Abstract
The aim of this study was to investigate the protective effects of juice powders from sweet orange [Citrus sinensis (L.) Osbeck], unshiu mikan (Citrus unshiu Marcow), and mini tomato (Solanum lycopersicum L.), and their major flavonoids, hesperidin, narirutin, and rutin in tert-butyl hydroperoxide (t-BHP)-induced oxidative stress in HepG2 cells. The increased reactive oxygen species and decreased glutathione levels observed in t-BHP-treated HepG2 cells were ameliorated by pretreatment with juice powders, indicating that the hepatoprotective effects of juice powders and their major flavonoids are mediated by induction of cellular defense against oxidative stress. Moreover, pretreatment with juice powders up-regulated phase-II genes such as heme oxygenase-1 (HO-1), thereby preventing cellular damage and the resultant increase in HO-1 expression. The high-performance liquid chromatography profiles of the juice powders confirmed that hesperidin, narirutin, and rutin were the key flavonoids present. Our results suggest that these fruit juice powders and their major flavonoids provide a significant cytoprotective effect against oxidative stress, which is most likely due to the flavonoid-related bioactive compounds present, leading to the normal redox status of cells. Therefore, these fruit juice powders could be advantageous as bioactive sources for the prevention of oxidative injury in hepatoma cells.
Keywords: fruit juice powder, flavonoids, hepatoprotection, heme oxygenase-1, t-BHP
INTRODUCTION
Oxidative stress is considered to play a prominent role in the etiology of many conditions such as inflammation, aging, and cancer (1). Because reactive oxygen species (ROS) formation is a naturally occurring process, mammalian cells have developed several protective mechanisms to prevent excessive ROS formation or to detoxify ROS. These mechanisms employ antioxidant molecules and protective enzymes. Phase II detoxifying genes provide a major mechanism through which cells combat the toxicities of ROS, and the induction of these genes is both highly effective and sufficient for protecting cells against oxidative stress and the toxic and neoplastic effects of many toxicants and carcinogens (2). Antioxidant enzymes like heme oxygenase-1 (HO-1) provide protection against the deleterious effects of ROS, and HO-1 in particular has been shown to have hepatoprotective roles (3). There is a large body of evidence suggesting that HO-1 plays a key role in maintaining antioxidant homeostasis during cellular stress (4). Therefore, induction of HO-1 is an important cellular mechanism in the battle against oxidative injury both in vitro and in vivo (5). In addition, glutathione (GSH) is the most abundant non-protein thiol in mammalian cells and has many critical functions, including defense against oxidative stress as a scavenger of ROS and electrophiles. GSH receives electrons and is converted to oxidative GSH, thereby preventing free radicals from releasing electrons. Thus, GSH plays a role in maintaining cellular redox homeostasis, scavenging lipid peroxides, and detoxifying reactive intermediates of xenobiotics (6). The synthesis of GSH in cells can be disrupted during aging and under pathologic conditions such as diabetes mellitus, drug-resistant tumor growth, and endotoxemia (7,8).
Citrus fruits have commercial importance due to their nutritional value and flavor. Currently, there is a great biomedical interest in citrus fruits because their consumption appears to be associated with reduced risks of colorectal, esophageal, gastric, and stomach cancers, as well as stroke (9). Citrus species contain many important phytochemicals, such as flavonoids, amino acids, triterpenes, phenolic acids, and carotenoids (10). Recently, it has been reported that orange juice concentrate has a greater amount of flavonoids, including polymethoxylated flavones, hesperidin, rutin, and narirutin, in comparison to fresh juice (11).
Sweet orange belongs to the family Rutacae and is botanically known as Citrus sinnensis L.. Sweet orange contains some important phytochemicals including liminoids, flavonoids, polyphenols, and others (12), which prevent arteriosclerosis, oxidative stress, cancer, kidney stones, stomach ulcers, inflammation, liver disease, and chronic and degenerative diseases, as well as reducing cholesterol and high blood glucose levels, thereby promoting human health (9,12). Unshiu mikan (Citrus unshiu Marcov), which also belongs to the family of Rutacae, is a seedless and easy-peeling Korean citrus fruit that accounts for 30% of the total fruits produced in Korea. Unshiu mikan has been widely used as a folk medicine in Korea, China, and Japan to improve bronchial and asthmatic conditions or blood circulation, and it has also been used as a skin-moisturizing agent (13,14). Unshiu mikan also contains many phytochemicals, such as auraptene, β-cryptoxanthin, limonine, flavonoids, and others (15), which exhibit antimutagenic (16), anti-inflammatory (17), anti-allergic (18), antioxidant (19), antitumor (20), and anti-atherosclerosis activities (21).
In addition, the mini tomato (Solanum lycopersicum L.) is one of the most popular and widely consumed vegetable crops worldwide and is also beneficial to human health because of its high contents of antioxidant and phytochemical compounds, including lycopene, β-carotene, flavonoids, hydroxycinnamic acid derivatives, and others (22). The mini tomato has achieved tremendous popularity, especially in recent years with the discovery of lycopene’s anti-oxidative activities and anti-cancer functions (23). In the present study, we investigated the protective effects of fruit juice powders and their bioactive compounds against tert-butyl hydroperoxide (t-BHP)-induced oxidative hepatotoxicity and sought to determine how fruit juice powders protect HepG2 cells from t-BHP-induced damage.
