Abstract
Objective
Sleep problems in bipolar disorder (BD) are common, but reported rates vary from 10% to 80%, depending on definitions, methodologies and management of potential confounding factors. This multicenter study seeks to address these issues and also compares BD cases with Hypersomnia as well as the more commonly investigated Insomnia and No Sleep Problem groups.
Method
A cross‐sectional comparison of sleep profiles in 563 BD I and II individuals who participated in a structured assessment of demographic, clinical, illness history and treatment variables.
Results
Over 40% cases met criteria for Insomnia and 29% for Hypersomnia. In univariate analysis, Insomnia was associated with BD II depression whilst Hypersomnia was associated with BD I depression or euthymia. After controlling for confounders and covariates, it was demonstrated that Hypersomnia cases were significantly more likely to be younger, have BD I and be prescribed antidepressants whilst Insomnia cases had longer illness durations and were more likely to be prescribed benzodiazepines and hypnotics.
Conclusion
Whilst Insomnia symptoms are common in BD, Hypersomnia is a significant, frequently underexplored problem. Detailed analyses of large representative clinical samples are critical to extending our knowledge of differences between subgroups defined by sleep profile.
Keywords: bipolar I disorder, bipolar II disorder, sleep, insomnia, hypersomnia
Significant outcomes.
In a cross‐sectional clinical study of bipolar disorder (BD) patients in Norway, we found high rates of Insomnia (40%), but also of Hypersomnia (29%).
Hypersomnia is a significant and underexplored problem in BD. In this study, Hypersomnia cases were significantly more likely to be younger, BD I and prescribed antidepressants.
This is one of the first studies of sleep in BD that adequately controls for confounding factors such as age, body mass index and prescribed medications.
Limitations.
The cross‐sectional design means we can only examine statistical associations, not predictors of sleep profile or long‐term consequences of the different patterns.
We used no objective sleep measurements in the study.
Introduction
Bipolar disorder (BD) is a serious, chronic and recurring condition in which sleep disturbance is a common symptom regardless of phase of illness 1, 2, 3. Sleep disturbance is the most recognized prodromal symptom of mania 4 and is one of its diagnostic criteria, and improvement in sleep is an important therapeutic target in mania as it often heralds the restabilization of the current mental state 5. Likewise, sleep problems are reported in 50–90% of patients with syndromal or subsyndromal depressive symptoms in BD 6. Sleep disturbance is frequently the last symptom to resolve as an affective episode finishes 7, although for many individuals, it does not fully remit and remains a persistent interepisode symptom 8, 9. In sum, changes in sleep pattern predict new episodes and are a prognostic marker for episode outcomes, for all polarities of BD 5, 8.
Given the ubiquitous nature of sleep disruptions in BD, it is unsurprising that they have begun to receive greater attention. Several clinical studies have explored the sleep profiles of BD cases and identified a range of individual characteristics associated with the presence or absence of sleep problems. Also, a number of research studies have employed actigraphy or other objective measures to record sleep patterns and their associations with circadian or other systemic disturbances 8, 10, 11, 12. In addition, exploring how sleep symptoms respond to the treatments prescribed for acute BD episodes, there is increasing interest in the adjunctive use of chronobiotics such as melatonin agonists and/or chronotherapeutics such as cognitive and behavioural therapies to specifically target sleep problems in BD cases 8, 10, 11, 12. However, these approaches have exposed the lack of a full understanding of the range of sleep profiles that may be observed in BD and as yet it is not known whether the reported sleep patterns are mainly associated with different phases or subtypes of BD and/or how much of the variance in sleep might be explained by other known influences such as age, gender, body mass index (BMI) or indeed be a result of the confounding effects of BD treatments 13.
Previous studies have reported differences in sleep problems between euthymic, depressed, hypomanic, manic or mixed states [e.g. Gruber et al. 14], and between BD subtypes such as BD I and BD II e.g. Brill et al. 15. However, attempts to define any other characteristics of BD cases with or without sleep problems have been undermined by the fact that studies rarely differentiate between cases with insomnia, hypersomnia and/or any other sleep disturbance, an exception is Soehner et al. 16. Furthermore, apart from the large‐scale study of sleep profiles from the STEP‐BD programme, the vast majority of clinical studies of sleep in BD include only a small numbers of participants (average around 50) who are often recruited from specialist clinics. Thus, there is a lack of robust evidence regarding differences in sleep profiles across mental states or BD subtypes in clinically representative populations. Furthermore, many studies have inadequate statistical power to explore how sleep profiles differ across mood states; BD subtypes; and/or lack the capacity to examine confounding factors or colinearity between putative discriminating variables.
Aims of the study
This study uses data from a sample of >500 patients with bipolar disorder (BD) recruited from general psychiatry services across Norway and explores three unresolved questions:
How common are Insomnia and Hypersomnia in BD?
Are symptoms of Insomnia or Hypersomnia associated with different subtypes of BD, current mental states or treatments, illness history or other factors (e.g. age and BMI)?
What combinations of variables best differentiate individuals with Insomnia or Hypersomnia from those with No Sleep Problems?
