Abstract
Ovarian sex cord-stromal tumors are clinically significant heterogeneous tumors that include several pathologic types. These tumors are often found in adolescents and young adults and can present with hormonal manifestations as well as signs and symptoms of a pelvic mass. Serum tumor markers may assist in preoperative diagnosis and surveillance. Several subtypes are associated with genetic predisposition, including those observed in patients with Peutz-Jegher syndrome. Recent studies have elucidated the relationship between Sertoli-Leydig cell tumors and DICER1 mutations. When classified as International Federation of Gynecology and Obstetrics stage Ia, most subtypes may be treated with surgery alone. Higher stage or recurrent tumors have variable prognoses that range from a usually rapid course in poorly differentiated Sertoli-Leydig cell tumor to an often prolonged course in adult granulosa cell tumors. New understanding of the molecular pathogenesis of these tumors may pave the way for novel therapeutics.
INTRODUCTION
Ovarian sex cord-stromal tumors are uncommon neoplasms that typically present in the first two to three decades of life, with the exception of adult granulosa cell tumors, which typically present later, with risk for development peaking at age 50 to 55 years. In aggregate, these tumors account for approximately 5% of ovarian malignancies in women age 15 to 24 years.1
CLINICAL PRESENTATION
As with other ovarian tumors, sex cord-stromal tumors usually present with the typical symptoms of an adnexal mass, including abdominal pain, distention, and, rarely, torsion. In contrast to epithelial and germ cell tumors, however, sex cord-stromal tumors frequently present with signs of hormonal production, such as hirsutism and virilization, menstrual changes, or precocious puberty.2 Initial evaluation should include a thorough history with careful attention to any individual or family history of possible tumor predisposition as well as physical examination with attention to presence of precocious puberty or delayed menarche, hyper pigmented macules that are suggestive of Peutz-Jegher, or thyroid nodules that are suggestive of DICER1 syndrome. Tumors are often large at diagnosis and may rupture, which can result in an acute presentation with hemoperitoneum.
PATHOLOGY
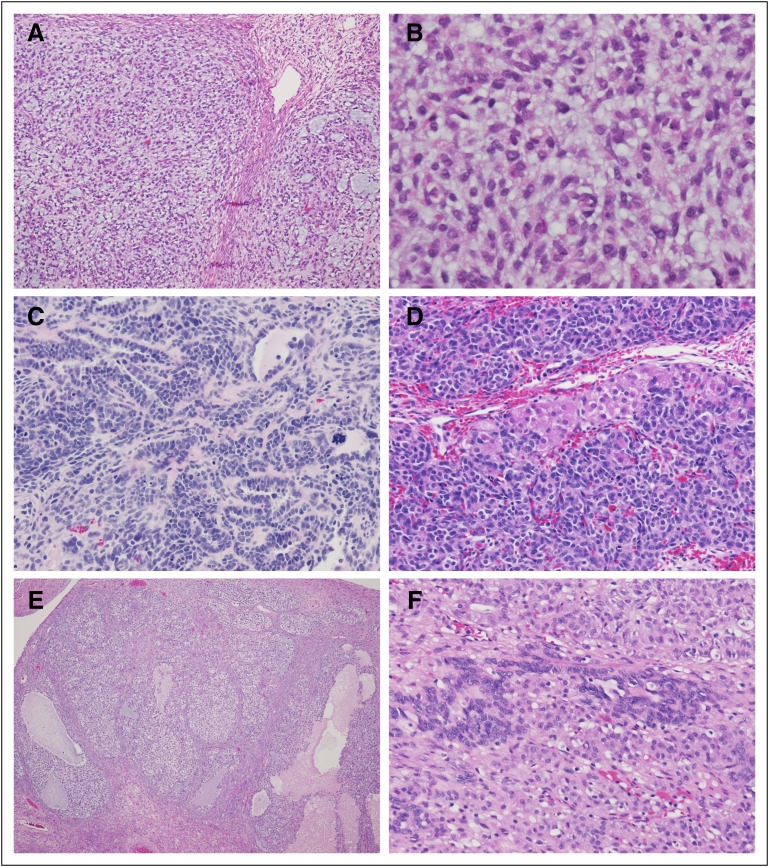
These tumors are typically unilateral, 10 to 15 cm in greatest dimension, and may vary from solid, firm, and lobulated to soft and friable, often with hemorrhage and/or necrosis. The following histologic subtypes are seen: adult granulosa cell tumor, juvenile granulosa cell tumor, Sertoli-Leydig cell tumor, sex cord tumor with annular tubules, and mixed forms, including gynandroblastoma. Figure 1 shows representative pathology images from juvenile granulosa cell tumor, Sertoli-Leydig cell tumors, and gynandroblastoma.