MATERIALS AND METHODS
Chemicals
Dimethyl sulfoxide (DMSO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), t-BHP, 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA), 6-hydroxy- 2,5,7,8-tetramethylchroman-2-carboxyl acid (Trolox), bovine serum albumin, quercetin, silymarin, rutin, narirutin, and hesperidin were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Antibodies against HO-1 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Minimum essential medium (MEM), penicillin-streptomycin, 0.25% trypsin-ethylenediaminetetraacetic acid (EDTA), fetal bovine serum (FBS), sodium pyruvate, and nonessential amino acids were purchased from Gibco-BRL Life Technologies (Grand Island, NY, USA). The GSH assay kit was purchased from Cayman Chemical (Ann Arbor, MI, USA). All chemicals and solvents used were of analytical grade.
Preparation of samples
Fresh sweet orange (Citrus sinensis L.), unshiu mikan (Citrus unshiu Marcow), and mini tomato (Solanum lycopersicum L.) were purchased from a commercial market in the months of March and April 2014 in Busan, Korea. First, the peel and seeds were removed from the fruits, and then the pulp portion was collected. Pulp sections were blended in an automated blender machine (HE-DBF04, Hurom, Gimhae, Korea), and the juice extract was collected and filtered to remove the debris. Filtered fruit juices were concentrated in an evaporator at a temperature of 60°C. A freeze-drying lyophilization machine (−52°C) was used to remove the water from the juice extracts. The dehydrated juice powders were then collected for further experiments. In the preparation of boiled mini tomato, the filtered tomato juice was transferred into a large pot and heated at a temperature of 70°C for 10~15 min with constant stirring. The boiled juice powders were then evaporated and lyophilized as described above, and the dehydrated juice powders were collected for further experiments.
HPLC quantitative analysis
Reverse-phase high-performance liquid chromatography (HPLC) was performed on a JASCO HPLC system (JASCO Corporation, Tokyo, Japan), consisting of a PU-1580 intelligent HPLC pump, an LG-1580-04 quaternary gradient unit, a UV-1575 intelligent UV/VIS detector, a PG-1580-54 4-line degasser, and a CO-1560 intelligent column thermostat. The BORWIN chromatographic system (JMBS Developments, Le Fontanil, France) was used for HPLC data analysis. Chromatographic separation was accomplished on a Phenomenex C18 reverse-phase column (4.6×250 cm, 5 μm, Phenomenex Inc., Torrance, CA, USA) at 30°C and was monitored at 280 nm. The linear gradient solvent system consisted of 0.5% acetic acid in water (solvent A) and 100% CH3CN (solvent B), and was adjusted from 75% (solvent A):25% (solvent B) to 0% (solvent A):100% (solvent B) over 60 min at a flow rate of 0.5 mL/min.
Cell culture
The HepG2 (human hepatocarcinoma) cell line was purchased from American Type Culture Collection (HB-8065, Manassas, VA, USA). Cells were maintained in MEM containing 2.0 mM L-glutamine, 0.1 mM non-essential amino acids, 1.0 mM sodium pyruvate, and 10% FBS at 37°C in a humidified atmosphere with 5% CO2. Medium was changed every 48 h. Samples were dissolved in DMSO before being added to cells, and the final concentration of DMSO did not exceed 0.1%.
Cell viability and cytoprotective assay in HepG2 cells
Cell viability was assessed using the MTT staining assay, as previously described (24). In brief, HepG2 cells were seeded into a 96-well plate at a density of 2.0×104 cells per well and incubated at 37°C for 24 h. The cells were then fed fresh serum-free MEM containing various concentrations of juice powders at concentrations up to 200 μg/mL, along with the flavonoids hesperidin, narirutin, and rutin at concentrations ranging from 2.5 to 100 μM, and incubation was continued for another 24 h. Control cells were treated with vehicle (<0.1% DMSO) at a concentration equal to that used in sample-treated cells. For the cytoprotective assay, after 24 h of incubation with tested samples, the medium was replaced with medium containing t-BHP (200 μM). Cells were then incubated for 2 h before the addition of 100 μL of MTT solution (0.5 mg/mL in phosphate-buffered saline, PBS). To measure the proportion of surviving cells, the medium was replaced with 100 μL of DMSO. Control cells were treated with 0.1% DMSO, at which concentration no cytotoxicity was determined by the assay. Absorbance was measured at 570 nm with a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Triplicate experiments were run for each set and averaged.
Measurement of the level of intracellular reactive oxygen species
The level of intracellular ROS was quantified using the oxidant-sensitive fluorescence probe DCFH-DA (25). The level of ROS was evaluated according to the procedure previously reported (26). The fluorescence intensities were measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using a fluorescence microplate reader (FL×800; Bio-Tek Instruments Inc., Winooski, UT, USA).
