Abstract
Non-alcoholic fatty liver disease (NAFLD) is one of the most prevalent liver diseases associated with an altered lifestyle, besides genetic factors. The control and management of NAFLD mostly depend on lifestyle modifications, due to the lack of a specific therapeutic approach. In this context, we assessed the effect of carrot juice on the development of high fructose-induced hepatic steatosis. For this purpose, male weanling Wistar rats were divided into 4 groups, fed either a control (Con) or high fructose (HFr) diet of AIN93G composition, with or without carrot juice (CJ) for 8 weeks. At the end of the experimental period, plasma biochemical markers, such as triglycerides, alanine aminotransferase, and β-hydroxy butyrate levels were comparable among the 4 groups. Although, the liver injury marker, aspartate aminotransferase, levels in plasma showed a reduction, hepatic triglycerides levels were not significantly reduced by carrot juice ingestion in the HFr diet-fed rats (HFr-CJ). On the other hand, the key triglyceride synthesis pathway enzyme, hepatic stearoyl-CoA desaturase 1 (SCD1), expression at mRNA level was augmented by carrot juice ingestion, while their protein levels showed a significant reduction, which corroborated with decreased monounsaturated fatty acids (MUFA), particularly palmitoleic (C16:1) and oleic (C18:1) acids. Notably, it also improved the long chain n-3 polyunsaturated fatty acid, docosahexaenoic acid (DHA; C22:6) content of the liver in HFr-CJ. In conclusion, carrot juice ingestion decreased the SCD1-mediated production of MUFA and improved DHA levels in liver, under high fructose diet-fed conditions. However, these changes did not significantly lower the hepatic triglyceride levels.
Keywords: PUFA, fatty liver, vegetables, carotenoids, elongases
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a disease that starts with simple fat/triglyceride accumulation in the liver (fatty liver) and can progress to hepatocellular carcinoma (HCC). NAFLD is one of the most prevalent health problems of affluent countries, which affects nearly 20~30% of adult populations (1,2). Although the patho-physiology remains unclear, genetic, environmental, and lifestyle factors are implicated in the development and progression from simple steatosis to carcinoma. Among various lifestyle factors, consumption of high fructose corn syrup-sweetened soft drinks, is positively associated with the development of NAFLD, independent of metabolic syndrome in humans and experimental models. However, there is no specific and/or effective therapy to treat NAFLD, except for weight loss (1,3–5).
In this context, fruits and vegetables, rich sources of various potent phytonutrients/phytochemicals, fiber, minerals and vitamins are known to offer protection against life-threatening diseases such as cardiovascular disease, and some types of cancers, due to their anti-inflammatory and anti-oxidant properties (6). Among commonly consumed vegetables, carrots are rich in fiber, carotenoids, vitamin E, C, and phenolic compounds (7). Epidemiological, clinical, and experimental studies have shown that carrot consumption offers protection against oxidative stress, DNA damage, cancer, and inflammation (8–14). Previously, Poudyal et al. (15) have demonstrated improved hepatic inflammatory condition and glucose tolerance after purple carrot juice administration in high carbohydrate and high fat diet-fed Wistar rats. In addition, Nicolle et al. (16,17) have shown hypocholesterolemic and hypolipdemic effects of carrots in mice fed a cholesterol-enriched diet, while increased fecal sterol excretion and reduction of cholesterol absorption in rats due to lyophilized carrot feeding. However, so far, no study has addressed the effect of carrot (either in whole or of juice form) on lipid metabolism in a fructose-induced steatosis model. Therefore, the present study was aimed at assessing the effect of carrot juice on hepatic lipid metabolism in a high fructose diet-induced steatosis rat model.
MATERIALS AND METHODS
Materials
All chemicals used were of analytical grade. Triglycerides and glucose assay kits were purchased from BioSystems SA (Barcelona, Spain). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and retinol-binding protein 4 (RBP4) measurement kits were procured from BioVision Inc. (Milpitas, CA, USA). Recombinant human insulin, β-hydroxy butyrate assay kit, secondary antibodies, various standards such as retinol, β-carotene, β-apo-8′-carotenal, and fatty acids were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). C-peptide (EMD Millipore Corporation, Billerica, MA, USA), insulin (Crystal Chem Inc., Downers Grove, IL, USA), and fibroblast growth factor 21 (FGF21) (R&D Systems, Minneapolis, MN, USA) quantitation kits were used. Total RNA isolation kits were obtained from Qiagen GmbH (Hilden, Germany). For quantitative real-time polymerase chain reaction analysis (qRT-PCR), first-strand cDNA synthesis kits (New England Biolabs, Ipswich, MA, USA) and pre-validated universal probe for rats (Roche Diagnostics GmbH, Mannheim, Germany) were used. Experimental diets were obtained from OpenSource Diets (Research Diets Inc., New Brunswick, NJ, USA).
Experimental design
Twenty four male weanling Wistar rats were received from the National Centre for Laboratory Animal Sciences, National Institute of Nutrition, Hyderabad, India. They were divided into 4 groups consisting of 6 rats and were given either the control diet (n=12) or high fructose diet (n=12) with or without carrot juice (CJ) administration. The groups were designated as control (Con), control diet with carrot juice (Con-CJ), high fructose (HFr), and high fructose diet with carrot juice (HFr-CJ). Animals were housed individually with an ambient temperature of 22.0±1°C, relative humidity of 50~60%, 12-h light/dark cycle, and animals were given “humane care” in accordance with the principles of the guide to the care and use of experimental animals. The Institutional Animal Ethics Committee (IAEC) of the National Institute of Nutrition, Hyderabad, India approved the study (No. P08F/IAEC/NIN/6/2013/SMJ/WNIN M62). The isocaloric diet of AIN93G composition is provided in the Table 1, and carrot juice containing 0.3 mg of β-carotene was administered through oral route daily for a period of 8 weeks. Daily food intake and weekly body weight data were recorded. At the end of the experimental period, over-night fasted animals were anesthetized using isoflurane (nasal inhalation), blood was drawn from the retro-orbital sinus in ethylenediaminetetraacetic acid-coated tubes, and rats were immediately sacrificed by cervical dislocation. Various tissues were collected, weighed and stored at −80°C for further analysis.