FIG 1.
Common histologic features of juvenile granulosa cell tumors, Sertoli-Leydig tumors, and gynandroblastoma ovarian neoplasms. (A and B) Low-power and high-power views of juvenile granulosa cell tumor showing nodular arrangement of tumor cells in pale blue background with mucoid pools. Cells have ovoid, regular nuclei and ill-defined cytoplasm. (C and D) Sertoli-Leydig tumors have multiple histologic patterns. Panel C shows tubular arrangements of Sertoli cells with hyperchromatic nuclei and high nuclear-cytoplasmic ratio. An anaplastic mitotic figure is visible on the right side of the photomicrograph. Panel D shows the typical nested arrangement of Sertoli cells admixed with larger Leydig cells with abundant pink cytoplasm. (E and F) Gynandroblastomas feature a combination of juvenile granulosa cell tumor (E) and Sertoli-Leydig cell tumor (F) features. These two photomicrographs are from different areas of the same tumor.
In one review of 72 pediatric patients with sex cord-stromal tumors, juvenile granulosa cell tumors and Sertoli-Leydig cell tumors together accounted for 85% of such neoplasms in children and adolescents.2 Almost two thirds of cases were juvenile granulosa cell tumors. Juvenile granulosa cell tumors are characterized by polygonal cells with usually abundant cytoplasm growing in nodules or diffusely. Follicles of varying size and shape typically punctuate the tumor. Tumor cells are typically immature, sometimes strikingly pleomorphic, and are usually briskly mitotic.2 The capsule of the ovary is intact in most cases, which accounts, in part, for the excellent outcome; however, in tumors with rupture or invasion beyond the capsule, juvenile granulosa cell tumors can pursue an aggressive clinical course. Adult granulosa cell tumor is uncommon in children just as juvenile granulosa cell tumor is uncommon in adults.
Approximately 20% of ovarian sex cord-stromal tumors in children are Sertoli-Leydig cell tumors. Sertoli-Leydig cell tumors may be well differentiated with Sertoli cell tubules separated by delicate stroma that contain clusters of Leydig cells, intermediately differentiated with lobules of hyperchromatic Sertoli cells that often grow focally as cords and tubules with stromal Leydig cells, or poorly differentiated. Poorly differentiated tumors are usually dominantly sarcomatoid, with only rudimentary differentiation of the types already listed. Heterologous elements include mucinous epithelial glands or rhabdomyosarcomatous and/or chondrosarcomatous elements, the latter being of adverse prognostic significance. Elevation of alpha-fetoprotein (AFP) may be observed but rarely to the degree seen in yolk sac tumor.3-5
Sex cord tumors with annular tubules are a distinctive histologic category and show tubules with Sertoli cells arranged around one or more hyaline bodies. In patients with Peutz-Jegher syndrome, these tubules may be scattered and admixed with normal ovarian tissue rather than forming a distinct mass.
MOLECULAR PATHOLOGY
Recent analyses have reshaped our understanding of the pathophysiology of some of these tumor subtypes. Mutations in DICER1, STK11, and FOXL2 influence the development of some of these neoplasms.