Measurement of GSH
Measurement of total intracellular glutathione (tGSH) was performed according to the procedure previously reported (26). In brief, measurement of tGSH [including the reduced and oxidized forms, GSH and glutathione disulfide (GSSG), respectively; i.e., tGSH=GSH+GSSG] was performed by the Tietze’s enzymatic recycling method using GSH reductase according to the instructions in the GSH assay kit (Cayman Chemical). In brief, the HepG2 cells were incubated in 6-well culture dishes and were treated with various juice powders at concentrations between 50 and 200 μg/mL, while the flavonoids hesperidin, narirutin, and rutin were used at concentrations ranging from 2.5 to 100 μM in MEM for 24 h. Subsequently, the cells were washed twice with cold PBS (pH 7.4), harvested using a rubber policeman, and homogenized by a freeze-thaw method with PBS in order to extract tGSH. Lysates were then cleared by centrifugation, and an aliquot of the resulting supernatant was reserved for the protein assay. For deproteinization, an equal amount of 5% w/v metaphosphoric acid was added to the residual supernatant. After centrifugation (10,000 g for 15 min at 4°C), the resultant supernatant (400 μL) was neutralized with 20 μL of 50% v/v triethanolamine to measure the tGSH levels in the sample. The tGSH concentration was then determined by the kinetic method according to the procedure described in the assay kit; concentrations are expressed as μmol/mg protein.
Western blotting analysis and HO-1 activity
Cells were treated with juice powders and flavonoids for 24 h. After treatment, cells were collected and washed with cold PBS. The harvested cells were then lysed in the ice-cold lysis buffer and kept on ice for 30 min. After centrifugation at 13,000 g for 15 min at 4°C, the supernatants were collected, and the protein contents were determined using the Bradford method with bovine serum albumin as the standard. Aliquots of the lysates (40 μg of protein) were boiled for 5 min, and electrophoresis was performed on a 10% sodium dodecyl sulfate-polyacrylamide gel. Proteins in the gels were transferred onto polyvinylidene difluoride membranes, which were then incubated with rabbit polyclonal HO-1 or mouse monoclonal β-actin antibodies (1:1,000 dilutions) at 4°C overnight and then with horseradish peroxidase-conjugated secondary antibody (1:2,000 dilutions) (Cell Signaling, Beverly, MA, USA) for 1 h. Finally, protein bands were detected using an enhanced chemiluminescence Western blotting detection kit (Pierce Biotechnology, Rockford, IL, USA). Bands were visualized using a LAS3000® Luminescent image analyzer (Fujifilm, Tokyo, Japan) and quantified by densitometry analysis using PDQuest software (Version 7.0, Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
Data are expressed as the mean±standard deviation (SD) of at least 3 separate experiments unless otherwise indicated. Data were analyzed using one-way ANOVA, followed by Student’s t-tests for multiple comparisons. In all statistical analyses performed, P-values were two-tailed, and P<0.05 was considered statistically significant.
RESULTS
Quantitative analysis of major flavonoids from juice powders
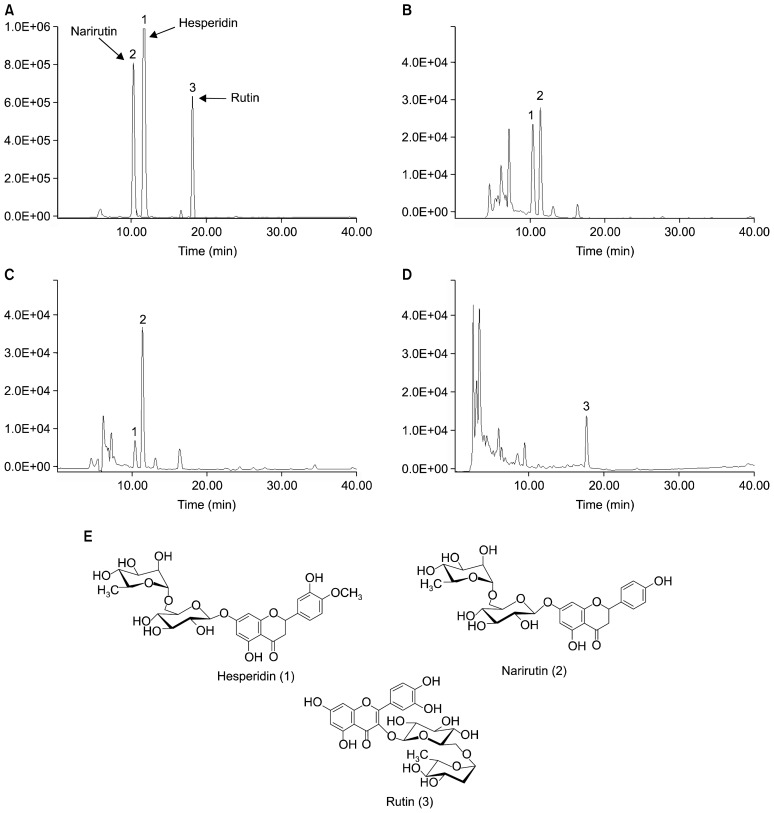
Simultaneous HPLC quantitative analysis was conducted to determine the relative presence of hesperidin, narirutin, and rutin in unshiu mikan, sweet orange, and tomato (Fig. 1). The retention times for the standard compounds of hesperidin, narirutin, and rutin were 11.15, 10.06, and 17.50 min, respectively. The unshiu mikan juice powder exhibited major peaks for hesperidin and narirutin with retention times of 11.32 and 10.13 min, respectively, while sweet orange exhibited major peaks for hesperidin and narirutin with retention times of 11.31 and 10.31 min. The major peak for boiled tomato was for rutin at a retention time of 17.57 min (Fig. 1). The quantitative amounts of flavonoids in unshiu mikan were 6.16 and 9.23 mg/g (hesperidin and narirutin, respectively), while those of sweet orange were 6.94 mg/g (hesperidin) and 0.372 mg/g (narirutin). In boiled tomato, the amount of the flavonoid rutin was found to be 5.77 mg/g.
