Abstract
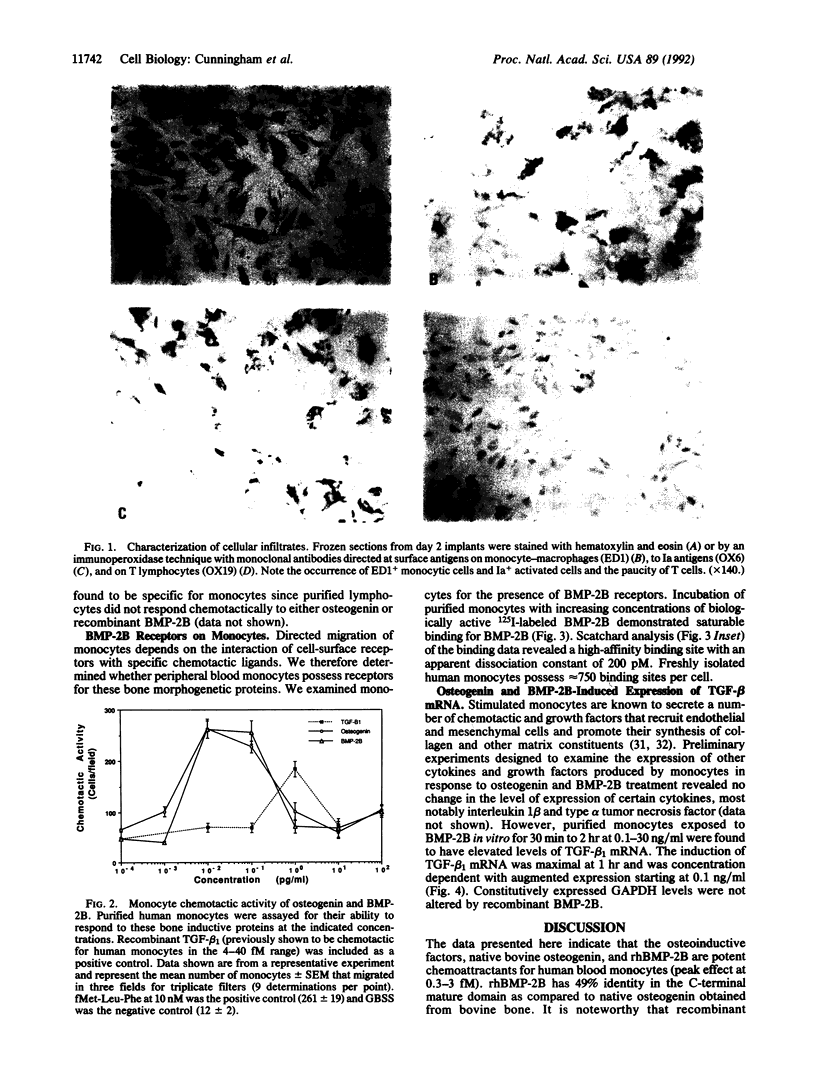
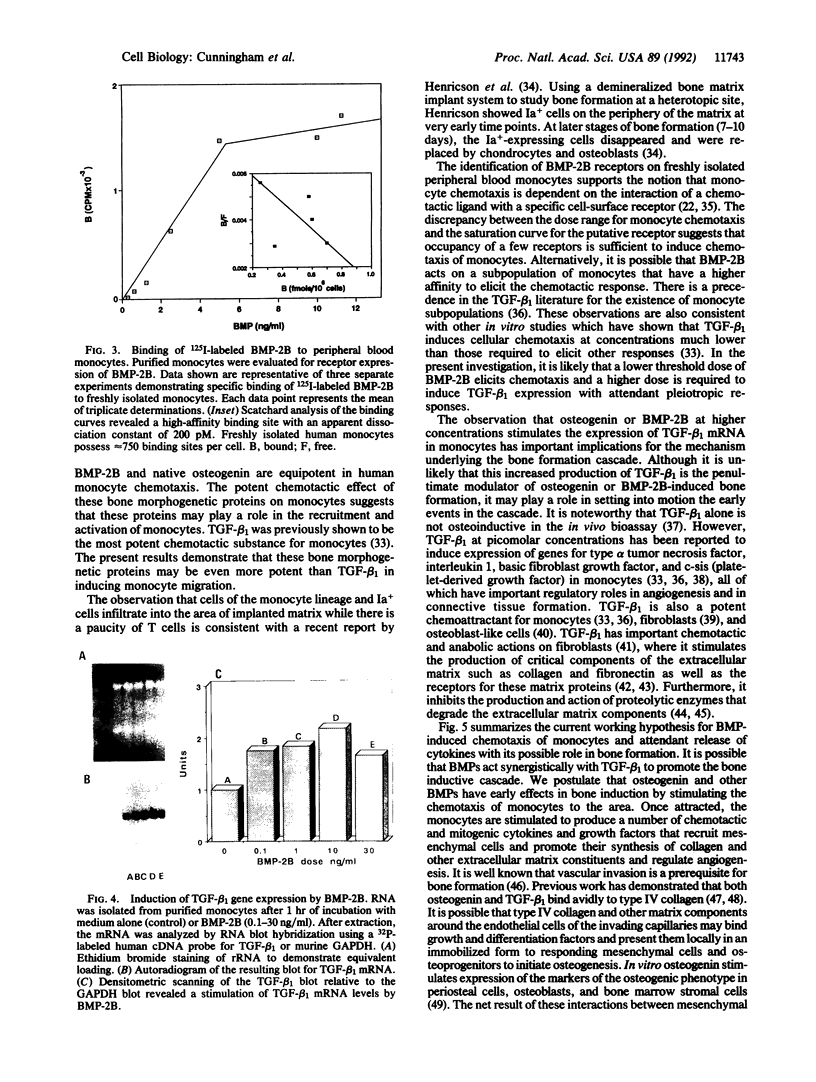
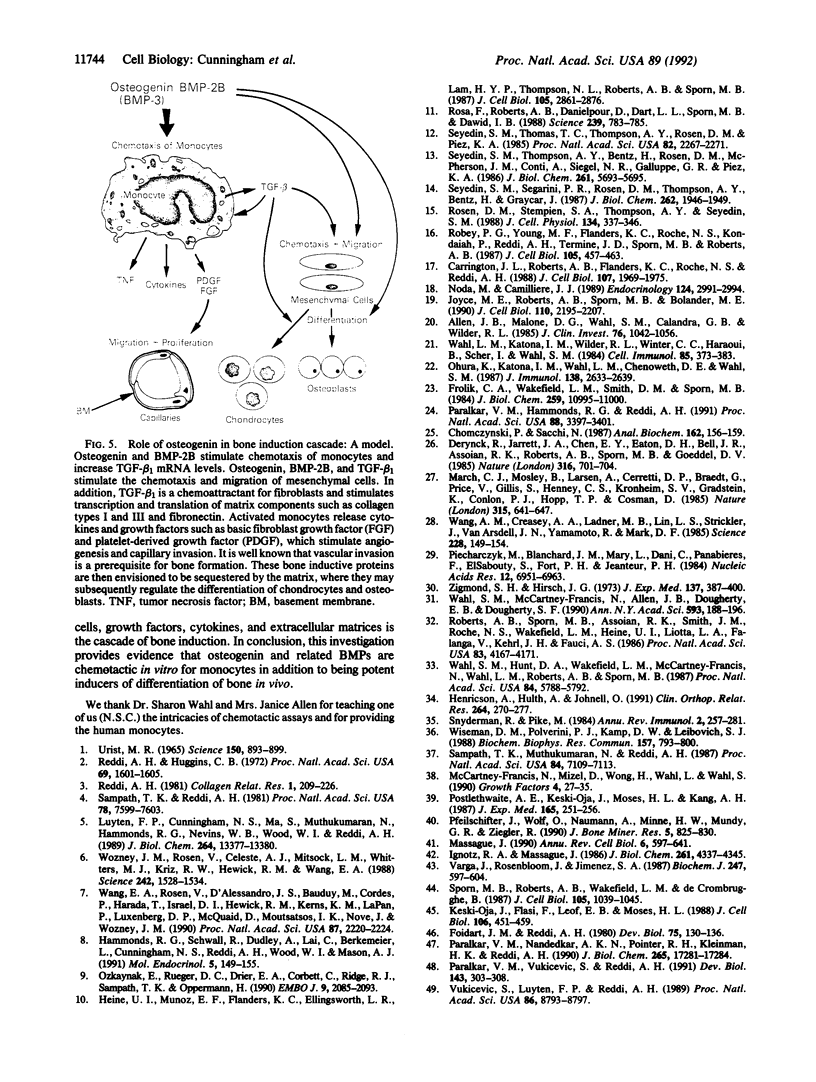
Subcutaneous implantation of demineralized bone matrix initiates a sequence of developmental events, which culminate in endochondral bone formation. During early stages of development of matrix-induced implants, ED1, Ia-positive monocytes-macrophages were observed, suggesting that in the initial phases of the endochondral bone formation cascade, the bone-inductive protein osteogenin and related bone morphogenetic proteins (BMPs) might serve as potent chemoattractants to recruit circulating monocytes. In this investigation, we demonstrate that at concentrations of 10-100 fg/ml (0.3-3 fM), native bovine osteogenin and recombinant human BMP-2B (rhBMP-2B) induce the directed migration of human blood monocytes in vitro. This chemotactic response was associated with expression of BMP binding sites (receptors) on monocytes. About 750 receptors per cell were detected with an apparent dissociation constant of 200 pM. Both osteogenin and rhBMP-2B at higher concentrations (0.1-30 ng/ml) stimulated mRNA expression for an additional regulatory molecule, type beta 1 transforming growth factor (TGF-beta 1) in human monocytes. TGF-beta 1, in turn, is known to induce a cascade of events leading to matrix generation. Monocytes stimulated by TGF-beta are known to secrete a number of chemotactic and mitogenic cytokines that recruit endothelial and mesenchymal cells and promote their synthesis of collagen and associated matrix constituents. TGF-beta 1 in concert with these other cytokines and matrix components regulates chemotaxis, mesenchymal proliferation, differentiation, angiogenesis, and controlled synthesis of extracellular matrix. Our results demonstrate that osteogenin and related BMPs through their profound effects on monocyte recruitment and cytokine synthesis may promote additional successive steps in the endochondral bone formation cascade.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B., Malone D. G., Wahl S. M., Calandra G. B., Wilder R. L. Role of the thymus in streptococcal cell wall-induced arthritis and hepatic granuloma formation. Comparative studies of pathology and cell wall distribution in athymic and euthymic rats. J Clin Invest. 1985 Sep;76(3):1042–1056. doi: 10.1172/JCI112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington J. L., Roberts A. B., Flanders K. C., Roche N. S., Reddi A. H. Accumulation, localization, and compartmentation of transforming growth factor beta during endochondral bone development. J Cell Biol. 1988 Nov;107(5):1969–1975. doi: 10.1083/jcb.107.5.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Foidart J. M., Reddi A. H. Immunofluorescent localization of type IV collagen and laminin during endochondral bone differentiation and regulation by pituitary growth hormone. Dev Biol. 1980 Mar;75(1):130–136. doi: 10.1016/0012-1606(80)90149-9. [DOI] [PubMed] [Google Scholar]