DICER1
Sertoli-Leydig cell tumors and gynandroblastomas are associated with DICER1 mutations.6-9 DICER1 encodes an endonuclease that is critical for microRNA processing. DICER1 mutations are associated with pleuropulmonary blastoma, which is the most common lung tumor of infancy and early childhood, as well as with embryonal rhabdomyosarcoma of the uterine cervix, renal tumors, thyroid nodules and carcinoma, nasal chondromesenchymal hamartoma, ciliary body medulloepithelioma, pineoblastoma, and pituitary blastoma.7,10-18 Typically, these tumors have biallelic DICER1 mutations that are composed of a loss of function in one allele and a missense mutation in the RNase IIIb domain. Biallelic loss of function and missense RNase IIIb DICER1 mutations result in systemic loss of 5p-microRNAs that precludes regulation of growth-promoting gene programs.19,20
The majority of individuals with DICER1 syndrome tumors have germline loss of function mutations in DICER1.19 Testing for DICER1 mutations may have important implications for individuals and familial tumor risk and may facilitate diagnosis of associated conditions. Of importance, individuals with tumor predisposition are at risk for development of contralateral, metachronous ovarian tumors after the general risk for recurrence has passed and, thus, prolonged monitoring is recommended in this setting.
In two young women with Sertoli-Leydig cell tumor who were enrolled in the International Ovarian and Testicular Stromal Tumor Registry, identification of a germline DICER1 mutation allowed screening for and diagnosis of pleuropulmonary blastoma in their children in its earliest and most curable form. Early diagnosis is critical to improving outcomes in children with pleuropulmonary blastoma as pleuropulmonary blastoma may progress from type I to type II to type III with a decrease in survival from 91% to 74% to 53%.8,21
STK11
Sex cord-stromal tumors with annular tubules may be associated with Peutz-Jegher syndrome and specifically with mutations in the STK11 gene. Individuals with clinical findings of Peutz-Jegher syndrome should undergo genetic testing that includes screening for deletion and/or duplication of STK11 and relevant organ-specific screening.22 A separate subtype of sex cord-stromal tumor with annular tubular is not associated with Peutz-Jegher syndrome and may present with progesterone secretion and show a complex growth pattern. These latter tumors carry a worse prognosis than those associated with Peutz-Jegher syndrome.
Ollier Disease and Mafucci Syndrome
Ollier disease includes enchondromatosis, whereas Mafucci syndrome includes enchondromatosis and hemangiomas. Enchondromas may be associated with bony deformities and chondrosarcomas. Somatic mosaic mutations in IDH1 and IDH2 may be observed.23 Both Ollier disease and Maffucci syndrome are associated with an increased risk of juvenile granulosa cell tumors.24
FOXL2
Nearly all adult granulosa cell tumors are characterized by missense somatic point mutations (402 C→G) in FOXL2, which may be useful diagnostically.25 This mutation may alter antiproliferative pathways and limit apoptosis, which contributes to the pathogenesis of adult granulosa cell tumors.25 New analyses of FOXL2 and clinical outcomes may alter the clinical approach to these generally indolent tumors.26
EPIDEMIOLOGY
Data from SEER suggest that the incidence of sex cord-stromal tumors is significantly lower among white women compared with black women (0.18 v 0.35 per 100,000 person years; relative risk, 0.53; 95% CI, 0.42 to 0.67).6,27 Unfortunately, many of these tumors, including higher stage juvenile granulosa cell tumors, are coded as benign or of uncertain malignant potential by using the International Classification of Disease–Oncology (ICD-O) coding system. Tumors thus classified are not collected by cancer registries; therefore, the incidence—especially for tumors other than adult granulosa cell tumors—are underestimated by cancer registry data. This underreporting limits our understanding of the epidemiology of these tumors, including the racial and ethnic predispositions.