Table 1.
Diet composition
| Ingredients | Control diet | High fructose diet | ||
|---|---|---|---|---|
|
|
|
|||
| g/kg | kcal/kg | g/kg | kcal/kg | |
| Casein | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 |
| Corn starch | 397.5 | 1,590 | 0 | 0 |
| Maltodextrin 10 | 132 | 528 | 0 | 0 |
| Sucrose | 100 | 400 | 0 | 0 |
| Fructose | 0 | 0 | 629.5 | 2,518 |
| Cellulose, BW200 | 50 | 0 | 50 | 0 |
| Soybean oil | 70 | 630 | 70 | 630 |
| t-Butyl hydroquinone | 0.014 | 0 | 0.014 | 0 |
| Mineral mix S10022G | 35 | 0 | 35 | 0 |
| Vitamin mix V10037 | 10 | 40 | 10 | 40 |
| Choline bitartrate | 2.5 | 0 | 2.5 | 0 |
| Total | 1,000 | 4,000 | 1,000 | 4,000 |
| Energy (kcal/g) | 4.0 | 4.0 | ||
Carrot juice extraction and β-carotene estimation
Carrots were purchased from a local market, washed in running tap water and juice was extracted using a mechanical juicer (Philips Juicer HR1861/00, Royal Philips, Amsterdam, The Netherlands). An average of 361 mL of juice was obtained per kg of carrots. Extracted juice was filtered through a nylon mesh and condensed nearly to 10% (i.e. ~36 mL) using a digital rotatory flash evaporator with a chiller (CH-9230, BÜCHI Labortechnik AG, Flawil, Switzerland). From the condensed juice, β-carotene was extracted and estimated by previously reported methods using β-apo-8′-carotenal as the internal standard (18,19). An average of 0.6 mL of juice containing 0.3 mg β-carotene was administered through an oral feeding tube. The variation in the β-carotene content of carrots from seasonal variation and varietal differences was controlled by maintaining a volume of juice that provided a dose of 0.3 mg β-carotene throughout the experiment.
Oral glucose and intra-peritoneal insulin tolerance tests (OGTT& IPITT)
At the end of week 6, over-night fasted animals were administered 2 g of glucose through oral route or 0.5 U of human recombinant insulin per kg body weight intra-peritoneally. Blood was drawn from the tail vein at 0, 15, 30, 60, 120, and 180 min intervals for glucose measurement using a glucometer (Roche Diagnostics GmbH), and the area under curve (AUC) was calculated.
Insulin-stimulated 2-deoxy-D-glucose uptake in isolated soleus muscle
Soleus muscles were removed immediately after the animals were sacrificed; muscle strips were tied in stainless steel holders at the tendon regions. Muscles were pre-incubated in pre-gassed Krebs-ringer-bicarbonate buffer containing 2 mM pyruvate for 20 min at 37°C. Then, the buffer was replaced with fresh buffer containing insulin (1 U/mL) or without insulin for 10 min at 30°C. Then, fresh buffer containing 2-deoxy-D-glucose (0.5 μCi/mL) was added and incubated for 10 min at 30°C. 2-Deoxy-D-glucose uptake was measured by counting radioactivity in 100 μL of muscle homogenate by liquid scintillation counting as described by Bruning et al. (20).
Plasma and liver biochemistry
Plasma biochemistry such as triglycerides, ALT, AST, glucose, insulin, C-peptide, RBP4, β-hydroxy butyrate (β-HB), and FGF21 levels was measured using commercially available kits. Retinol levels in plasma and liver were quantified as reported earlier (21). Total lipids from liver were extracted and used to analyze the triglycerides contents and also for fatty acid composition by gas-liquid chromatography, after converting them into fatty acid methyl esters as previously reported (21).
Histology and immunohistochemistry
Immediately after removal, liver tissue was placed in formalin solution and processed for histological examination, using hematoxylin and eosin (H&E) stained sections. Further, for immunohistochemistry, formalin-embedded sections were used for liver lipid droplet-associated proteins; abhydrolase domain containing 5 (ABHD5) and perilipin, as described earlier (21).
Liver protein expression by immunoblotting
Liver tissue (100~250 mg) was homogenized in T-PER tissue protein extraction reagent (Thermo Fisher Scientific, Rockford, IL, USA) supplemented with 5% protease inhibitor and 1% phosphotase inhibitor cocktails. Cell debris was discarded after a brief low-speed centrifugation at 200 g for 1 min at 4°C. Then, the supernatant was used for immunoblotting by using antibodies against various desaturases [such as fatty acid desaturase (FADS) 1, FADS2, and stearoyl-CoA desaturase 1 (SCD1)] and elongases [namely, very long chain fatty acid elongase (ELOVL) 2 and ELOVL6]. β-Actin was used as a loading control and images were analyzed using the Image J 1.49 software (National Institutes of Health, Bethesda, MD, USA) (21).
Gene expression by qRT-PCR
Total RNA from liver was isolated, and a reverse transcription reaction was performed according to the instructions provided with the kit using 1.0 μg of total RNA. From the synthesized cDNA, gene amplification was carried out in LightCycler480 Real Time-PCR system (Roche Diagnostics GmbH), using pre-validated probes for rats (UPL probes; Roche Diagnostics GmbH) and gene-specific primers. Relative expression was calculated by normalizing the expression data, using the endogenous expression of acidic ribosomal phosphoprotein (ARPP) (21).