Fig. 1.
HPLC chromatograms of standard (A), unshiu mikan (B), sweet orange (C), and boiled tomato (D). 1, hesperidin; 2, narirutin; 3, rutin (E).
Cytotoxicities of juice powders and their major flavonoids
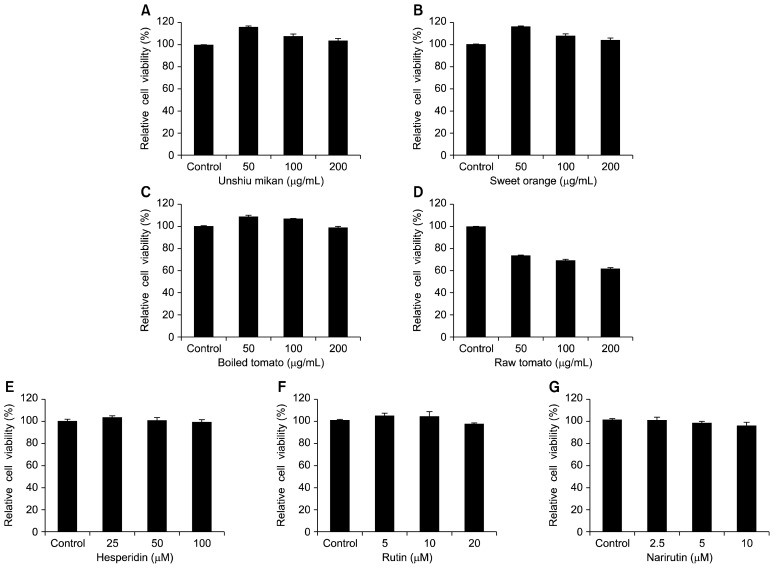
Before determining whether the juice powders of unshiu mikan, sweet orange, and boiled tomato and the flavonoids hesperidin, narirutin, and rutin possessed any hepatoprotective activity, the cytotoxicities of these juice powders and flavonoids on HepG2 cells were first measured by the MTT assay. HepG2 cells were pretreated with the unshiu mikan, sweet orange, and boiled tomato juice powders at concentrations up to 200 μg/mL, as well as with the flavonoids hesperidin, narirutin, and rutin in a concentration range of 0~100 μM, followed by incubation for 24 h. Unshiu mikan, sweet orange, and boiled tomato juice powders did not exhibit any cytotoxicity up to 200 μg/mL, as shown in Fig. 2A~C. However, raw tomato exhibited cytotoxicity at concentrations above 50 μg/mL, as shown in Fig. 2D. The flavonoids hesperidin, at concentrations up to 100 μM, and rutin, at a concentration of 20 μM, did not cause any cytotoxic effects, as shown in Fig. 2E~F. Furthermore, narirutin also did not cause any cytotoxic effects at concentrations up to 10 μM, as shown in Fig. 2G. Therefore, these concentrations were used in subsequent hepatoprotective assays with unshiu mikan, sweet orange, and boiled tomato juice powders.
Fig. 2.
Effects of unshiu mikan (A), sweet orange (B), boiled tomato (C), raw tomato (D), hesperidin (E), rutin (F), and narirutin (G) on cell viability in HepG2 cells. Cells were pretreated with the indicated concentrations of 50~200 μg/mL of juice powders from oranges and boiled tomatoes and with hesperidin, rutin, and narirutin at concentrations of 2.5 to 100 μM for 24 h. Data shown represent mean±standard deviation of triplicate experiments.