This ICD-O designation of benign (/0) or low malignant potential (/1) for many of the sex cord-stromal tumors is misleading because higher stage or recurrent disease often follows a more aggressive course and may be associated with a fatal outcome. Patients may experience recurrence of some types—especially adult granulosa cell tumors—decades after the original diagnosis; thus, individual cancer registry data may not document the full clinical course of these tumors. Because of the establishment of dedicated registries of these tumors in the United States and in Europe, there is increased evidence of the potential malignant behavior of these tumors28 as well as genetic links to other tumors. Neoplasms with /0 or /1 designations are usually not reportable to cancer registries but exceptions have been made. For instance, several CNS tumor types, including tumors of the brain and meninges, are reportable, even with a behavior code of /0 or /1. Conversely, juvenile pilocytic astrocytomas, although often considered benign, are coded as 9421/3 (malignant), and early endometrial carcinomas, which are largely curable with a benign course when treated early, are coded as invasive malignancies. The decision to collect data on these tumors was made, in part, because they have been deemed of significance to the understanding of the disease burdens of cancer, as these conditions—as with many ovarian sex cord-stromal tumors—can cause morbidity and mortality.
PREOPERATIVE EVALUATION AND INITIAL TREATMENT
When an ovarian sex cord-stromal tumor is suspected, levels of inhibin, estradiol, testosterone, and AFP should be obtained. Inhibin levels may be elevated in granulosa cell tumors; inhibin B may be more predictive than inhibin A.29 Granulosa cell tumors may also present with elevated estradiol, and Sertoli-Leydig cell tumors may present with elevated testosterone or, rarely, AFP.
Ultrasound is the most common initial imaging modality. A large mass is commonly seen. Cross-sectional imaging, either computed tomography or magnetic resonance imaging, may show an adnexal mass with a heterogeneous appearance. As in other ovarian tumors, laterality may be difficult to determine from initial imaging and is best determined intraoperatively.
Fertility-sparing surgery is preferred in children, adolescents, and women of reproductive age as long as the contralateral tube and ovary and the uterus are unaffected, which is usually the case. Fertility-sparing surgery does not refer to ovarian cystectomy alone, but implies the complete removal of the affected adnexa.30 Women who are past reproductive age usually undergo total hysterectomy and bilateral salpingo-oophorectomy. Comprehensive staging includes sampling of peritoneal fluid, examination of the contralateral ovary, biopsies of the peritoneum and any suspicious lesions, omental biopsy, and palpation of lymph nodes, with resection of any lymph nodes that have concerning features upon imaging or intraoperative examination. Complete lymphadenectomy is typically excluded from the procedure, as the risk of nodal metastasis with primary disease is low.31 Operative records should clearly state whether there is any evidence for intraoperative (International Federation of Gynecology and Obstetrics [FIGO] stage Ic1) or preoperative (FIGO stage Ic2) rupture.
If confirmed as FIGO stage Ia, most tumors may be treated with surgical resection alone. Tumors that are staged higher than Ia may require chemotherapy and/or additional surgery. Treatment with cisplatin, etoposide, and bleomycin over 5 days, usually for four cycles, is often administered to children and adolescents in the United States who require chemotherapy. In Europe, treatment with cisplatin, etoposide, and ifosfamide is more common. In adult patients, the standard of care has been considered bleomycin, etoposide, and cisplatin, but evidence suggests that paclitaxel and carboplatin have activity in this setting and may be equivalent and less toxic.32,33 A randomized phase II cooperative group trial is currently underway to compare these two regimens.
Prognosis is heavily dependent on stage. In pediatric patients with juvenile granulosa cell tumor, preoperative rupture or malignant ascites may confer a worse prognosis than intraoperative rupture.34 In Sertoli-Leydig cell tumor, the level of differentiation has major prognostic significance.29
Recurrent disease may be treated with surgery, but other modalities have efficacy. Radiation therapy may be useful in settings of recurrent disease.35 Combination therapy with taxanes or cisplatin, etoposide, and bleomycin may be used,33 and hormonal therapy with leuprolide acetate may demonstrate activity.36 Bevacizumab has also shown activity and provides another option for treatment, either alone or in combination.37,38
Separate from recurrent disease, in some individuals—for example, those with Sertoli-Leydig cell tumor in the context of predisposing DICER1 mutations—there is a risk of metachronous, contralateral Sertoli-Leydig cell tumor. These metachronous tumors are generally identified as stage Ia and are often treated with surgery alone.