Statistical analyses
Values are expressed as means±standard error (SE). Data were analyzed by one-way ANOVA with post-hoc least significant difference test (post-hoc LSD). Expression data were log transformed and subjected to one-way ANOVA with post-hoc LSD test. Paired sample t-test was performed for muscle glucose uptake analysis. P-value≤0.05 was considered significant. IBM SPSS Statistics 19.0 software (IBM Corp., Armonk, NY, USA) was used for analyses.
RESULTS
Impact of carrot juice on body weight and plasma biochemistry
The gain in body weight was comparable among the treatment groups, despite a significant reduction in food intake in carrot juice administered groups. Further, liver weights were not influenced by either high fructose or carrot juice feeding for eight weeks (Table 2).
Table 2.
Effects of carrot juice on physical and plasma biochemical parameters
| Experimental groups1) | ||||
|---|---|---|---|---|
|
|
||||
| Con | Con-CJ | HFr | HFr-CJ | |
| Initial weight (g) | 38±0.76 | 38.0±0.73 | 39±0.71 | 38±0.76 |
| Final weight (g) | 279±4.7 | 258±7.3 | 266±15.2 | 254±2.0 |
| Weight gain (g) | 241±4.5 | 220±7.3 | 225±14.3 | 217±2.12 |
| Food intake (g) | 15.5±0.7 | 13.6±0.37* | 16.6±0.92 | 14.9±0.25* |
| Liver weight (g) | 9.0±0.32 | 8.3±0.59 | 8.6±0.51 | 9.0±0.64 |
| Plasma biochemistry2) | ||||
| Triglycerides (mg/dL) | 93.8±11.3 | 76.9±9.0 | 95.1±19.7 | 108.9±13.1 |
| ALT (mU/mL) | 41.0±3.5 | 38.8±4.6 | 32.5±5.3 | 33.6±1.4 |
| AST (mU/mL) | 79.9±4.5 | 88.7±5.5 | 93.3±7.0 | 61.6±12.4* |
| FGF21 (ng/mL) | 234±32.8 | 258±36.4 | 175±47.7 | 253±40.8 |
| β-hydroxy butyrate (ng/mL) | 105±6.7 | 95±6.5 | 103±7.1 | 99±7.9 |
Values are represented as means±SE of 6 rats, except for FGF21 and β-hydroxy butyrate, 4 rats from each group. Data were analyzed by one way ANOVA with post-hoc least significance difference test.
Significantly different at P≤0.05 compared between with or without carrot juice groups of respective diet.
Con, control diet; Con-CJ, control diet with carrot juice; HFr, high fructose diet; HFr-CJ, high fructose diet with carrot juice.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; FGF21, fibroblast growth factor 21.
Eight weeks of high fructose and/or carrot juice feeding from weaning did not alter the levels of plasma triglycerides, FGF21, liver fatty acid oxidation marker, β-hydroxy butyrate and the liver injury marker ALT, possibly due to shorter duration. However, compared to the HFr diet, another liver injury marker, AST levels significantly decreased in the HFr-CJ group (Table 2).
Impact of carrot juice on liver biochemistry
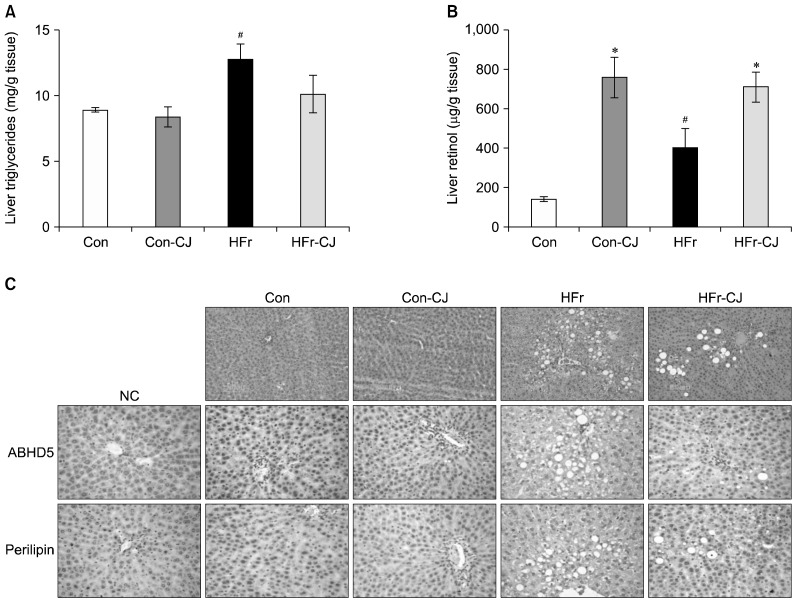
Compared to the control, feeding of HFr diet elevated triglyceride (P<0.013) and retinol (P<0.029) contents of the liver. On the other hand, carrot juice administration to HFr diet-fed rats increased the retinol levels significantly (P<0.001); however, reduction in triglycerides contents of liver was not statistically different when compared to the HFr diet-fed group (Fig. 1A and 1B). In addition, histological examination of H&E-stained liver sections also showed similar changes in liver lipid accumulation, which was reflected in the lipid droplet-associated proteins, ABHD5 and perilipin of the liver by immunohistological staining (Fig. 1C).
Fig. 1.
Impacts of carrot juice on liver biochemistry and histology. Liver triglyceride (A) and retinol (B) levels. Representative liver photomicrographs of hematoxylin & eosin stained liver sections (C), immnuohistological staining of liver lipid droplet-associated proteins [abhydrolase domain containing 5 (ABHD5) and perilipin]. 1st row, 20×; 2nd and 3rd row, 40× magnification. Data were analyzed by one way ANOVA with post-hoc least significant difference test. Results are expressed as means±SE of 6 rats from each group. Significantly different at P≤0.05 compared with the #control and high fructose group and *with or without carrot juice groups. NC, negative control; Con, control diet; Con-CJ, control diet with carrot juice; HFr, high fructose diet; HFr-CJ, high fructose diet with carrot juice.