Material and methods
Sample
The BD cases were recruited via two established Norwegian research networks exploring the clinical course and outcome of severe mental disorders, namely the TOP programme (Thematically Organized Psychosis) and BRAIN (Bipolar Research and Innovation Network). Whilst ‘BRAIN’ primarily includes patients who have been admitted or recently discharged from inpatient units throughout Norway, the ‘TOP’ programme primarily includes patients from outpatient clinics, most of whom were recruited from services in the largest city, namely Oslo 17, 18, 19, 20. The planned overlaps in protocols and procedures have allowed cases from both cohorts to be included in a range of previously published studies, for example Schoeyen et al. 21. The research programmes are approved by ‘The Regional Committee for Research Ethics’ and ‘The Norwegian Data Inspectorate’, and all the patients included in the current project gave written informed consent for participation.
The main inclusion criteria for the study were age ≥18 years and a diagnosis of bipolar disorder type I or II (BD I; BD II) which was confirmed via the Structural Clinical Interview for the DSM‐IV (SCID) 22. The exclusion criteria were as follows: evidence of a primary alcohol or substance abuse problem and/or a medical disorder that may account for a sleep disturbance, clinical evidence of cognitive impairments incompatible with informed consent for participation and/or absence of key data regarding BD subtype, mental state or sleep profile (see below).
Assessments
The BRAIN and TOP programmes record the same demographic and illness characteristics. Clinicians trained in the use of the study assessment protocol collect information from participants, which can be supplemented by the patients’ families and from clinical records as appropriate. For this study, information on demographics, BD history and current treatment were selected from the database, and current mental state and sleep profile were determined using the symptom ratings recorded on established, reliable and valid observer‐rated scales:
- Baseline Characteristics:
- Demographics: current age, gender, years of education and body mass index (BMI).
- History of BD: as in previous TOP‐BRAIN studies, age of BD onset is defined as the age at which the first mood episode occurred, and illness duration is defined as the difference between current age and age of onset 21. In addition, we report the total number of BD episodes, the total number of episodes by polarity (depression, hypomania or mania) and the number of episodes per annum (calculated from total number of episodes divided by illness duration in years).
- Prescribed medications: the total number of psychotropic medications currently prescribed to the individual and the classes of those medications were recorded. The latter were categorized as follows: lithium or anti‐epileptics (i.e. traditional mood stabilizers), antipsychotics, antidepressants, benzodiazepines and/or hypnotics.
Current Mental State & Sleep Profile:
The clinician version of the Inventory of Depressive Symptomatology (IDS‐C) and Young Mania Rating Scale (YMRS) are interview‐based assessments that can be used to record the frequency and severity of depressive and manic symptoms, respectively 23, 24. As both questionnaires assess all the criterion symptom domains designated to diagnose major mood episodes in DSM‐IV and five, they are frequently used to classify cases as euthymic, depressed, (hypo)manic or mixed e.g. Gopal et al., Sussman et al. 25, 26. Furthermore, several key studies of sleep patterns in mood disorders use the relevant items of the IDS‐C or other symptom rating scales to identify different diagnostic subtypes of sleep problems [e.g. Gruber et al. 14, Kaplan et al. 27, Sylvia et al. 28 and Soehner et al. 16]. We used these approaches to define current mental state and sleep profiles:
Current Mental State:
The cut‐off scores used to operationalize current mental states and the rationale for their selection were based on those employed in a clinical intervention trial for sleep problems in BD 12. To briefly summarize, the goal was to maximize the specificity of episode identification (i.e. categorization of cases as depressed, hypomanic/manic or mixed) and minimize misclassifications that may arise because of conflation of rating scale scores in those with co‐occurring sleep difficulties (due to the number of sleep items that might be endorsed in those who were ‘euthymic’ but, e.g., also had insomnia). The cut‐offs selected for hypomania on the YMRS and depression on the IDS‐C are therefore at the higher end of those quoted in the literature, to take into account the number and potential range of scores on the sleep‐related items. Thus, whilst a standard cut‐off on the Hamilton Rating Scale for Depression (HRSD) for mild depression is a score of about eight, we selected the slightly higher level of 11 (which is equivalent to about 18–19 on the IDS‐C; www.ids-qids.org). On the YMRS, we used a cut‐off of seven as used in recent sleep studies in BD 29, 30. Thus, current mental state categories are as follows:
Euthymia/Not in Major Episode = IDS‐C <18 and YMRS <7; Depression = IDS‐C ≥18 and YMRS <7; (Hypo)Mania = YMRS ≥8 and IDS‐C <18; Mixed State = YMRS ≥8 and IDS‐C ≥18.
Sleep Profile:
Whilst the symptom rating scales are not specifically designed to identify sleep disorders, individual items are considered to be reliable and valid ‘proxy’ measures of insomnia or hypersomnia meeting recognized diagnostic criteria as described in the International Classification of Sleep Disorders and in DSM 5 28, 31, 32. Also, the sleep items in the IDS have been validated as measures of insomnia symptom severity and hypersomnia severity and shown agreement with multimethod assessments such as weekly sleep diary recordings, clinical interviews 16, 32, 33 and objective measures such as actigraphy 34.
In this study, sleep profiles (Insomnia, Hypersomnia and No Sleep Problems) were operationalized from the four sleep items in IDS‐C 14, 16, 28, 32, 35: difficulty falling asleep (item 1), difficulty maintaining sleep (item 2), early morning awakening (item 3) and hypersomnia (item 4). The following definitions were used:
Insomnia was regarded as present if the individual demonstrated one or more of the following scores: Sleep Onset Insomnia ≥2 (takes at least 30 min to fall asleep, more than half the time), Mid‐nocturnal Insomnia = 3 (awakens more than once a night and stays awake for 20 min or more, more than half the time) or Early Morning Insomnia ≥1 (more than half the time, awakens more than 30 min before need be) and they scored zero on the Hypersomnia item (0 = sleeps no longer than 7–8 h a night, without naps).