ONGOING STUDIES
The International Ovarian and Testicular Stromal Tumor Registry was established in 2011 to study these rare tumors. Since that time, 140 patients have been enrolled.39 In Europe, individuals with ovarian sex cord-stromal tumors can be registered with the European Cooperative Study Group on Pediatric Rare Tumors.40 International collaboration continues to advance the understanding of these tumors.
The GOG 264 trial randomly assigned women with newly diagnosed and recurrent chemonaive ovarian sex cord-stromal tumors to primary therapy with carboplatin and paclitaxel versus cisplatin, etoposide, and bleomycin. Thus far, the study has accrued 40 patients with plans to expand to a juvenile cohort. In addition, combined treatment with taxanes and bevacizumab is being investigated in the recurrent setting in an ongoing trial in Europe.41
SUGGESTIONS FOR FUTURE STUDY
Ovarian sex cord-stromal tumors are underreported but clinically significant neoplasms. As a result of coding and reporting issues, these tumors are often not reported to state or national cancer registries. These methodologic limitations do not reflect our current understanding of the biology and clinical relevance of these tumors. A /1 ICD-O behavior code may result in underestimation of risks. For example, with Sertoli-Leydig cell tumors, careful staging and assessment of level of differentiation is required to determine whether adjuvant therapy is needed. Referral to an oncology specialist for evaluation, possible treatment, surveillance, and a discussion of potential genetic implications is recommended. In addition, limitations in national reporting prevent an epidemiologic understanding of these tumors, including the risk for subsequent malignancies. These tumors exhibit interesting epidemiologic patterns, disproportionately affect young women—with implications for fertility and hormone function—and are now known to be an important component of specific tumor predisposition syndromes as described above.42
Reconsideration of the criteria for the inclusion of these tumors in national cancer registries would allow for a more population-based approach to the study of sex cord-stromal neoplasms. Benefits would include the ability to calculate true incidence and survival rates, which, given the long interval to recurrence of these tumors, is difficult to determine from single-institution analyses. Another benefit would be the determination of any geographic or racial and/or ethnic differences in incidence.
Changes to coding designations would facilitate clinically relevant epidemiologic investigations. Including all Sertoli-Leydig cell tumors, juvenile granulosa cell tumors, and gynandroblastomas in national registries through one or both of the above modifications will allow accurate tracking of incidence as well as subsequent neoplasms to aid in the understanding of the full spectrum of disease. As cancer epidemiologic investigations advance, it is critical to understand not only what is histologically malignant, but also what is clinically significant and what will ultimately contribute to our understanding of the impact of cancer and its therapies.
TREATMENT
Our understanding of the pathophysiology of these tumors continues to advance, yet, at this point, this knowledge has not yet been translated into novel therapeutic approaches. Currently, individuals with sex cord-stromal tumors receive treatment that is similar to that in individuals with germ cell or epithelial tumors. As translational efforts advance, it is likely that treatment will evolve and that therapies directed at the underlying genetic aberrations may replace more generic treatment.
Acknowledgment
This work is supported by National Institutes of Health, National Cancer Institute Grant No. R01-CA143167 and The Parson’s Foundation (to D.A.H. and Y.H.M.). D.T.S. is supported by the Barbara and Hubertus Trettner Foundation and the German Childhood Cancer Foundation. The International Ovarian and Testicular Stromal Tumor Registry is supported by St Baldrick’s Foundation, Pine Tree Apple Tennis Classic, Hyundai Hope on Wheels, and the Randy Shaver Cancer Research and Community Fund. We thank Mercedes Wilhelm for assistance with manuscript preparation.