Cytoprotective effects of juice powders and their key flavonoids on t-BHP-induced hepatotoxicity in HepG2 cells
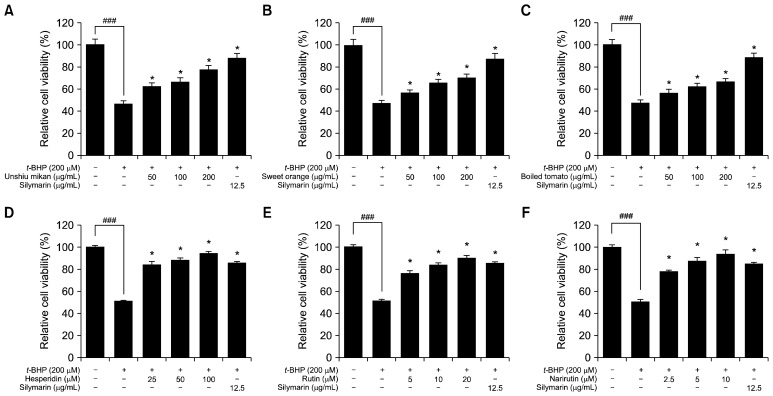
To evaluate the hepatoprotective effect on t-BHP-treated HepG2 cells, cells were pretreated with powders from oranges and boiled tomato at various concentrations (50, 100, and 200 μg/mL) as well as with flavonoids within the concentration range of 2.5~100 μM for 24 h before being treated with t-BHP (200 μM). Thereafter, cells were incubated for 2 h. Exposure to t-BHP (200 μM) significantly decreased the viability of cells to about 49%, while pretreatment of HepG2 cells with oranges and boiled tomato at concentrations of 50, 100, and 200 μg/mL significantly increased the cell viability and protected cells against t-BHP-induced cytotoxicity in a dose-dependent manner, as shown in Fig. 3A~C. Additionally, when HepG2 cells were treated with t-BHP only (200 μM), the viability levels of the cells were reduced sharply by up to 51%, whereas pretreatment of HepG2 cells with hesperidin, narirutin, and rutin at concentrations up to 100 μM significantly increased the cell viability and demonstrated marked dose-dependent cytoprotective effects against t-BHP-induced cytotoxicity, as shown in Fig. 3D~F. To protect against t-BHP-induced cell damage, cells were treated with unshiu mikan at concentrations of 200, 100, and 50 μg/mL, and the cell viabilities were 71±0.1%, 66±0.5%, and 62±0.04%, respectively. Treatment with sweet orange yielded cell viabilities of 69±0.04%, 65± 0.51%, and 61±1.0% at concentrations of 200, 100, and 50 μg/mL, respectively, and boiled tomato treatment resulted in viabilities of 62±0.2%, 58±0.7%, and 55±0.04 %, respectively, when used at 200, 100, and 50 μg/mL. Moreover, cell viabilities achieved upon treatment with hesperidin at concentrations of 100, 50, and 25 μM were found to be 94±1.0%, 88±2.0%, and 84±1.0%, respectively. Additionally, there was no significant difference in cell viabilities achieved with treatment of hesperidin at concentrations of 25 and 50 μM. In the case of rutin, the relative cell viabilities achieved with concentrations of 5, 10, and 20 μM were 75±2.0%, 83±1.1%, and 90±1%, respectively. Pretreatment with narirutin also increased the viability of HepG2 cells at concentrations of 2.5, 5, and 10 μM, the rates of viability were found to be 78± 1.0%, 87±2.0%, and 93±3.1%, respectively. The positive control silymarin increased cell viabilities by 85±0.5% when used at a concentration of 12.5 μg/mL. Thus, it is clear that pretreatment of HepG2 cells with unshiu mikan, sweet orange, boiled tomato, and bioactive flavonoids markedly protected cells against t-BHP-induced cytotoxicity.
Fig. 3.
Cytoprotective effects of unshiu mikan (A), sweet orange (B), boiled tomato (C), hesperidin (D), rutin (E), and narirutin (F) on tert-butyl hydroperoxide (t-BHP)-treated HepG2 cells. Cells were pretreated with the indicated concentrations (50 to 200 μg/mL) of juice powders and with hesperidin, rutin, and narirutin at concentrations of 2.5 to 100 μM for 24 h. After that time, cells were treated with t-BHP (200 μM) and incubated for 2 h. Control values were obtained in the absence of t-BHP and juice powders and flavonoids. Silymarin was used as a positive control. Data are presented as mean±SD of triplicate experiments. Significantly different from the t-BHP-treated group at *P<0.05 and the control group at ### P<0.001.