Fatty acid composition of liver showed ~2 fold increase in the monounsaturated fatty acids (MUFA) [palmitoleic (C16:1), and oleic (C18:1) acids] due to HFr diet consumption, while n-3 polyunsaturated fatty acid (PUFA) levels such as α-linolenic (C18:3) and docosahexaenoic acids (DHA, C22:6) decreased by 0.3 fold and 0.7 fold, respectively, compared to the control diet-fed group. On the contrary, the group that received the HFr diet with carrot juice, palmitic (C16:0), palmitoleic (C16:1), and oleic (C18:1) acids decreased significantly by 0.7, 0.5, and 1.3 fold, respectively, while DHA (C22:6) levels increased by 1.5 fold, when compared to the HFr diet alone (Table 3).
Table 3.
Effects of carrot juice on liver fatty acid composition
| Major fatty acids (%) | Experimental groups1) | |||
|---|---|---|---|---|
|
| ||||
| Con | Con-CJ | HFr | HFr-CJ | |
| Palmitic acid (C16:0) | 19.3±0.28 | 19.9±1.10 | 22.1±0.53 | 15.9±2.77* |
| Palmitoleic acid (C16:1) | 1.7±0.13 | 1.7±0.37 | 3.4±0.26# | 1.7±0.37* |
| Stearic acid (C18:0) | 13.6±0.46 | 12.8±1.61 | 12.4±0.60 | 16.3±0.85* |
| Oleic acid (C18:1) | 15.3±0.55 | 18.3±4.95 | 24.8±1.78# | 16.5±1.69* |
| Linoleic acid (C18:2) | 22.5±0.69 | 22.9±0.44 | 14.7±0.74# | 18.5±1.05* |
| α-Linolenic acid (C18:3, n-3) | 0.92±0.11 | 0.74±0.10 | 0.31±0.07* | 0.26±0.09 |
| Eicosadienoic acid (C20:2) | 1.7±0.14 | 1.6±0.29 | 1.6±0.19 | 2.1±0.21 |
| Dihomo-γ-linolenic acid (C20:3) | 0.63±0.13 | 0.52±0.10 | 0.54±0.06 | 0.72±0.15 |
| Arachidonic acid (C20:4) | 16.9±0.53 | 15.1±2.84 | 15.3±0.90 | 19.1±1.18 |
| Eicosapentaenoic acid (C20:5) | 0.87±0.27 | 0.91±0.25 | 0.76±0.08 | 1.1±0.21 |
| Docosaenoic acid (C22:0) | 0.89±0.06 | 0.86±0.16 | 0.92±0.11 | 1.2±0.11 |
| Docosatetraenoic acid (C22:4) | 1.5±0.13 | 1.4±0.27 | 1.8±0.31 | 2.1±0.30 |
| Docosapentaenoic acid (C22:5, n-3) | 0.45±0.11 | 0.24±0.09 | 0.31±0.05 | 0.34±0.04 |
| Docosahexaenoic acid (C22:6, n-3) | 3.7±0.13 | 3.05±0.59 | 2.69±0.29# | 4.05±0.14* |
Values are represented as means±SE of 6 rats from each group. Data were analyzed by one way ANOVA with post-hoc least significance difference test. Significantly different at P≤0.05 compared with the #control and high fructose group and *with or without carrot juice groups.
Con, control diet; Con-CJ, control diet with carrot juice; HFr, high fructose diet; HFr-CJ, high fructose diet with carrot juice.
Impact of carrot juice on insulin sensitivity
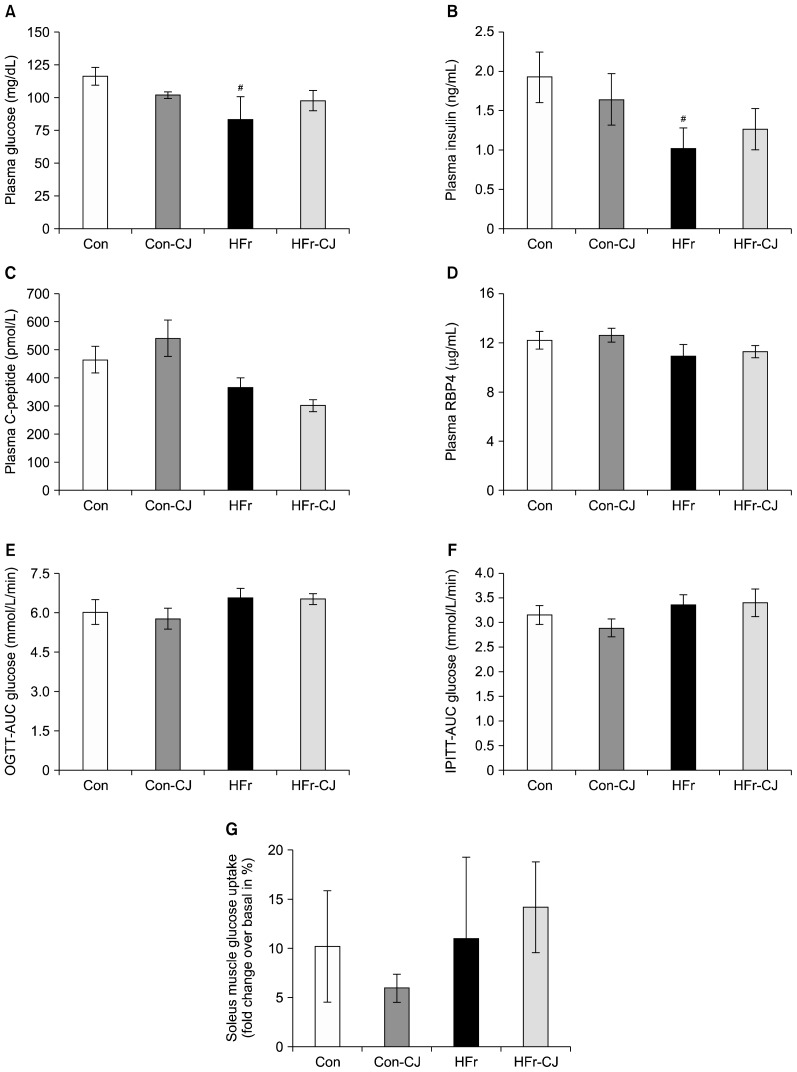
Compared to the control, plasma glucose and insulin levels were found lower in the HFr diet-fed group; however, C-peptide and RBP4 levels were comparable. Further, the calculated AUG of glucose levels for both OGTT and IPITT remained unchanged across the various groups. In addition, basal and insulin stimulated-glucose uptake of soleus muscle were also comparable among all the groups (Fig. 2A~G).