AUTHOR CONTRIBUTIONS
Conception and design: Kris Ann P. Schultz, Dominik T. Schneider, Robert H. Young, David M. Gershenson, D. Ashley Hill, A. Lindsay Frazier
Collection and assembly of data: Kris Ann P. Schultz, Anne K. Harris, D. Ashley Hill
Data analysis and interpretation: Kris Ann P. Schultz, Anne K. Harris, Dominik T. Schneider, Jubilee Brown, David M. Gershenson, Louis P. Dehner, D. Ashley Hill, Yoav H. Messinger, A. Lindsay Frazier
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ovarian Sex Cord-Stromal Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jop.ascopubs.org/site/misc/ifc.xhtml.
Kris Ann P. Schultz
Research Funding: St Baldrick’s
Anne K. Harris
No relationship to disclose
Dominik T. Schneider
No relationship to disclose
Robert H. Young
No relationship to disclose
Jubilee Brown
No relationship to disclose
David M. Gershenson
Stock or Other Ownership: Johnson & Johnson, Pfizer, Biogen Idec, Celgene
Honoraria: Merck
Consulting or Advisory Role: Clovis Oncology
Patents, Royalties, Other Intellectual Property: Elsevier, UpToDate
Louis P. Dehner
No relationship to disclose
D. Ashley Hill
No relationship to disclose
Yoav H. Messinger
Honoraria: Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
A. Lindsay Frazier
Consulting or Advisory Role: Decibel Therapeutics
References
- 1.Morowitz M, Huff D, von Allmen D. Epithelial ovarian tumors in children: A retrospective analysis. J Pediatr Surg. 2003;38:331–335, discussion 331-335. doi: 10.1053/jpsu.2003.50103. [DOI] [PubMed] [Google Scholar]
- 2.Schneider DT, Calaminus G, Wessalowski R, et al. Ovarian sex cord-stromal tumors in children and adolescents. J Clin Oncol. 2003;21:2357–2363. doi: 10.1200/JCO.2003.05.038. [DOI] [PubMed] [Google Scholar]
- 3.Motoyama I, Watanabe H, Gotoh A, et al. Ovarian Sertoli-Leydig cell tumor with elevated serum alpha-fetoprotein. Cancer. 1989;63:2047–2053. doi: 10.1002/1097-0142(19890515)63:10<2047::aid-cncr2820631029>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 4.Kurman RJ, Carcangiu ML, Herrington S, et al. World Health Organization Classification of Tumors of Female Reproductive Organs. Lyon, France: IARC Press; 2014. , pp 18-21. [Google Scholar]
- 5.Young RH. Sex cord-stromal tumors of the ovary and testis: Their similarities and differences with consideration of selected problems. Mod Pathol. 2005;18:S81–S98. doi: 10.1038/modpathol.3800311. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 6.Schultz KA, Pacheco MC, Yang J, et al. Ovarian sex cord-stromal tumors, pleuropulmonary blastoma and DICER1 mutations: A report from the International Pleuropulmonary Blastoma Registry. Gynecol Oncol. 2011;122:246–250. doi: 10.1016/j.ygyno.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heravi-Moussavi A, Anglesio MS, Cheng SW, et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med. 2012;366:234–242. doi: 10.1056/NEJMoa1102903. [DOI] [PubMed] [Google Scholar]
- 8.Schultz KA, Harris A, Williams GM, et al. Judicious DICER1 testing and surveillance imaging facilitates early diagnosis and cure of pleuropulmonary blastoma. Pediatr Blood Cancer. 2014;61:1695–1697. doi: 10.1002/pbc.25092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conlon N, Schultheis AM, Piscuoglio S, et al. A survey of DICER1 hotspot mutations in ovarian and testicular sex cord-stromal tumors. Mod Pathol. 2015;28:1603–1612. doi: 10.1038/modpathol.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart DR, Messinger YHM, Williams GM, et al. Nasal chondromesenchymal hamartomas arise secondary to germline and somatic mutations of DICER1 in the pleuropulmonary blastoma tumor predisposition disorder. Hum Genet. 