABCB4 heterozygous gene mutations associated with fibrosing cholestatic liver disease in adults. Gastroenterology 2008;135:131‐141. [DOI] [PubMed] [Google Scholar]
- 13. Lucena JF, Herrero JI, Quiroga J, Sangro B, Garcia‐Foncillas J, Zabalegui N, et al. A multidrug resistance 3 gene mutation causing cholelithiasis, cholestasis of pregnancy, and adulthood biliary cirrhosis. Gastroenterology 2003;124:1037‐1042. [DOI] [PubMed] [Google Scholar]
- 14. Smit JJ, Schinkel AH, Oude Elferink RP, Groen AK, Wagenaar E, van Deemter L, et al. Homozygous disruption of the murine mdr2 P‐glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 1993;75:451‐462. [DOI] [PubMed] [Google Scholar]
- 15. Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology 2004;127:261‐274. [DOI] [PubMed] [Google Scholar]
- 16. Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med 1995;332:924‐933. [DOI] [PubMed] [Google Scholar]
- 17. Jacquemin E, De Vree JM, Cresteil D, Sokal EM, Sturm E, Dumont M, et al. The wide spectrum of multidrug resistance 3 deficiency: from neonatal cholestasis to cirrhosis of adulthood. Gastroenterology 2001;120:1448‐1458. [DOI] [PubMed] [Google Scholar]
- 18. Lindor KD. Ursodiol for primary sclerosing cholangitis. Mayo Primary Sclerosing Cholangitis‐Ursodeoxycholic Acid Study Group. N Engl J Med 1997;336:691‐695. [DOI] [PubMed] [Google Scholar]
- 19. Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, et al. High‐dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology 2009;50:808‐814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Olsson R, Boberg KM, de Muckadell OS, Lindgren S, Hultcrantz R, Folvik G, et al. High‐dose ursodeoxycholic acid in primary sclerosing cholangitis: a 5‐year multicenter, randomized, controlled study. Gastroenterology 2005;129:1464‐1472. [DOI] [PubMed] [Google Scholar]
- 21. Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev 2012;26:312‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Angelin B, Larsson TE, Rudling M. Circulating fibroblast growth factors as metabolic regulators—a critical appraisal. Cell Metab 2012;16:693‐705. [DOI] [PubMed] [Google Scholar]
- 23. Cicione C, Degirolamo C, Moschetta A. Emerging role of fibroblast growth factors 15/19 and 21 as metabolic integrators in the liver. Hepatology 2012;56:2404‐2411. [DOI] [PubMed] [Google Scholar]
- 24. Russell DW. Fifty years of advances in bile acid synthesis and metabolism. J Lipid Res 2009;50(Suppl.):S120‐S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nicholes K, Guillet S, Tomlinson E, Hillan K, Wright B, Frantz GD, et al. A mouse model of hepatocellular carcinoma: ectopic expression of fibroblast growth factor 19 in skeletal muscle of transgenic mice. Am J Pathol 2002;160:2295‐2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sawey ET, Chanrion M, Cai C, Wu G, Zhang J, Zender L, et al. Identification of a therapeutic strategy targeting amplified FGF19 in liver cancer by oncogenomic screening. Cancer Cell 2011;19:347‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahn SM, Jang SJ, Shim JH, Kim D, Hong SM, Sung CO, et al. Genomic portrait of resectable hepatocellular carcinomas: implications of RB1 and FGF19 aberrations for patient stratification. Hepatology 2014;60:1972‐1982. [DOI] [PubMed] [Google Scholar]
- 28. Hyeon J, Ahn S, Lee JJ, Song DH, Park CK. Expression of fibroblast growth factor 19 is associated with recurrence and poor prognosis of hepatocellular carcinoma. Dig Dis Sci 2013;58:1916‐1922. [DOI] [PubMed] [Google Scholar]
- 29. Uriarte I, Fernandez‐Barrena MG, Monte MJ, Latasa MU, Chang HC, Carotti S, et al. Identification of fibroblast growth factor 15 as a novel mediator of liver regeneration and its application in the prevention of post‐resection liver failure in mice. Gut 2013;62:899‐910. [DOI] [PubMed] [Google Scholar]
- 30. Uriarte I, Latasa MU, Carotti S, Fernandez‐Barrena MG, Garcia‐Irigoyen O, Elizalde M, et al. Ileal FGF15 contributes to fibrosis‐associated hepatocellular carcinoma development. Int J Cancer 2015;136:2469‐2475. [DOI] [PubMed] [Google Scholar]
- 31. Zhou M, Wang X, Phung V, Lindhout DA, Mondal K, Hsu JY, et al. Separating tumorigenicity from bile acid regulatory activity for endocrine hormone FGF19. Cancer Res 2014;74:3306‐3316. [DOI] [PubMed] [Google Scholar]
- 32. Luo J, Ko B, Elliott M, Zhou M, Lindhout DA, Phung V, et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med 2014;6:247ra100. [DOI] [PubMed] [Google Scholar]
- 33. Zhang H, Xie J, Xie Q, Wilson JM, Gao G. Adenovirus‐adeno‐associated virus hybrid for large‐scale recombinant adeno‐associated virus production. Hum Gene Ther 2009;20:922‐929. [DOI] [PubMed] [Google Scholar]