Hypersomnia was regarded as present if the individual had a score ≥1 on the Hypersomnia item.
An individual was classified with ‘No Sleep Problems’ if they showed the following scoring pattern: Sleep Onset Insomnia <2, Mid‐nocturnal Insomnia <3, Early Morning Insomnia <2 and Hypersomnia = 0.
Statistical analyses
All analyses were undertaken using spss version 21, (IBM Corp., Armonk, NY, USA) and a significance level of P < 0.05 (two‐tailed tests) was employed unless otherwise stated.
Means (and standard deviations; SD) and/or medians are reported for continuous variables as appropriate, and numbers and percentages are reported for categorical variables. Differences between subgroups were examined using anova for normally distributed continuous variables, Kruskal–Wallis tests for non‐normal distributions (statistics reported as X2) and X2 tests for differences between groups for categorical variables.
Multinomial logistic regression (MNLR) was used to examine the best combination of variables that correctly classified cases into three groups defined by sleep profile (No Sleep Problems, Insomnia and Hypersomnia). The group without any sleep problems was selected as the reference category and the forward (likelihood ratio) procedure was used for the regression. Variables entered into the model were selected a priori if (a) they have been reported to be associated with sleep problems in BD in prior publications (e.g. BD subtype, gender, current mental state, BMI and medication regime) and/or (b) if they had a significance level <0.05 in the univariate analyses. To avoid confounding, the YMRS and IDS‐C scores were excluded (as these scales were used to operationalize current mental state and sleep categories). The factors and variables in the MLRN analysis were as follows: current age, gender, BD subtype, age at onset of BD, illness duration, number of BD episodes per annum, current mental state, BMI, and number and classes of medications prescribed. The variables included in the final classification model are reported using odds ratios (OR) with 95% confidence intervals (CI).
Results
Demographics and illness characteristics
As shown in Table 1, the sample comprised of 563 individuals (TOP = 309; BRAIN = 254), of whom 66% met diagnostic criteria for BD I (n = 373). The mean age at interview was 38 years and the majority of the sample was female (59%). The mean body mass index (BMI) was about 26 (SD 4.4). The mean duration of illness was nearly 17 years, with a median of nine BD episodes in total, of which about four were depressive episodes and three were elevated mood episodes (medians: Mania = 1; Hypomania = 2). At interview, most cases were categorized as euthymic (40%) or depressed (38%), with 13% meeting criteria for hypomania or mania, and nine per cent were in a mixed state. The median total IDS score was 17 and the median total YMRS score was two. Less than 10% of cases were reported to be currently medication‐free. The mean number of medications being prescribed per person was 2.43 (SD 1.42; range 0–8). The most commonly prescribed medications were lithium or an antiepileptic mood stabilizer (68%), followed by antipsychotics (58%); just over a third of the sample was prescribed antidepressants and just under a third benzodiazepines or hypnotics.
Table 1.
Demographics and illness characteristics
| Demographics and clinical history | n (%) |
| Total sample | 563 |
| Bipolar I: bipolar II | 373 (66.3%): 190 (33.7%) |
| Female: Male | 333 (59.1%): 230(40.9%) |
| Mean (SD) | |
| Age at interview | 38.0 (13.4) |
| Education in years | 14.1 (3.0) |
| Body mass index | 25.7 (4.4) |
| Age at onset of bipolar disorder | 21.2 (10.7) |
| Illness duration in years | 16.7 (13.3) |
| Number of episodes | Median (IQR) |
| Bipolar disorder | 9 (4–22) |
| Per annum (episodes/illness duration) | 0.9 (0.5–1.8) |
| Depression | 4 (2–10) |
| Mania | 1 (0–2) |
| Hypomania | 2 (0–6) |
| Current mental state | n (%) |
| Euthymic | 226 (40.1%) |
| Depressed | 212 (37.7%) |
| (Hypo)manic | 73 (13.0%) |
| Mixed state | 52 (9.2%) |
| Median (IQR) | |
| Inventory of depressive symptoms | 17 (10–27) |
| Young mania rating scale | 2 (0–6) |
| Currently prescribed medications | n (%) |
| No medication | 44 (7.8%) |
| 1 mood stabilizera | 381 (67.7%) |
| ≥2 mood stabilizersa | 158 (28.1%) |
| Antipsychotics | 329 (58.4%) |
| Antidepressants | 190 (33.7%) |
| Benzodiazepines | 150 (26.6%) |
| Hypnotics | 156 (27.7%) |
| ≥3 medications | 244 (43.4%) |
Mood stabilizer refers to Lithium or Anti‐epileptics (see text for details).
Current sleep profiles
Just less than one‐third of the sample had No Sleep Problems (28%), whilst a similar proportion met criteria for Hypersomnia (29%). Overall, 43% of BD cases met criteria for Insomnia, with 29% meeting criteria for at least two Insomnia subtypes. As shown in Table 2, the most common Insomnia symptoms recorded were sleep onset difficulty (early insomnia = 36%), or waking up too early (late insomnia = 35%).
Table 2.