- Frolik C. A., Wakefield L. M., Smith D. M., Sporn M. B. Characterization of a membrane receptor for transforming growth factor-beta in normal rat kidney fibroblasts. J Biol Chem. 1984 Sep 10;259(17):10995–11000. [PubMed] [Google Scholar]
- Hammonds R. G., Jr, Schwall R., Dudley A., Berkemeier L., Lai C., Lee J., Cunningham N., Reddi A. H., Wood W. I., Mason A. J. Bone-inducing activity of mature BMP-2b produced from a hybrid BMP-2a/2b precursor. Mol Endocrinol. 1991 Jan;5(1):149–155. doi: 10.1210/mend-5-1-149. [DOI] [PubMed] [Google Scholar]
- Heine U., Munoz E. F., Flanders K. C., Ellingsworth L. R., Lam H. Y., Thompson N. L., Roberts A. B., Sporn M. B. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987 Dec;105(6 Pt 2):2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricson A., Hulth A., Johnell O. The occurrence of accessory immunologic cells in bone induction. Clin Orthop Relat Res. 1991 Mar;(264):270–277. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Joyce M. E., Roberts A. B., Sporn M. B., Bolander M. E. Transforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol. 1990 Jun;110(6):2195–2207. doi: 10.1083/jcb.110.6.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Blasi F., Leof E. B., Moses H. L. Regulation of the synthesis and activity of urokinase plasminogen activator in A549 human lung carcinoma cells by transforming growth factor-beta. J Cell Biol. 1988 Feb;106(2):451–459. doi: 10.1083/jcb.106.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten F. P., Cunningham N. S., Ma S., Muthukumaran N., Hammonds R. G., Nevins W. B., Woods W. I., Reddi A. H. Purification and partial amino acid sequence of osteogenin, a protein initiating bone differentiation. J Biol Chem. 1989 Aug 15;264(23):13377–13380. [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N., Mizel D., Wong H., Wahl L., Wahl S. TGF-beta regulates production of growth factors and TGF-beta by human peripheral blood monocytes. Growth Factors. 1990;4(1):27–35. doi: 10.3109/08977199009011007. [DOI] [PubMed] [Google Scholar]
- Noda M., Camilliere J. J. In vivo stimulation of bone formation by transforming growth factor-beta. Endocrinology. 1989 Jun;124(6):2991–2994. doi: 10.1210/endo-124-6-2991. [DOI] [PubMed] [Google Scholar]
- Ohura K., Katona I. M., Wahl L. M., Chenoweth D. E., Wahl S. M. Co-expression of chemotactic ligand receptors on human peripheral blood monocytes. J Immunol. 1987 Apr 15;138(8):2633–2639. [PubMed] [Google Scholar]