Fig. 2.
Impacts of carrot juice on insulin sensitivity markers. Results are expressed as means±SE of 7~8 rats, except for muscle glucose uptake, 4 rats from each group. Plasma glucose, insulin, plasma C-peptide, and retinol-binding protein 4 (RBP4) levels, respectively (A~D). Area under curve (AUC) of glucose for respective oral glucose tolerance test (OGTT) (E) and intra-peritoneal insulin tolerance test (IPITT) (F). Soleus muscle insulin-stimulated glucose uptake (fold change in % over basal glucose uptake) (G). Data were analyzed by one way ANOVA with post-hoc least significant difference test. #Significantly different at P≤0.05 compared between control and high fructose group. Con, control diet; Con-CJ, control diet with carrot juice; HFr, high fructose diet; HFr-CJ, high fructose diet with carrot juice.
Impact of carrot juice on liver fatty acid desaturases and elongases
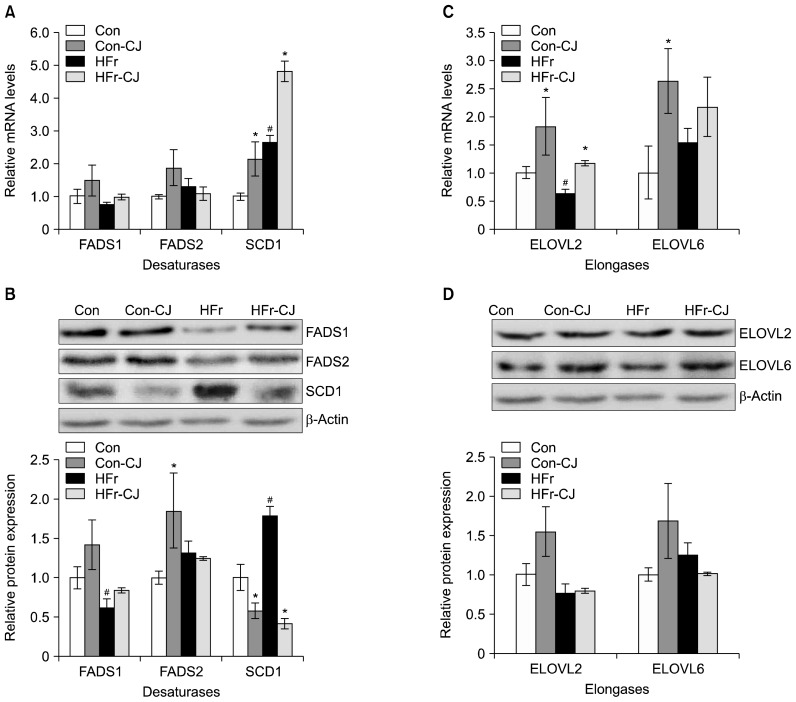
From the gene expression data, it may be noted that among the various fatty acid desaturases, such as FADS1, FADS2, and SCD1, carrot juice administration along with either the control or HFr diet resulted in augmentation of SCD1 expression at mRNA level (2 and 1.8 fold, respectively), while no changes were observed with FADS1 and FADS2 (Fig. 3A). On the contrary, SCD1 protein levels decreased by 0.4 fold and 4 fold, respectively in the control and HFr diet-fed groups (Con-CJ and HFr-CJ), due to carrot juice ingestion, when compared to their respective controls (Fig. 3B).
Fig. 3.
Impacts of carrot juice on liver fatty acid desaturases and elongases. Results are expressed as means±SE of 3 rats for gene expression and 3~4 rats for protein expression. Respective hepatic mRNA and representative immunoblots with densitometry analyses for FADS1, FADS2 and SCD1 levels (A, B). Respective hepatic mRNA and representative immunoblots with densitometry analyses for ELOVL2 and ELOVL6 levels (C, D). Data were analyzed by one way ANOVA with post-hoc least significant difference test. Significantly different at P≤0.05 compared with the #control and high fructose group and *with or without carrot juice groups. Con, control diet; Con-CJ, control diet with carrot juice; HFr, high fructose diet; HFr-CJ, high fructose diet with carrot juice; FADS, fatty acid desaturase; SCD1, stearoyl-CoA desaturase 1; ELOVL, very long chain fatty acid elongase.
ELOVL2 gene expressions in terms of mRNA levels showed significant increases by ~2.0 fold in the carrot juice administered group, fed with control (Con-CJ) and HFr (HFr-CJ) diet, while a 1.4 fold increase in ELOVL6 expression was observed only in control diet-fed group (Con-CJ) (Fig. 3C). However, the protein expressions of both ELOVL 2 and 6 were not statistically different (Fig. 3D).
DISCUSSION
The main aim of this study was to assess whether carrot juice ingestion regulates the liver lipid metabolism in high fructose-induced hepatic steatosis rat model. It was observed that the administration of carrot juice for 8 weeks from weaning did not bring down the hepatic triglyceride levels significantly, which corroborated with liver histology and immunohistological findings on lipid droplet-associated proteins, ABHD5 and perilipin. However, there was an improvement in liver function as indicated by decreased AST levels, particularly in the group that received high fructose diet.