Prevalence of different sleep profiles (no sleep problems, insomnia or hypersomnia)
| Sleep profile (n = 563) | n (%) |
|---|---|
| No sleep problems | 159 (28.2%) |
| Hypersomnia | 164 (29.1%) |
| Insomnia | 240 (42.6%) |
| Early (sleep onset) | 205 (36.4%) |
| Middle (mid‐nocturnal) | 104 (18.5%) |
| Late (early awakening) | 197 (35.0%) |
| ≥2 Insomnias | 161 (28.6%) |
Sleep profiles, bipolar subtypes and current mental state
Sleep profiles of bipolar subtypes
As shown in Fig. 1, within the BD I group (n = 373), 30% cases (n = 113) reported No Sleep Problems, 38% met criteria for Insomnia (n = 141), whilst 32% reported Hypersomnia (n = 119). In BD II (n = 190), the proportions were as follows: 24% No Sleep Problems (n = 46), 52% Insomnia (n = 99) and 26% Hypersomnia (n = 45). The prevalence of the three sleep profiles differs significantly between BD I and BD II (X2 10.61; df 2; P = 0.005)
Figure 1.
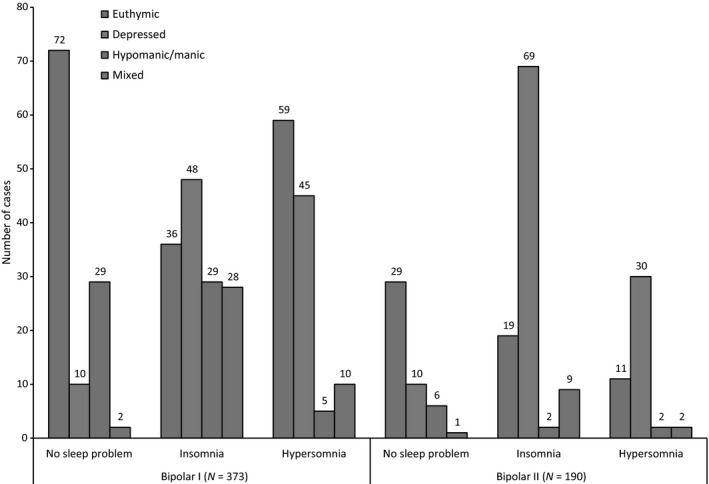
Sleep profile according to mental state in cases with BD I and BD II. As noted in the text and also in Table 3, the prevalence rates for each sleep profile sub‐group differs significantly according to BD subtype (Insomnia vs. Hypersomnia vs. No Sleep Problems: X2 10.61; df 2; P 0.005).
Current mental state and bipolar subtypes in groups defined by sleep profile
Within the No Sleep Problem group, there were no statistically significant differences in the proportion of BD I or II cases who were euthymic, depressed, (hypo) manic or in a mixed state, about two‐thirds of BD I and II cases were euthymic (see Table 3). There was a non‐significant trend for more BD I cases to be categorized as (hypo) manic (26%) and BD II cases as depressed (22%), but mixed states were rare in both BD I and BD II cases.
Table 3.
Comparison of sleep profile and mental state between BD I and BD II
| Sleep profile | Current mental state | Bipolar I (n = 373) | Bipolar II (n = 190) | Chi‐square | P value |
|---|---|---|---|---|---|
| No sleep problems | n = 113 | n = 46 | 6.71 | ns | |
| Euthymic | 72 (63.7%) | 29 (63.0%) | |||
| Depressed | 10 (8.8%) | 10 (21.7%) | |||
| (Hypo) manic | 29 (25.7%) | 6 (13.0%) | |||
| Mixed | 2 (1.8%) | 1 (2.2%) | |||
| Insomnia | n = 141 | n = 99 | 36.05 | 0.001 | |
| Euthymic | 36 (25.5%) | 19 (19.2%) | |||
| Depressed | 48 (34.0%) | 69 (69.7%) | |||
| (Hypo) manic | 29 (20.6%) | 2 (2.0%) | |||
| Mixed | 28 (19.9%) | 9 (9.1%) | |||
| Hypersomnia | n = 119 | n = 45 | 11.48 | 0.009 | |
| Euthymic | 59 (49.6%) | 11 (24.4%) | |||
| Depressed | 45 (37.8%) | 30 (66.7%) | |||
| (Hypo) manic | 5 (4.2%) | 2 (4.4%) | |||
| Mixed | 10 (8.4%) | 2 (4.4%) |
The italic values refer to the total n in the cell.
Within the Insomnia group, depression was twice as common in the BD II as compared to the BD I cases (70% v 34%), whilst (hypo) manic (BD I = 21%, BD II = 2%) and mixed states (BD I = 20%; BD II = 9%) were both significantly more prevalent in BD I cases with Insomnia (X2 36.05; df 3; P = 0.001).
As shown in Table 3, within the Hypersomnia group, the proportion of BD I and BD II cases with euthymia (BD I = 50%, BD II = 24%) or depression (BD I = 38%, BD II = 67%) accounted for the significant differences that were observed (X2 11.48; df 3; P 0.009).