Effects of juice powders and their key flavonoids on the levels of intracellular ROS in t-BHP-induced HepG2 cells
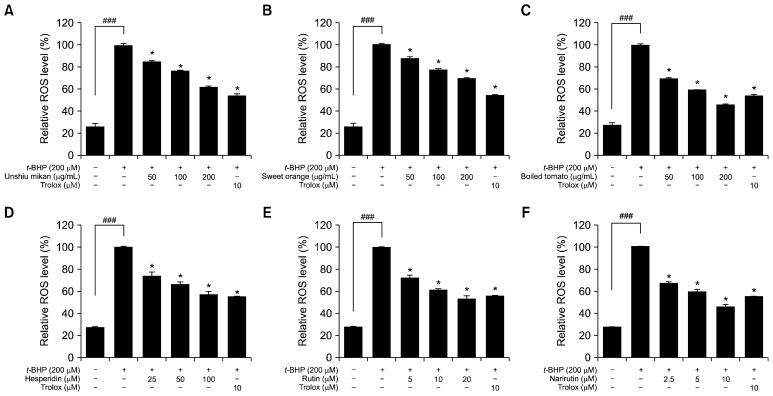
To determine if the observed cytoprotective effects of unshiu mikan, sweet orange, and boiled tomato can be attributed to a reduction in oxidative stress, we next determined the effects of these juice powders and their flavonoids on ROS generation in HepG2 cells exposed to t-BHP (200 μM). Exposure to t-BHP (200 μM) modified the redox status of the cells, resulting in generation of ROS, which was estimated using the ROS-sensitive fluorescence indicator DCFH-DA. ROS generation increased significantly when the cells were treated with t-BHP (200 μM) to levels of about 100%, indicating that t-BHP had a strong effect on ROS generation in HepG2 cells. Pretreatment of HepG2 cells with unshiu mikan, sweet orange, and boiled tomato in a concentration range of 0~200 μg/mL and pretreatment with hesperidin, rutin, and narirutin in a concentration range of 0~100 μM, significantly inhibited ROS generation and protected cells against ROS-induced oxidative stress in a concentration-dependent manner compared to t-BHP-induced HepG2 cells, as shown in Fig. 4. The relative ROS levels achieved with treatment of unshiu mikan at the concentrations of 50, 100, and 200 μg/mL were found to be 84%, 76%, and 61%, respectively. Sweet orange at concentrations of 50, 100, and 200 μg/mL inhibited the ROS level to 88%, 78%, and 69%, respectively. Pretreatment with boiled tomato powder significantly inhibited ROS level to 69%, 58%, and 46% when used at concentrations of 50, 100, and 200 μg/mL, respectively. In addition, the relative ROS level achieved with treatment of hesperidin at concentrations of 25, 50, and 100 μM was found to be 74%, 66%, and 57%, respectively. Rutin at the concentrations of 5, 10, and 20 μM decreased ROS level to 72%, 61%, and 52%, respectively. Pretreatment with narirutin significantly inhibited ROS level at the concentrations of 2.5, 5, and 10 μM to the levels of 67%, 59%, and 46%, respectively. The positive control Trolox at a concentration of 10 μM inhibited ROS production to about 54%. These results clearly demonstrate that the orange and tomato juice powders, as well as their major flavonoids, act as scavengers of ROS induced by t-BHP in HepG2 cells.
Fig. 4.
Effects of unshiu mikan (A), sweet orange (B), boiled tomato (C), hesperidin (D), rutin (E), and narirutin (F) on tert-butyl hydroperoxide (t-BHP)-induced reactive oxygen species (ROS) generation in HepG2 cells. Cells pretreated with different concentrations (50 to 200 μg/mL) of juice powders and hesperidin, rutin, and narirutin at concentrations of 2.5 to 100 μM or Trolox (10 μM) for 1 h were stimulated with 200 μM t-BHP for 30 min. ROS levels were measured by 2′,7′-dichlorodihydrofluorescein diacetate with fluorescent analysis. The control values were obtained in the absence of t-BHP (200 μM), juice powders, and flavonoids and after the addition of t-BHP (200 μM). Trolox was used as a positive control. Data are expressed as the mean±SD of three independent experiments. Significantly different from the t-BHP-treated group at *P<0.05 and the control group at ###P<0.001.
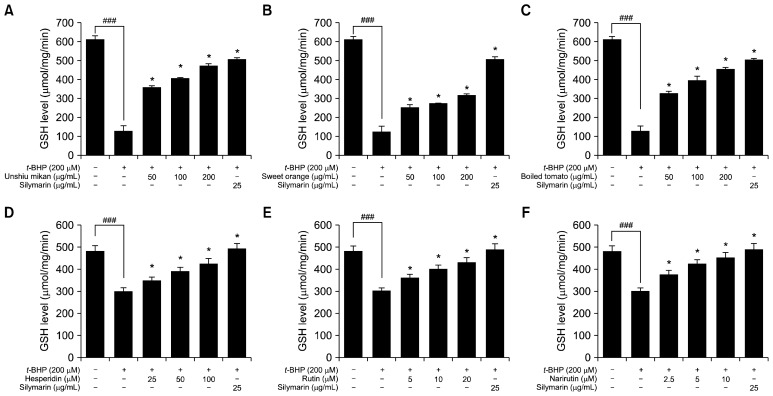
Effects of juice powders and their key flavonoids on intracellular GSH level in t-BHP-treated HepG2 cells
To further examine the antioxidant enzymes of cultured HepG2 cells exposed to t-BHP (200 μM), the activity level of GSH was determined. Exposure of t-BHP (200 μM) to HepG2 cells greatly decreased the GSH level. Nevertheless, the reduced level of intracellular GSH was significantly rescued and increased when cells were treated with increasing concentrations of unshiu mikan, sweet orange, and boiled tomato juice powders in a dose-dependent manner, as shown in Fig. 5A~C. Unshiu mikan, sweet orange, and boiled tomato treatments markedly increased the depleted GSH level in t-BHP-treated (200 μM) HepG2 cells. Moreover, unshiu mikan and boiled tomato at concentrations of 100 μg/mL and 200 μg/mL restored the GSH level near to that of the untreated control group. In addition, the reduced level of intracellular GSH was also significantly rescued and increased by increasing concentrations of hesperidin, rutin, and narirutin in a dose-dependent manner, as shown in Fig. 5D~F. All of the flavonoids markedly increased the GSH level depleted by t-BHP treatment (200 μM) in HepG2 cells. Particularly, narirutin at concentrations of 5 μM and 10 μM restored the GSH level near to that of the untreated control group. Pretreatment with 25 μg/mL of the positive control silymarin significantly increased the GSH levels. These findings clearly illustrate that juice powders from oranges and tomato, as well as their major flavonoids hesperidin, rutin, and narirutin, positively regulated the GSH content in t-BHP-treated HepG2 cells and provide an antioxidant defense system against oxidative stress.