The MUFA, such as palmitoleic (C16:1) and oleic (C18:1) acids are endogenously synthesized by SCD1, which inserts a cis double bond at delta 9 position of respective saturated fatty acids [palmitic (C16:0) and stearic (C18:0) acids] and therefore, SCD1 is also known as delta-9 desaturase. These MUFAs play a key determinant role in triglyceride synthesis. Studies have demonstrated the role of SCD1 in high fructose diet-induced hepatic steatosis (22–25). It is well known that vitamin A and its metabolites are potent transcriptional regulators of SCD1 (26, 27). Previously, we also reported up- and down-regulation of hepatic SCD1 in response to vitamin A-enriched and vitamin A-deficient diets, respectively (21,28). Hence, the finding of improved hepatic vitamin A status clearly affirms the involvement of vitamin A-mediated SCD1 up-regulation in carrot juice-ingested group at the transcriptional level.
On the contrary, despite increased SCD1, its protein levels decreased due to carrot juice feeding with the high fructose diet, which corroborated with the reduced hepatic MUFAs content, particularly, the SCD1-catalyzed products, such as palmitoleic (C16:1) and oleic (C18:1) acids. Besides hormones, various dietary factors/components, such as glucose, fructose, cholesterol, and retinoic acid induce SCD1 gene expression in liver. On the other hand, PUFA of n-6 and n-3 series and conjugated linoleic acid inhibit the expression of hepatic SCD1 gene (21–29). Previously, studies by Heinemann and Ozols have demonstrated that the SCD1 is a short-lived protein, which undergoes rapid changes/degradation in response to hormonal and dietary factors. Studies have also reported that liver microsomal endopeptidase and plasminogen-like protein preferentially and selectively degrade the hepatic SCD1 protein respectively. Further, the regulation of SCD1 is considered to be highly diverse and its expression affected by various physiological and dietary factors (30–33). It is known that in eukaryotes, cellular protein and enzyme levels are tightly controlled by different mechanisms that include transcription/post-transcription and translational/post-translational modifications (34–36). Although the data on SCD1 protein stability are lacking, we speculate that possibly other than the β-carotene (as the activity of β-carotene monooxygenase 1 is higher in rodents, mostly it is converted into retinol), the phyto-nutrients such as phytoene, phytofluene, chlorogenic acid, caffeic acid, and p-coumaric acid of the carrot juice may play a regulatory role in controlling the expression of SCD1 protein levels through the translational and/or post-translational modifications, including its protein stability. Perhaps, the precise mechanism is not addressed here. Nevertheless, this warrants further investigations, which may shed the light on the underlying molecular mechanisms that regulate the SCD1 protein levels, at least by some of these dietary factors.
Contrary to our previous study, where down-regulation of SCD1 both at protein and gene levels was associated with improved high fructose-induced hepatic steatosis (21), in the present study, no such significant improvement in hepatic steatosis was observed. These findings rise a question that why the reduction in SCD1 did not bring down the hepatic triglycerides/steatosis? In general, age and duration largely affect the outcome of dietary factors, which also derive the support from our ongoing work, wherein the treatment of carrot juice to the younger rats significantly improved the high fructose diet-induced hepatic triglyceride content (unpublished data). Therefore, it is plausible that the ingestion of carrot juice from weanling period did not have pronounced effects and may require a longer duration.
Synthesis of long-chain PUFA involves various fatty acid desaturases and elongases (37). In a recent study, we found that high fat diet feeding resulting in the elevation of liver DHA levels through ELOVL2 regulation in female mice, which also had high hepatic retinol levels (38). Pauter et al. (39) have shown ablation of ELOVL2 resulting in impaired hepatic DHA synthesis. However, in the present study, increase in liver DHA levels by carrot juice did not accompany with an increase in the expression of desaturases (FADS1 and FADS2) and elongases (ELOVL2 and ELOVL6), particularly at protein levels and thus the data are apparently inconclusive. Nevertheless, the intracellular activities of these enzymes and the role of other elongases such as ELOVL1, 3, 4, 5, and 7, in DHA synthesis cannot be ruled out. On the other hand, several studies have reported the DHA-mediated attenuation of hepatic steatosis in clinical and experimental conditions including mouse primary hepatocytes (40–45). However, the increase in DHA levels did not elicit such response in the present study, which again implies that possibly the duration of treatment may not be sufficient to bring out the favorable changes, particularly in weaning rats.
In conclusion, carrot juice administration decreases the hepatic SCD1 expression at protein and improves the long-chain n-3 PUFA [DHA (C22:6)] content. However, these changes did not reduce the high fructose-induced hepatic steatosis significantly. Hence, the study underscores the role of age and duration in eliciting the beneficial effect or outcome of the dietary factors. Nevertheless, the data clearly show that the bioactive component(s) of carrot have a potent regulatory role on SCD1 expression at protein level, and thus, may pave a way for identifying and developing nutraceuticals against the metabolic diseases such as obesity, type 2 diabetes, and NAFLD, wherein SCD1 is a key player.