Other key characteristics of groups defined by sleep profile
As shown in Table 4, we found no significant differences in gender distribution, mean BMI or episodes per annum between the three sleep subgroups. Age at interview was significantly lower in the Hypersomnia group (35.1 years) compared with the other two subgroups (F 5.67; df 2,560; P = 0.004), whilst age of onset was significantly lower in the Insomnia group (19.7 years) compared with the other groups (F 4.21; df 2,560; P = 0.02). The Hypersomnia group had the shortest illness duration compared with the Insomnia group (13 vs. 19 years; F 10.54; df 2,560; P = 0.001).
Table 4.
Comparison of characteristics and prescribed medications between sleep profile groups
| No sleep problems (n = 159) | Insomnia (n = 240) | Hypersomnia (n = 164) | Statistica | P value | |
|---|---|---|---|---|---|
| Mean age at interview | 38.7 (13.0) | 39.5 (13.6) | 35.1 (13.2) | 5.67 | 0.004 |
| Females | 89 (56%) | 138 (57.5%) | 106 (64.6%) | 2.97 | ns |
| Mean body mass index | 25.9 (4.9) | 25.4 (4.0) | 25.9 (4.5) | 0.77 | ns |
| Mean age at onset | 22.7 (12.1) | 19.7 (10.2) | 21.8 (9.7) | 4.21 | 0.02 |
| Mean illness duration | 16.0 (12.7) | 19.4 (14.0) | 13.4 (12.3) | 10.54 | 0.001 |
| Episodes per annum: Mean (SD)/Median | 1.5 (2.1)/0.9 | 1.7 (2.6)/1.0 | 1.4 (2.2)/1.0 | 1.36 | ns |
| Medications | |||||
| None | 16 (10.0%) | 17 (7.1%) | 12 (7.1%) | 0.73 | ns |
| 1 mood stabilizerb | 106 (66.7%) | 165 (68.8%) | 110 (67.1%) | 0.22 | ns |
| ≥2 mood stabilizersb | 42 (26.4%) | 71 (29.5%) | 46 (28%) | 0.89 | ns |
| Antipsychotics | 95 (59.7%) | 119 (53.8%) | 105 (64.0%) | 4.39 | ns |
| Antidepressants | 37 (23.3%) | 84 (35.0%) | 69 (42.1%) | 13.05 | 0.001 |
| Benzodiazepines | 41 (25.8%) | 81 (33.8%) | 28 (17.1%) | 13.94 | 0.001 |
| Hypnotics | 37 (23.3%) | 83 (34.6%) | 36 (22.0%) | 9.94 | 0.007 |
| Mean number of medications | 2.26 (1.47) | 2.57 (1.50) | 2.38 (1.24) | 1.61 | ns |
anova, chi‐square or Kruskal–Wallis test.
Mood stabilizer refers to lithium or anti‐epileptics (see text for details).
The proportion of cases who were medication‐free, and the mean number of medications per person did not differ between groups. Likewise, rates of prescription of lithium and anti‐epileptic mood stabilizers and of antipsychotics were not significantly different in the three groups. Although the proportion of individuals in the Hypersomnia group who were currently depressed did not differ significantly from the other subgroups, more Hypersomnia cases were being prescribed antidepressants than individuals in the other groups (Hypersomnia = 42%; Insomnia = 35%; No Sleep Problems = 23%; X2 13.05, P = 0.01). In contrast, benzodiazepines and hypnotics were prescribed significantly more for the Insomnia group (benzodiazepines = 34%; hypnotics = 35%) than the groups with Hypersomnia (benzodiazepines = 17%; hypnotics = 22%) or No Sleep Problems (benzodiazepines = 26%; hypnotics = 24%).
Multinominal logistic regression (MNLR) model
A MNLR analysis was used to explore which combination of variables best differentiated the Insomnia and Hypersomnia groups from the reference group (No Sleep Problems). Age, gender, BD subtype and current mental state were entered into the model, followed by age of onset of BD, number of episodes per year of illness, BMI and number and classes of psychotropics prescribed. As shown in Table 5, only two variables significantly differentiated cases with Insomnia from those with No Sleep Problems: the Insomnia group were significantly more likely to be depressed (OR 9.46; 95% CI 5.13–17.47; P = 0.001) or in a mixed state (OR 21.38; 95% CI 6.15–74.36; P = 0.001) than the No Sleep Problems group. Compared to the group with No Sleep Problems, the Hypersomnia group was also much more likely to be in a depressed (OR 6.26; 95% CI 3.29–11.90; P = 0.001) or mixed state (OR 7.21; 95% CI 1.89–27.49; P = 0.004), but they showed several additional differences. The Hypersomnia group was significantly younger (OR 0.97; 95% CI 0.95–0.99; P = 0.011), was more than twice as likely to meet criteria for BD I (OR 2.12; 95% CI 1.15–3.93; P = 0.016) and to be prescribed antidepressants (OR 2.66; 95% CI 1.23–5.77; P = 0.013) than the group with No Sleep Problems. The Hypersomnia group was significantly less likely to be in a hypomanic/manic episode (OR 0.31; 95% CI 0.12–0.77; P = 0.012).
Table 5.
Multinominal logistic regression model of the best combination of variables that differentiate Insomnia and Hypersomnia groups from the ‘No Sleep Problems’ group
| Insomnia | Hypersomnia | |
|---|---|---|
| Age at Interview | OR 0.97 (95% CI 0.95–0.99) | |
| Diagnosis of bipolar I disorder | OR 2.12 (95% CI 1.15–3.93) | |
| Prescribed antidepressants | OR 2.66 (95% CI 1.23–5.77) | |
| Depressive episode | OR 9.46 (95% CI 5.13–17.47) | OR 6.26 (95% CI 3.29–11.90) |
| Mixed state | OR 21.38 (95% CI 6.15–74.36) | OR 7.21 (95% CI 1.89–27.49) |
| Hypomanic/Manic episode | OR 0.31 (95% CI 0.12–0.77) |
OR, odds ratio; 95% CI, 95% confidence intervals.