- 34. Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno‐associated virus vectors. J Virol 2002;76:4580‐4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rivera VM, Ye X, Courage NL, Sachar J, Cerasoli F Jr, Wilson JM, et al. Long‐term regulated expression of growth hormone in mice after intramuscular gene transfer. Proc Natl Acad Sci USA 1999;96:8657‐8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tomlinson E, Fu L, John L, Hultgren B, Huang X, Renz M, et al. Transgenic mice expressing human fibroblast growth factor‐19 display increased metabolic rate and decreased adiposity. Endocrinology 2002;143:1741‐1747. [DOI] [PubMed] [Google Scholar]
- 37. Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2005;2:217‐225. [DOI] [PubMed] [Google Scholar]
- 38. Holt JA, Luo G, Billin AN, Bisi J, McNeill YY, Kozarsky KF, et al. Definition of a novel growth factor‐dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev 2003;17:1581‐1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Oude Elferink RP, Jansen PL. The role of the canalicular multispecific organic anion transporter in the disposal of endo‐ and xenobiotics. Pharmacol Ther 1994;64:77‐97. [DOI] [PubMed] [Google Scholar]
- 40. Cai SY, Mennone A, Soroka CJ, Boyer JL. Altered expression and function of canalicular transporters during early development of cholestatic liver injury in Abcb4‐deficient mice. Am J Physiol Gastrointest Liver Physiol 2014;306:G670‐G676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moustafa T, Fickert P, Magnes C, Guelly C, Thueringer A, Frank S, et al. Alterations in lipid metabolism mediate inflammation, fibrosis, and proliferation in a mouse model of chronic cholestatic liver injury. Gastroenterology 2012;142:140‐151. [DOI] [PubMed] [Google Scholar]
- 42. Acalovschi M, Tirziu S, Chiorean E, Krawczyk M, Grunhage F, Lammert F. Common variants of ABCB4 and ABCB11 and plasma lipid levels: a study in sib pairs with gallstones, and controls. Lipids 2009;44:521‐526. [DOI] [PubMed] [Google Scholar]
- 43. Voshol PJ, Havinga R, Wolters H, Ottenhoff R, Princen HM, Oude Elferink RP, et al. Reduced plasma cholesterol and increased fecal sterol loss in multidrug resistance gene 2 P‐glycoprotein‐deficient mice. Gastroenterology 1998;114:1024‐1034. [DOI] [PubMed] [Google Scholar]
- 44. Lammert F, Wang DQ, Hillebrandt S, Geier A, Fickert P, Trauner M, et al. Spontaneous cholecysto‐ and hepatolithiasis in Mdr2−/− mice: a model for low phospholipid‐associated cholelithiasis. Hepatology 2004;39:117‐128. [DOI] [PubMed] [Google Scholar]
- 45. Debray D, Pariente D, Urvoas E, Hadchouel M, Bernard O. Sclerosing cholangitis in children. J Pediatr 1994;124:49‐56. [DOI] [PubMed] [Google Scholar]
- 46. Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, et al. Research resource: Comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol 2010;24:2050‐2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schaap FG, van der Gaag NA, Gouma DJ, Jansen PL. High expression of the bile salt‐homeostatic hormone fibroblast growth factor 19 in the liver of patients with extrahepatic cholestasis. Hepatology 2009;49:1228‐1235. [DOI] [PubMed] [Google Scholar]
- 48. Huang L, Zhao A, Lew JL, Zhang T, Hrywna Y, Thompson JR, et al. Farnesoid X receptor activates transcription of the phospholipid pump MDR3. J Biol Chem 2003;278:51085‐51090. [DOI] [PubMed] [Google Scholar]
- 49. Ghonem NS, Ananthanarayanan M, Soroka CJ, Boyer JL. Peroxisome proliferator‐activated receptor alpha activates human multidrug resistance transporter 3/ATP‐binding cassette protein subfamily B4 transcription and increases rat biliary phosphatidylcholine secretion. Hepatology 2014;59:1030‐1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fickert P, Zollner G, Fuchsbichler A, Stumptner C, Weiglein AH, Lammert F, et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct‐ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology 2002;123:1238‐1251. [DOI] [PubMed] [Google Scholar]
- 51. Baghdasaryan A, Claudel T, Gumhold J, Silbert D, Adorini L, Roda A, et al. Dual farnesoid X receptor/TGR5 agonist INT‐767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO‐ 3 output. Hepatology 2011;54:1303‐1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 2000;102:731‐744. [DOI] [PubMed] [Google Scholar]
- 53. Zweers SJ, Booij KA, Komuta M, Roskams T, Gouma DJ, Jansen PL, et al. The human gallbladder secretes fibroblast growth factor 19 into bile: towards defining the role of fibroblast growth factor 19 in the enterobiliary tract. Hepatology 2012;55:575‐583. [DOI] [PubMed] [Google Scholar]
- 54. Naugler WE, Tarlow BD, Fedorov LM, Taylor M, Pelz C, Li B, et al. Fibroblast growth factor signaling controls liver size in mice with humanized livers. Gastroenterology 2015;149:728‐740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Choi M, Moschetta A, Bookout AL, Peng L, Umetani M, Holmstrom SR, et al. Identification of a hormonal basis for gallbladder filling. Nat Med 2006;12:1253‐1255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.28257/suppinfo.
Supporting Information