- Ozkaynak E., Rueger D. C., Drier E. A., Corbett C., Ridge R. J., Sampath T. K., Oppermann H. OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. EMBO J. 1990 Jul;9(7):2085–2093. doi: 10.1002/j.1460-2075.1990.tb07376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar V. M., Hammonds R. G., Reddi A. H. Identification and characterization of cellular binding proteins (receptors) for recombinant human bone morphogenetic protein 2B, an initiator of bone differentiation cascade. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3397–3401. doi: 10.1073/pnas.88.8.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar V. M., Nandedkar A. K., Pointer R. H., Kleinman H. K., Reddi A. H. Interaction of osteogenin, a heparin binding bone morphogenetic protein, with type IV collagen. J Biol Chem. 1990 Oct 5;265(28):17281–17284. [PubMed] [Google Scholar]
- Paralkar V. M., Vukicevic S., Reddi A. H. Transforming growth factor beta type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev Biol. 1991 Feb;143(2):303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J., Wolf O., Naumann A., Minne H. W., Mundy G. R., Ziegler R. Chemotactic response of osteoblastlike cells to transforming growth factor beta. J Bone Miner Res. 1990 Aug;5(8):825–830. doi: 10.1002/jbmr.5650050805. [DOI] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Moses H. L., Kang A. H. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987 Jan 1;165(1):251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A. H. Cell biology and biochemistry of endochondral bone development. Coll Relat Res. 1981 Feb;1(2):209–226. doi: 10.1016/s0174-173x(81)80021-0. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey P. G., Young M. F., Flanders K. C., Roche N. S., Kondaiah P., Reddi A. H., Termine J. D., Sporn M. B., Roberts A. B. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol. 1987 Jul;105(1):457–463. doi: 10.1083/jcb.105.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa F., Roberts A. B., Danielpour D., Dart L. L., Sporn M. B., Dawid I. B. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988 Feb 12;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- Rosen D. M., Stempien S. A., Thompson A. Y., Seyedin S. M. Transforming growth factor-beta modulates the expression of osteoblast and chondroblast phenotypes in vitro. J Cell Physiol. 1988 Mar;134(3):337–346. doi: 10.1002/jcp.1041340304. [DOI] [PubMed] [Google Scholar]
- Sampath T. K., Muthukumaran N., Reddi A. H. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7109–7113. doi: 10.1073/pnas.84.20.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath T. K., Reddi A. H. Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7599–7603. doi: 10.1073/pnas.78.12.7599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedin S. M., Segarini P. R., Rosen D. M., Thompson A. Y., Bentz H., Graycar J. Cartilage-inducing factor-B is a unique protein structurally and functionally related to transforming growth factor-beta. J Biol Chem. 1987 Feb 15;262(5):1946–1949. [PubMed] [Google Scholar]