Discussion
This study used definitions of sleep profiles employed in previous research and applied these to a large, clinically representative sample of BD cases recruited from general adult psychiatry services. We ascertained that 43% of this sample met published criteria for Insomnia and 29% for Hypersomnia, and these sleep profiles differed significantly, not only across phases of illness (euthymia, depression, mania or mixed states), but also by BD subtype (I or II). Previous clinical studies of sleep problems (vaguely defined) in euthymic BD reported rates of sleep problems that ranged from 15% 24 to 83% 32; whilst other research has reported that sleep problems are associated with BD depression 31, 32, anxiety symptoms 1, BD subtype 11, 12 and with mental state 11.
None of the available studies have reported the link between different sleep profiles, BD subtypes and mental states, and so our study offers additional important insights. For example, BD I cases in our study reported high rates of Hypersomnia in depression and/or euthymia than BD II cases. Whilst not answering the question of whether sleep profile is a state or trait marker of BD spectrum or of BD I 1, these pattern variations highlight two important aspects of this study. First, sleep problems in BD may be better understood if the analyses explore associations across combinations of factors (such as subtypes and mood state), rather than employing item by item analyses, and second, that the use of three distinct sleep categories may explain some of the previous differences in prevalence estimates and some apparently contradictory findings. With the exception of Soehner et al. 13 (who studied unipolar and BD depressions), previous clinical studies of sleep patterns in BD have usually employed only two categories, namely no sleep problems vs. either (non‐specific) sleep problems or insomnia.
Previous publications rarely controlled for potential confounders that might explain some of the apparent associations between sleep patterns and clinical or individual characteristics 1, 14, 15, 16, 28, 35, 36. When group comparisons and/or logistic regression analyses take account of other variables (such as age, gender, BMI, medications, and clinical and illness characteristics), the only features that differentiated Insomnia from the No Sleep Problems group were being depressed (OR >9) or being in a mixed state (OR >20). Also, the 95% confidence intervals for these odds ratios were wide, suggesting some caution is needed in interpreting these findings. Some support for these findings comes from Gruber et al. 14, who defined cases according to sleep duration and found that short sleepers had higher levels of mood elevation and of depressive symptoms, whilst long sleepers were characterized by more depressive symptoms.
The Hypersomnia group compared with the Insomnia group was younger, had a shorter mean illness duration and was less likely to be prescribed hypnotics and benzodiazepines. In contrast, the Insomnia group had a younger age of onset than the Hypersomnia group. However, it should be noted that whilst the definitions employed in this study preclude the possibility that individuals meeting the criteria for Insomnia would simultaneously have Hypersomnia, it is potentially feasible for a subpopulation of the Hypersomnia group to have some symptoms of Insomnia. Whilst we have no evidence that this attenuated any differences between the groups with sleep problems, the potential Insomnia–Hypersomnia constellation warrants further examination. Cases with Hypersomnia were also significantly more likely to be in a depressed or a mixed state compared to the No Sleep Problems group, but Hypersomnia was not associated with pure (hypo) mania. In this study, it appears that Hypersomnia can be associated with any mental state that includes a significant depressive component (both depression and mixed states). Interestingly, the Hypersomnia group was significantly younger and was twice as likely to have BD I compared with the No Sleep Problems group. There are few clinical studies of sleep profiles in BD that have examined Hypersomnia; but in a mixed unipolar and BD sample, Soehner et al. 16 also reported that hypersomnia was associated with younger age. The final factor that characterized the Hypersomnia group was that the cases were more likely to be prescribed antidepressants compared with the No Sleep Problems group and antidepressants are associated with both increased somnolence and insomnia. The only previous study that examined prescribing in individuals with BD and sleep problems did not find any differences in medication class or load between individuals with or without hypersomnia 32. It is unclear why the Hypersomnia cases in our study were more often prescribed antidepressants, especially as the overall rate of depression did not appear to differ significantly from the other groups. One possible, but speculative, explanation is that the Hypersomnia cases may have had more persistent and severe depressive symptoms than other cases (and were therefore prescribed more medication), whilst another possibility is that some antidepressants may actually contribute to the excessive sleepiness 37. Whilst this cannot be proven on the basis of our data, it is noteworthy that Gruber et al. reported that long sleepers showed significantly higher levels of disability, than other BD cases 14.