Fig. 5.
Effects of unshiu mikan (A), sweet orange (B), boiled tomato (C), hesperidin (D), rutin (E), and narirutin (F) on intracellular glutathione (GSH) level in tert-butyl hydroperoxide (t-BHP)-treated HepG2 cells. Cells were pretreated with the indicated concentrations (50 to 200 μg/mL) of juice powders and with hesperidin, rutin, and narirutin at concentrations of 2.5 to 100 μM for 24 h. Then t-BHP (200 μM) was added, and cells were incubated for 2 h. Whole cell proteins were isolated and used for GSH assays. The data shown represent the mean±SD of triplicate experiments. Significantly different from the t-BHP-treated group at *P<0.05 and the control group at ### P<0.001.
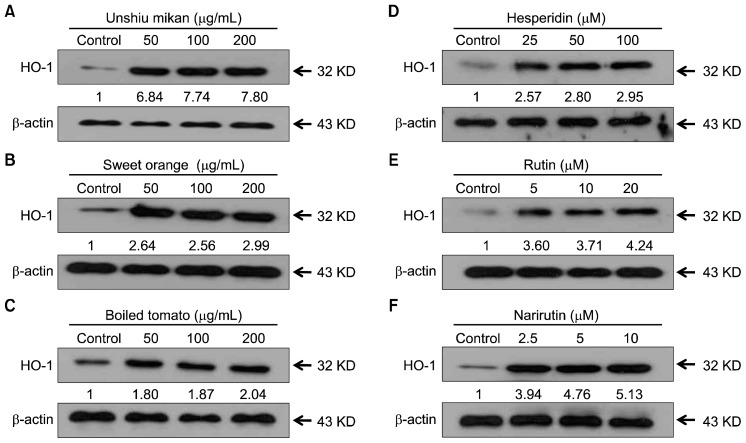
Effects of juice powders and their key flavonoids on HO-1 expression
HO-1, an enzyme essential for heme degradation, has been shown to exert anti-oxidative effects under various conditions (5). We first determined the effects of various nontoxic concentrations of orange and tomato juice powders on HO-1 induction. Cells treated with these juice powders for 24 h showed a concentration-dependent increase in HO-1 protein expression (Fig. 6A~C). This increased expression level suggests that juice powders enhance the expression of HO-1. In addition, we also determined the effects of various nontoxic concentrations of flavonoids on HO-1 induction. Cells were treated with hesperidin, rutin, and narirutin for 24 h and showed a concentration-dependent increase in HO-1 protein expression (Fig. 6D~F). This increase suggests that flavonoids also enhance the expression of HO-1. Overall, our data provide evidence to support the view that expression of HO-1, a key phase II detoxifying enzyme, is involved in the mechanism responsible for the hepatoprotective effects of juice powders from oranges and tomatoes as well as their flavonoids, hesperidin, rutin, and narirutin.
Fig. 6.
Effects of unshiu mikan (A), sweet orange (B), boiled tomato (C), hesperidin (D), rutin (E), and narirutin (F) on heme oxygenase-1 (HO-1) protein expression. Cells were exposed to various concentrations of juice powders and hesperidin, rutin, and narirutin for 24 h, and expression levels of proteins, including HO-1, were analyzed by Western blotting.
DISCUSSION
Clinical trials and epidemiological studies have established an inverse correlation between fruit and vegetable dietary intake and the occurrence of inflammatory diseases, cardiovascular diseases, cancer, and aging-related disorders (27,28). Dietary antioxidants, including vitamin C, polyphenols, and carotenoids, are believed to be effective nutrients in the prevention of these oxidative stress-related diseases (29). Although fruits and vegetables are primary sources for these “nutrient” antioxidants, other dietary components may also be important protective agents. Citrus fruits have commercial importance due to their nutritional value and special flavor. Citrus fruit juice is rich in vitamin C and other bioactive compounds including flavonoids and phenolic acids, which are all potentially health-promoting (10). Among citrus fruit juices, orange juice is the most popular fruit juice worldwide. Consumers perceive orange juice as being a healthy and natural source of vitamins and other health-promoting nutrients, resulting in increasing worldwide demand and production (30). Additionally, the convenient packaging and long shelf life of juices are advantageous compared to fresh fruits (30). Recent intervention studies have demonstrated the health benefits of long-term orange juice consumption, such as an increased total antioxidant status, lower total cholesterol levels, and the prevention of endotoxin increases after meals high in fat and carbohydrate (31–33). It has previously been reported that concentrated orange juice has greater flavonoid content, including the polymethoxylated flavones hesperidin, narirutin, and naringin, in comparison to fresh juice (11).