ACKNOWLEDGEMENTS
The authors acknowledge the Department of Biotechnology (DBT) India for the financial support for this study (Ref No. BT/PR6145/FNS/20/566/2012). Mr. Raja Gopal Reddy gratefully acknowledge the DST for the INSPIRE Fellowship. The authors thank Mrs. K. Sharada for the support in preparing histology sections.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Laguna JC, Alegret M, Roglans N. Simple sugar intake and hepatocellular carcinoma: epidemiological and mechanistic insight. Nutrients. 2014;6:5933–5954. doi: 10.3390/nu6125933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cave M, Deaciuc I, Mendez C, Song Z, Joshi-Barve S, Barve S, McClain C. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem. 2007;18:184–195. doi: 10.1016/j.jnutbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Dongiovanni P, Lanti C, Riso P, Valenti L. Nutritional therapy for nonalcoholic fatty liver disease. J Nutr Biochem. 2016;29:1–11. doi: 10.1016/j.jnutbio.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Goran MI, Walker R, Allayee H. Genetic-related and carbohydrate-related factors affecting liver fat accumulation. Curr Opin Clin Nutr Metab Care. 2012;15:392–396. doi: 10.1097/MCO.0b013e3283544477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferder L, Ferder MD, Inserra F. The role of high-fructose corn syrup in metabolic syndrome and hypertension. Curr Hypertens Rep. 2010;12:105–112. doi: 10.1007/s11906-010-0097-3. [DOI] [PubMed] [Google Scholar]
- 6.Pool-Zobel BL, Bub A, Liegibel UM, Treptow-van Lishaut S, Rechkemmer G. Mechanisms by which vegetable consumption reduces genetic damage in humans. Cancer Epidemiol Biomarkers Prev. 1998;7:891–899. [PubMed] [Google Scholar]
- 7.Sharma KD, Karki S, Thakur NS, Attri S. Chemical composition, functional properties and processing of carrot–a review. J Food Sci Technol. 2012;49:22–32. doi: 10.1007/s13197-011-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potter AS, Foroudi S, Stamatikos A, Patil BS, Deyhim F. Drinking carrot juice increases total antioxidant status and decreases lipid peroxidation in adults. Nutr J. 2011;10:96. doi: 10.1186/1475-2891-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Törrönen R, Lehmusaho M, Häkkinen S, Hänninen O, Mykkänen H. Serum β-carotene response to supplementation with raw carrots, carrot juice or purified β-carotene in healthy non-smoking women. Nutr Res. 1996;16:565–575. doi: 10.1016/0271-5317(96)00035-8. [DOI] [Google Scholar]
- 10.Bub A, Watzl B, Abrahamse L, Delincée H, Adam S, Wever J, Müller H, Rechkemmer G. Moderate intervention with carotenoid-rich vegetable products reduces lipid peroxidation in men. J Nutr. 2000;130:2200–2206. doi: 10.1093/jn/130.9.2200. [DOI] [PubMed] [Google Scholar]
- 11.He Y, Root MM, Parker RS, Campbell TC. Effects of carotenoid-rich food extracts on the development of preneoplastic lesions in rat liver and on in vivo and in vitro antioxidant status. Nutr Cancer. 1997;27:238–244. doi: 10.1080/01635589709514532. [DOI] [PubMed] [Google Scholar]
- 12.Kobaek-Larsen M, Christensen LP, Vach W, Ritskes-Hoitinga J, Brandt K. Inhibitory effects of feeding with carrots or (−)-falcarinol on development of azoxymethane-induced preneoplastic lesions in the rat colon. J Agric Food Chem. 2005;53:1823–1827. doi: 10.1021/jf048519s. [DOI] [PubMed] [Google Scholar]
- 13.Pool-Zobel BL, Bub A, Müller H, Wollowski I, Rechkemmer G. Consumption of vegetables reduces genetic damage in humans: first results of a human intervention trial with carotenoid-rich foods. Carcinogenesis. 1997;18:1847–1850. doi: 10.1093/carcin/18.9.1847. [DOI] [PubMed] [Google Scholar]
- 14.Wehbe K, Mroueh M, Daher CF. The potential role of Daucus carota aqueous and methanolic extracts on inflammation and gastric ulcers in rats. J Complementary Integr Med. 2009;6:7. doi: 10.2202/1553-3840.1159. [DOI] [Google Scholar]
- 15.Poudyal H, Panchal S, Brown L. Comparison of purple carrot juice and β-carotene in a high-carbohydrate, high-fat diet-fed rat model of the metabolic syndrome. Br J Nutr. 2010;104:1322–1332. doi: 10.1017/S0007114510002308. [DOI] [PubMed] [Google Scholar]
- 16.Nicolle C, Gueux E, Lab C, Jaffrelo L, Rock E, Mazur A, Amouroux P, Rémésy C. Lyophilized carrot ingestion lowers lipemia and beneficially affects cholesterol metabolism in cholesterol–fed C57BL/6J mice. Eur J Nutr. 2004;43:237–245. doi: 10.1007/s00394-004-0465-3. [DOI] [PubMed] [Google Scholar]
- 17.Nicolle C, Cardinault N, Aprikian O, Busserolles J, Grolier P, Rock E, Demigné C, Mazur A, Scalbert A, Amouroux P, Rémésy C. Effect of carrot intake on cholesterol metabolism and on antioxidant status in cholesterol-fed rat. Eur J Nutr. 2003;42:254–261. doi: 10.1007/s00394-003-0419-1. [DOI] [PubMed] [Google Scholar]
- 18.Sulaeman A, Keeler L, Giraud DW, Taylor SL, Wehling RL, Driskell JA. Carotenoid content and physicochemical and sensory characteristics of carrot chips deep-fried in different oils at several temperatures. J Food Sci. 2001;66:1257–1264. doi: 10.1111/j.1365-2621.2001.tb15198.x. [DOI] [Google Scholar]
- 19.Nierenberg DW, Nann SL. A method for determining concentrations of retinol, tocopherol, and five carotenoids in human plasma and tissue samples. Am J Clin Nutr. 1992;56:417–426. doi: 10.1093/ajcn/56.2.417. [DOI] [PubMed] [Google Scholar]
- 20.Brüning JC, Michael MD, Winnay JN, Hayashi T, Hörsch D, Accili D, Goodyear LJ, Kahn CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol Cell. 1998;2:559–569. doi: 10.1016/S1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 21.Raja Gopal Reddy M, Pavan Kumar C, Mahesh M, Sravan Kumar M, Mullapudi Venkata S, Putcha UK, Vajreswari A, Jeyakumar SM. Vitamin A deficiency suppresses high fructose-induced triglyceride synthesis and elevates resolvin D1 levels. Biochim Biophys Acta. 2016;1861:156–165. doi: 10.1016/j.bbalip.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Karahashi M, Ishii F, Yamazaki T, Imai K, Mitsumoto A, Kawashima Y, Kudo N. Up-regulation of stearoyl-CoA desaturase 1 increases liver MUFA content in obese zucker but not Goto-Kakizaki rats. Lipids. 2013;48:457–467. doi: 10.1007/s11745-013-3786-2. [DOI] [PubMed] [Google Scholar]