Although this study tried to learn methodological lessons from earlier clinical studies of sleep profiles in BD; several limitations should be acknowledged. First, the cross‐sectional design means we can only examine statistical associations, not predictors of sleep profile or the downstream consequences of these different patterns. Second, although we could identify cases with symptom levels indicative of euthymia, we could not determine how long they had been euthymic. Third, we relied on definitions of sleep profiles that have been employed in previous studies, which categorize the problems according to item ratings on established symptom scales, rather than using formal diagnostic criteria 16, 28. As an example, our definition of Hypersomnia did not include an assessment of daytime sleepiness. As such, our groups are more accurately regarded as signifying sleep profiles, or symptoms of sleep problems, not diagnostic categories or full syndromes. Having said that, research comparing the reliability and validity of these ratings to objective measures or other assessments of sleep patterns demonstrates that these profiles offer acceptable representations of the underlying diagnostic categories 16, 38. This could be further improved by supplementing the ratings derived from the mood symptom scales with specific sleep questionnaires (e.g. PSQI) 39. In addition, we did not know the percentage of patients with obstructive sleep apnoea which is frequent in patients with BD and can potentially explain parts of the rates of sleep problems 40. Lastly, we did not include any objective sleep measurements. However, our rationale was that techniques such as actigraphy and polysomnography are more costly and resource intensive, and our primary goal was to reflect the measures used to evaluate sleep profiles in BD from the perspective of day to day clinical practice.
As noted, strengths of this study include the use of a large sample recruited from routine clinical settings and that data collection was undertaken by independent trained interviewers who were blind to the hypotheses being tested. Also, we examined combinations of variables that best classified cases into groups according to their sleep profile and took into account potential confounders that have not been routinely examined in other studies. However, we are aware that additional characteristics will need to be considered in future research, such as seasonality, obstructive sleep apnoea, physical illnesses and their treatments.
Sleep problems may be a cause of BD episodes or a core symptom of an episode 1. Studies of psychopathology also highlight that sleep profiles, such as hypersomnia in ‘atypical’ depression, or insomnia as a (transdiagnostic) marker of sympathetic arousal, may be important indicators of a bipolar or unipolar course of illness and/or predict response to treatment in BD cases 10, 11. Such findings highlight the importance of undertaking regular, detailed assessments of sleep profiles in individuals with mood disorders and giving due consideration to how the treatment of BD may impact (positively or negatively) on sleep patterns. However, further impetus is given to this issue because DSM 5 has now recommended that sleep disorders are no longer classified as primary or secondary. The likely implication is that sleep problems in individuals with BD are more likely to receive specific clinical attention and that treatments directly targeting a particular sleep profile will be introduced (rather than relying on indirect effects on sleep of generic BD treatments). To target interventions effectively, we will need to build on this and other clinical research studies to develop a more detailed understanding of the range and nature of sleep problems that occur within and across BD subtypes and mental states. Only then will it be possible to ensure that more sophisticated, evidence‐based treatments are instigated.
Declaration of interest
The authors have no conflict of interest to declare in regard to the research work presented in this manuscript.
Steinan MK, Scott J, Lagerberg TV, Melle I, Andreassen OA, Vaaler AE, Morken G. Sleep problems in bipolar disorders: more than just insomnia.
References
- 1. Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry 2008;165:820–829. [DOI] [PubMed] [Google Scholar]
- 2. Johnson SL, Morriss R, Scott J et al. Depressive and manic symptoms are not opposite poles in bipolar disorder. Acta Psychiatr Scand 2011;123:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hayes JF, Miles J, Walters K, King M, Osborn DP. A systematic review and meta‐analysis of premature mortality in bipolar affective disorder. Acta Psychiatr Scand 2015;131:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. J Affect Disord 2003;74:209–217. [DOI] [PubMed] [Google Scholar]
- 5. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry 2008;165:830–843. [DOI] [PubMed] [Google Scholar]
- 6. Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry 2005;66:1254–1269. [DOI] [PubMed] [Google Scholar]
- 7. Wichniak A, Wierzbicka A, Jernajczyk W. Sleep and antidepressant treatment. Curr Pharm Des 2012;18:5802–5817. [DOI] [PubMed] [Google Scholar]
- 8. Kaplan KA, Harvey AG. Behavioral treatment of insomnia in bipolar disorder. Am J Psychiatry 2013;170:716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geoffroy PA, Scott J, Boudebesse C et al. Sleep in patients with remitted bipolar disorders: a meta‐analysis of actigraphy studies. Acta Psychiatr Scand 2015;131:89–99. [DOI] [PubMed] [Google Scholar]
- 10. Scott J. Clinical parameters of circadian rhythms in affective disorders. Eur Neuropsychopharmacol 2011;21(Suppl 4):S671–S675. [DOI] [PubMed] [Google Scholar]