2014;133:1443–1450. doi: 10.1007/s00439-014-1474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doros L, Yang J, Dehner L, et al. DICER1 mutations in embryonal rhabdomyosarcomas from children with and without familial PPB-tumor predisposition syndrome. Pediatr Blood Cancer. 2012;59:558–560. doi: 10.1002/pbc.24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foulkes WD, Bahubeshi A, Hamel N, et al. Extending the phenotypes associated with DICER1 mutations. Hum Mutat. 2011;32:1381–1384. doi: 10.1002/humu.21600. [DOI] [PubMed] [Google Scholar]
- 13.de Kock L, Sabbaghian N, Druker H, et al. Germ-line and somatic DICER1 mutations in pineoblastoma. Acta Neuropathol. 2014;128:583–595. doi: 10.1007/s00401-014-1318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rio Frio T, Bahubeshi A, Kanellopoulou C, et al. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA. 2011;305:68–77. doi: 10.1001/jama.2010.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Witkowski L, Mattina J, Schönberger S, et al. DICER1 hotspot mutations in non-epithelial gonadal tumours. doi: 10.1038/bjc.2013.637. Br J Cancer 109:2744-2750, 2013 [Erratum: Br J Cancer 109:3131, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Kock L, Druker H, Weber E, et al. Ovarian embryonal rhabdomyosarcoma is a rare manifestation of the DICER1 syndrome. Hum Pathol. 2015;46:917–922. doi: 10.1016/j.humpath.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Doros LA, Rossi CT, Yang J, et al. DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod Pathol. 2014;27:1267–1280. doi: 10.1038/modpathol.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doros L, Schultz KA, Stewart DR, et al. GeneReviews: DICER1-related disorders. http://www.ncbi.nlm.nih.gov/books/NBK196157/ [Google Scholar]
- 19.Brenneman M, Field A, Yang J, et al. Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in DICER1 syndrome: A unique variant of the two-hit tumor suppression model. F1000Res. 2015;4:214. doi: 10.12688/f1000research.6746.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pugh TJ, Yu W, Yang J, et al. Exome sequencing of pleuropulmonary blastoma reveals frequent biallelic loss of TP53 and two hits in DICER1 resulting in retention of 5p-derived miRNA hairpin loop sequences. Oncogene. 2014;33:5295–5302. doi: 10.1038/onc.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messinger YH, Stewart DR, Priest JR, et al. Pleuopulmonary blastoma: A report on 350 central pathology-confirmed pleuropulmonary blastoma cases by the International Pleuropulmonary Blastoma Registry. Cancer. 2015;121:276–285. doi: 10.1002/cncr.29032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: A systematic review and recommendations for management. Gut. 2010;59:975–986. doi: 10.1136/gut.2009.198499. [DOI] [PubMed] [Google Scholar]
- 23.Pansuriya TC, van Eijk R, d’Adamo P, et al. Somatic mosaic IDH1 and IDH2 mutations are associated with enchondroma and spindle cell hemangioma in Ollier disease and Maffucci syndrome. Nat Genet. 2011;43:1256–1261. doi: 10.1038/ng.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka Y, Sasaki Y, Nishihira H, et al. Ovarian juvenile granulosa cell tumor associated with Maffucci’s syndrome. Am J Clin Pathol. 1992;97:523–527. doi: 10.1093/ajcp/97.4.523. [DOI] [PubMed] [Google Scholar]