- Seyedin S. M., Thomas T. C., Thompson A. Y., Rosen D. M., Piez K. A. Purification and characterization of two cartilage-inducing factors from bovine demineralized bone. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2267–2271. doi: 10.1073/pnas.82.8.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedin S. M., Thompson A. Y., Bentz H., Rosen D. M., McPherson J. M., Conti A., Siegel N. R., Galluppi G. R., Piez K. A. Cartilage-inducing factor-A. Apparent identity to transforming growth factor-beta. J Biol Chem. 1986 May 5;261(13):5693–5695. [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. Chemoattractant receptors on phagocytic cells. Annu Rev Immunol. 1984;2:257–281. doi: 10.1146/annurev.iy.02.040184.001353. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urist M. R. Bone: formation by autoinduction. Science. 1965 Nov 12;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Varga J., Rosenbloom J., Jimenez S. A. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987 Nov 1;247(3):597–604. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S., Luyten F. P., Reddi A. H. Stimulation of the expression of osteogenic and chondrogenic phenotypes in vitro by osteogenin. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8793–8797. doi: 10.1073/pnas.86.22.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Katona I. M., Wilder R. L., Winter C. C., Haraoui B., Scher I., Wahl S. M. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984 May;85(2):373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Hunt D. A., Wakefield L. M., McCartney-Francis N., Wahl L. M., Roberts A. B., Sporn M. B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., McCartney-Francis N., Allen J. B., Dougherty E. B., Dougherty S. F. Macrophage production of TGF-beta and regulation by TGF-beta. Ann N Y Acad Sci. 1990;593:188–196. doi: 10.1111/j.1749-6632.1990.tb16111.x. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Wang E. A., Rosen V., D'Alessandro J. S., Bauduy M., Cordes P., Harada T., Israel D. I., Hewick R. M., Kerns K. M., LaPan P. Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2220–2224. doi: 10.1073/pnas.87.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman D. M., Polverini P. J., Kamp D. W., Leibovich S. J. Transforming growth factor-beta (TGF beta) is chemotactic for human monocytes and induces their expression of angiogenic activity. Biochem Biophys Res Commun. 1988 Dec 15;157(2):793–800. doi: 10.1016/s0006-291x(88)80319-x. [DOI] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]