- 11. Hickie IB, Naismith SL, Robillard R, Scott EM, Hermens DF. Manipulating the sleep‐wake cycle and circadian rhythms to improve clinical management of major depression. BMC Med 2013;11:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinan MK, Krane‐Gartiser K, Langsrud K, Sand T, Kallestad H, Morken G. Cognitive behavioral therapy for insomnia in euthymic bipolar disorder: study protocol for a randomized controlled trial. Trials 2014;15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Boudebesse C, Geoffroy PA, Henry C et al. Links between sleep and body mass index in bipolar disorders: an exploratory study. Eur Psychiatry 2014;30:89–93. [DOI] [PubMed] [Google Scholar]
- 14. Gruber J, Harvey AG, Wang PW et al. Sleep functioning in relation to mood, function, and quality of life at entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP‐BD). J Affect Disord 2009;114:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brill S, Penagaluri P, Roberts RJ, Gao Y, El‐Mallakh RS. Sleep disturbances in euthymic bipolar patients. Ann Clin Psychiatry 2011;23:113–116. [PubMed] [Google Scholar]
- 16. Soehner AM, Kaplan KA, Harvey AG. Prevalence and clinical correlates of co‐occurring insomnia and hypersomnia symptoms in depression. J Affect Disord 2014;167:93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morken G, Vaaler AE, Folden GE, Andreassen OA, Malt UF. Age at onset of first episode and time to treatment in in‐patients with bipolar disorder. Br J Psychiatry 2009;194:559–560. [DOI] [PubMed] [Google Scholar]
- 18. Ringen PA, Lagerberg TV, Birkenaes AB et al. Differences in prevalence and patterns of substance use in schizophrenia and bipolar disorder. Psychol Med 2008;38:1241–1249. [DOI] [PubMed] [Google Scholar]
- 19. Birkenaes AB, Sogaard AJ, Engh JA et al. Sociodemographic characteristics and cardiovascular risk factors in patients with severe mental disorders compared with the general population. J Clin Psychiatry 2006;67:425–433. [DOI] [PubMed] [Google Scholar]
- 20. Finseth PI, Morken G, Malt UF, Andreassen OA, Vaaler AE. Risk factors of cycle acceleration in acutely admitted patients with bipolar disorder. Acta Psychiatr Scand 2014;130:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schoeyen HK, Vaaler AE, Auestad BH et al. Despite clinical differences, bipolar disorder patients from acute wards and outpatient clinics have similar educational and disability levels compared to the general population. J Affect Disord 2011;132:209–215. [DOI] [PubMed] [Google Scholar]
- 22. First M, Spitzer R, Gibbon M, Williams J. Structured clinical interview for DSM‐IV‐TR axis I disorders, research version. Patient edn. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 23. Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med 1996;26:477–486. [DOI] [PubMed] [Google Scholar]
- 24. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 25. Gopal S, Steffens DC, Kramer ML, Olsen MK. Symptomatic remission in patients with bipolar mania: results from a double‐blind, placebo‐controlled trial of risperidone monotherapy. J Clin Psychiatry 2005;66:1016–1020. [DOI] [PubMed] [Google Scholar]
- 26. Sussman N, Mullen J, Paulsson B, Vagero M. Rates of remission/euthymia with quetiapine in combination with lithium/divalproex for the treatment of acute mania. J Affect Disord 2007;100(Suppl 1):S55–S63. [DOI] [PubMed] [Google Scholar]
- 27. Kaplan KA, Talbot LS, Gruber J, Harvey AG. Evaluating sleep in bipolar disorder: comparison between actigraphy, polysomnography, and sleep diary. Bipolar Disord 2012;14:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sylvia LG, Dupuy JM, Ostacher MJ et al. Sleep disturbance in euthymic bipolar patients. J Psychopharmacol 2012;26:1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Boudebesse C, Geoffroy PA, Bellivier F et al. Correlations between objective and subjective sleep and circadian markers in remitted patients with bipolar disorder. Chronobiol Int 2014;31:698–704. [DOI] [PubMed] [Google Scholar]
- 30. Geoffroy PA, Boudebesse C, Bellivier F et al. Sleep in remitted bipolar disorder: a naturalistic case‐control study using actigraphy. J Affect Disord 2014;158:1–7. [DOI] [PubMed] [Google Scholar]
- 31. Welmer AK, von Arbin M, Murray V, Holmqvist LW, Sommerfeld DK. Determinants of mobility and self‐care in older people with stroke: importance of somatosensory and perceptual functions. Phys Ther 2007;87:1633–1641. [DOI] [PubMed] [Google Scholar]
- 32. Kaplan KA, Gruber J, Eidelman P, Talbot LS, Harvey AG. Hypersomnia in inter‐episode bipolar disorder: does it have prognostic significance? J Affect Disord 2011;132:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Manber R, Blasey C, Arnow B et al. Assessing insomnia severity in depression: comparison of depression rating scales and sleep diaries. J Psychiatr Res 2005;39:481–488. [DOI] [PubMed] [Google Scholar]
- 34. Gonzalez R, Tamminga C, Tohen M, Suppes T. Comparison of objective and subjective assessments of sleep time in subjects with bipolar disorder. J Affect Disord 2013;149:363–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Eidelman P, Talbot LS, Gruber J, Harvey AG. Sleep, illness course, and concurrent symptoms in inter‐episode bipolar disorder. J Behav Ther Exp Psychiatry 2010;41:145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rocha PM, Neves FS, Correa H. Significant sleep disturbances in euthymic bipolar patients. Compr Psychiatry 2013;54:1003–1008. [DOI] [PubMed] [Google Scholar]
- 37. Ketter TA, Miller S, Dell'osso B, Calabrese JR, Frye MA, Citrome L. Balancing benefits and harms of treatments for acute bipolar depression. J Affect Disord 2014;169(Suppl 1):S24–S33. [DOI] [PubMed] [Google Scholar]
- 38. Harvey AG, Talbot LS, Gershon A. Sleep disturbance in bipolar disorder across the lifespan. Clin Psychol 2009;16:256–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Steinan MK, Krane‐Gartiser K, Morken G, Scott J Sleep Problems in Euthymic Bipolar Disorders: A Review of Clinical Studies. Curr Psychiatry Rev 2015;11:1–9. [Google Scholar]
- 40. Kelly T, Douglas L, Denmark L, Brasuell G, Lieberman DZ. The high prevalence of obstructive sleep apnea among patients with bipolar disorders. J Affect Disord 2013;151:54–58. [DOI] [PubMed] [Google Scholar]