- 25.Rosario R, Cohen PA, Shelling AN. The role of FOXL2 in the pathogenesis of adult ovarian granulosa cell tumours. Gynecol Oncol. 2014;133:382–387. doi: 10.1016/j.ygyno.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 26.McConechy MK, Färkkilä A, Horlings HM, et al. Molecularly defined adult granulosa cell tumor of the ovary: The clinical phenotype. J Natl Cancer Inst. 2016;108:djw134. doi: 10.1093/jnci/djw134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mink PJ, Sherman ME, Devesa SS. Incidence patterns of invasive and borderline ovarian tumors among white women and black women in the United States. Results from the SEER Program, 1978-1998. Cancer. 2002;95:2380–2389. doi: 10.1002/cncr.10935. [DOI] [PubMed] [Google Scholar]
- 28.Kalfa N, Sultan C. Juvenile ovarian granulosa cell tumor: A benign or malignant condition? Gynecol Endocrinol. 2009;25:299–302. doi: 10.1080/09513590802630153. [DOI] [PubMed] [Google Scholar]
- 29.Schneider DT, Orbach D, Cecchetto G, et al. Ovarian Sertoli Leydig cell tumours in children and adolescents: An analysis of the European Cooperative Study Group on Pediatric Rare Tumors (EXPeRT) Eur J Cancer. 2015;51:543–550. doi: 10.1016/j.ejca.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M, Cheung MK, Shin JY, et al. Prognostic factors responsible for survival in sex cord stromal tumors of the ovary—An analysis of 376 women. Gynecol Oncol. 2007;104:396–400. doi: 10.1016/j.ygyno.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 31.Brown J, Sood AK, Deavers MT, et al. Patterns of metastasis in sex cord-stromal tumors of the ovary: Can routine staging lymphadenectomy be omitted? Gynecol Oncol. 2009;113:86–90. doi: 10.1016/j.ygyno.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Brown J, Shvartsman HS, Deavers MT, et al. The activity of taxanes in the treatment of sex cord-stromal ovarian tumors. J Clin Oncol. 2004;22:3517–3523. doi: 10.1200/JCO.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 33.Brown J, Shvartsman HS, Deavers MT, et al. The activity of taxanes compared with bleomycin, etoposide, and cisplatin in the treatment of sex cord-stromal ovarian tumors. Gynecol Oncol. 2005;97:489–496. doi: 10.1016/j.ygyno.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Schneider DT, Calaminus C, Göbel U. In reply. J Clin Oncol. 2004;22:2033–2035. [Google Scholar]
- 35.Wolf JK, Mullen J, Eifel PJ, et al. Radiation treatment of advanced or recurrent granulosa cell tumor of the ovary. Gynecol Oncol. 1999;73:35–41. doi: 10.1006/gyno.1998.5287. [DOI] [PubMed] [Google Scholar]
- 36.Fishman A, Kudelka AP, Tresukosol D, et al. Leuprolide acetate for treating refractory or persistent ovarian granulosa cell tumor. J Reprod Med. 1996;41:393–396. [PubMed] [Google Scholar]
- 37.Tao X, Sood AK, Deavers MT, et al. Anti-angiogenesis therapy with bevacizumab for patients with ovarian granulosa cell tumors. Gynecol Oncol. 2009;114:431–436. doi: 10.1016/j.ygyno.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown J, Brady WE, Schink J, et al. Efficacy and safety of bevacizumab in recurrent sex cord-stromal ovarian tumors: Results of a phase 2 trial of the Gynecologic Oncology Group. Cancer. 2014;120:344–351. doi: 10.1002/cncr.28421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ovarian and Testicular Stromal Tumor Registry Home. http://www.otstregistry.org/
- 40.European Cooperative Study Group for Pediatric Rare Tumors Home. http://www.raretumors-children.eu/
- 41.Ray-Coquard I, Brown J, Harter P, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for ovarian sex cord stromal tumors. Int J Gynecol Cancer. 2014;24:S42–S47. doi: 10.1097/IGC.0000000000000249. (suppl 3) [DOI] [PubMed] [Google Scholar]
- 42.Schultz KA, Williams G, Hill D, et al. Ovarian tumors in association with pleuropulmonary blastoma: A new manifestation of the PPB Tumor syndrome. Pediatr Blood Cancer. 2010;54(6):305. [Google Scholar]