- 23.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeyakumar SM, Lopamudra P, Padmini S, Balakrishna N, Giridharan NV, Vajreswari A. Fatty acid desaturation index correlates with body mass and adiposity indices of obesity in Wistar NIN obese mutant rat strains WNIN/Ob and WNIN/GR-Ob. Nutr Metab. 2009;6:27. doi: 10.1186/1743-7075-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki M, Dobrzyn A, Man WC, Chu K, Sampath H, Kim HJ, Ntambi JM. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 26.Stone RL, Bernlohr DA. The molecular basis for inhibition of adipose conversion of murine 3T3-L1 cells by retinoic acid. Differentiation. 1990;45:119–127. doi: 10.1111/j.1432-0436.1990.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 27.Miller CW, Waters KM, Ntambi JM. Regulation of hepatic stearoyl-CoA desaturase gene 1 by vitamin A. Biochem Biophys Res Commun. 1997;231:206–210. doi: 10.1006/bbrc.1997.6070. [DOI] [PubMed] [Google Scholar]
- 28.Jeyakumar SM, Vajreswari A, Giridharan NV. Vitamin A regulates obesity in WNIN/Ob obese rat; independent of stearoyl-CoA desaturase-1. Biochem Biophys Res Commun. 2008;370:243–247. doi: 10.1016/j.bbrc.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 29.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res. 2004;43:91–104. doi: 10.1016/S0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 30.Heinemann FS, Ozols J. Degradation of stearoyl-coenzyme A desaturase: endoproteolytic cleavage by an integral membrane protease. Mol Biol Cell. 1998;9:3445–3453. doi: 10.1091/mbc.9.12.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinemann FS, Ozols J. Stearoyl-CoA desaturase, a short-lived protein of endoplasmic reticulum with multiple control mechanisms. Prostaglandins Leukot Essent Fatty Acids. 2003;68:123–133. doi: 10.1016/S0952-3278(02)00262-4. [DOI] [PubMed] [Google Scholar]
- 32.Heinemann FS, Korza G, Ozols J. A plasminogen-like protein selectively degrades stearoyl-CoA desaturase in liver microsomes. J Biol Chem. 2003;278:42966–42975. doi: 10.1074/jbc.M306240200. [DOI] [PubMed] [Google Scholar]
- 33.Heinemann FS, Mziaut H, Korza G, Ozols J. A microsomal endopeptidase from liver that preferentially degrades stearoyl-CoA desaturase. Biochemistry. 2003;42:6929–6937. doi: 10.1021/bi034071x. [DOI] [PubMed] [Google Scholar]
- 34.Fan J, Krautkramer KA, Feldman JL, Denu JM. Metabolic regulation of histone post-translational modifications. ACS Chem Biol. 2015;10:95–108. doi: 10.1021/cb500846u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanes SD. Prolyl isomerases in gene transcription. Biochim Biophys Acta. 2015;1850:2017–2034. doi: 10.1016/j.bbagen.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee J, Ozcan U. Unfolded protein response signaling and metabolic diseases. J Biol Chem. 2014;289:1203–1211. doi: 10.1074/jbc.R113.534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guillou H, Zadravec D, Martin PG, Jacobsson A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: insights from transgenic mice. Prog Lipid Res. 2010;49:186–199. doi: 10.1016/j.plipres.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Raja Gopal, Reddy M, Asha GV, Sravan Kumar M, Uday Kumar P, Vajreswari A, Jeyakumar SM. High fat diet feeding elevates liver retinol, docosahexaenoic acid and very long chain fatty acid elongase 2 levels in C57BL/6J mice. Int J Vitam Nutr Res 2016 [Google Scholar]
- 39.Pauter AM, Olsson P, Asadi A, Herslöf B, Csikasz RI, Zadravec D, Jacobsson A. Elovl2 ablation demonstrates that systemic DHA is endogenously produced and is essential for lipid homeostasis in mice. J Lipid Res. 2014;55:718–728. doi: 10.1194/jlr.M046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng J, Peng C, Ai Y, Wang H, Xiao X, Li J. Docosahexaenoic acid ameliorates fructose-induced hepatic steatosis involving ER stress response in primary mouse hepatocytes. Nutrients. 2016;8:55. doi: 10.3390/nu8010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soni NK, Nookaew I, Sandberg A, Gabrielsson BG. Eicosapentaenoic and docosahexaenoic acid-enriched high fat diet delays the development of fatty liver in mice. Lipids Health Dis. 2015;14:74. doi: 10.1186/s12944-015-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Depner CM, Philbrick KA, Jump DB. Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a Ldlr−/− mouse model of western diet-induced nonalcoholic steatohepatitis. J Nutr. 2013;143:315–323. doi: 10.3945/jn.112.171322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fedor DM, Adkins Y, Mackey BE, Kelley DS. Docosahexaenoic acid prevents trans-10, cis-12-conjugated linoleic acid-induced nonalcoholic fatty liver disease in mice by altering expression of hepatic genes regulating fatty acid synthesis and oxidation. Metab Syndr Relat Disord. 2012;10:175–180. doi: 10.1089/met.2011.0113. [DOI] [PubMed] [Google Scholar]
- 44.Nobili V, Bedogni G, Alisi A, Pietrobattista A, Risé P, Galli C, Agostoni C. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011;96:350–353. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 45.Janczyk W, Socha P, Lebensztejn D, Wierzbicka A, Mazur A, Neuhoff-Murawska J, Matusik P. Omega-3 fatty acids for treatment of non-alcoholic fatty liver disease: design and rationale of randomized controlled trial. BMC Pediatr. 2013;13:85. doi: 10.1186/1471-2431-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]