Several epidemiological studies have indicated a beneficial effect of tomato consumption in the prevention of some major chronic diseases, such as some types of cancer and cardiovascular disease (34). In the present study, we investigated the hepatoprotective activity of mini tomato, one of the most important types of fresh fruit consumed in South Korea. Previously, Crozier et al. (35) found higher concentrations of flavonoids in mini tomatoes compared to normal-sized tomatoes. Stewart et al. (36) also suggested that the greater skin/volume ratio of mini tomatoes enhanced their flavonoid content because these compounds are present within the skin of the fruit. The cytotoxicity of raw tomato juice powder on HepG2 cells can be explained on the basis of previous reports. Raiola et al. (37) has reported that 3 varieties of tomato juice powder showed significant anticancer activities in different types of cancer cells. It has also been reported that raw tomato juice extracts exhibit a significant cytotoxicity activity in HepG2 cells (38). Thus, we proceeded with boiled mini tomatoes for our experiments. Previously, many researchers have reported that boiled tomato is more effective than raw tomato for human health (39–41). Chang et al. (41) have also reported that freeze-drying or hot-air-drying tomatoes increases the antioxidative properties due to the enhanced availability of ascorbic acid, total phenolics, total flavonoids, and lycopene content compared to raw tomato. In this study, we noted that the boiled mini tomato had increased total phenolics and total flavonoid content compared to raw mini tomato.
Hepatic injury induced by t-BHP has been well characterized and is commonly used as a model for screening the hepatoprotective activities of drugs (42). Numerous studies have noted that t-BHP, an organic hydroperoxide, induces an array of cellular dysfunctions, including generation of peroxyl radicals, peroxidation of membrane lipids, deletion of GSH and protein thiol, and DNA damage, eventually leading to cell death (43). In addition, t-BHP has been implicated in oxidative stress that results from intracellular production of ROS (44). Furthermore, in hepatocyte cultures and in livers, t-BHP can be metabolized to free radical intermediates by cytochrome P-450, and these intermediates can initiate lipid peroxidation and oxidative stress (45). The antioxidant and free radical scavenging activities of many substances have been assessed, and many substances that possess anti-hepatotoxic activity also show strong antioxidant activity (46). In this study, an intracellular system was employed to test the cytotoxic effects of juice powders from oranges and tomatoes, as well as their key flavonoids. In the case of cell viability following oxidative damage by t-BHP, unshiu mikan, sweet orange, and boiled tomato showed significant protective activities in HepG2 cells. However, in the case of raw tomato, cell viability was not increased to a significant level; thus, raw tomato was not found to exhibit any cytoprotective effects at the indicated concentrations. However, unshiu mikan, sweet orange, and boiled tomato prevented t-BHP-induced cell death, evidenced by the MTT test indicating that they have cytoprotective activities. Moreover, hesperidin, rutin, and narirutin, which are major flavonoids present in juice powders of unshiu mikan, sweet orange, and boiled tomato, displayed cell protection against t-BHP-induced oxidative stress. These protective effects of hesperidin, rutin, and narirutin may be, in part, responsible for the cytoprotection of the juice powders.
Oxidative stress can be defined as an imbalance between the oxidant and antioxidant systems. Under normal circumstances, the levels of ROS are low enough to be removed by the natural defense systems of the cell. However, when ROS is induced by oxidants to such an extent that cellular defenses are overwhelmed, the cells are exposed to oxidative stress, consequently leading to cell injury (47). Thus, phytochemical or antioxidant therapy is therefore regarded as a promising strategy to prevent cells from oxidative damage (48). In addition, apoptosis of the cells can be induced by ROS, leading to pathological cell death. According to the results, treatment with 200 μM t-BHP induced ROS generation. As expected, pretreatment with unshiu mikan, sweet orange, and boiled tomato juice powders or with hesperidin, rutin, and narirutin decreased t-BHP-mediated ROS generation. These results suggest that the protective effects of orange and tomato juice powders and hesperidin, rutin, and narirutin on the cytotoxicity of HepG2 cells may be, in part, attributed to the scavenging of ROS, consequently preventing t-BHP-induced oxidative damage in HepG2 cells. Chen et al. (49) have previously reported that sweet orange peel extract and bioactive flavonoid hesperidin decreased ROS generation, findings that are similar to our current results.
GSH, which is widely distributed in animal tissues, plants, and microorganisms, is well known to function both as a reductant and as a nucleophile due to its sidechain sulfhydryl residue in the cysteine of GSH (50). Numerous studies have shown that GSH expression level is increased by some extracts and naturally occurring phenolic and flavonoid compounds (49,51). In an attempt to further explain the observed cytoprotective effect of juice powders from oranges and tomatoes, we determined the GSH level in t-BHP-induced HepG2 cells co-incubated with unshiu mikan, sweet orange, and boiled tomato juice powders and their key flavonoids, hesperidin, rutin, and narirutin. According to the data obtained, 200 μM t-BHP was significantly cytotoxic to HepG2 cells, accompanied by ROS generation and a marked depletion in GSH level. Indeed, severe depletion of GSH level makes cells more vulnerable to oxidative damage and is normally associated with calcium homeostasis disruption, which ultimately causes cell death. However, treatment with juice powders and their flavonoids, hesperidin, rutin, and narirutin, prevented the decrease in GSH level induced by t-BHP. Among the juice powders, unshiu mikan, sweet orange, and boiled tomato markedly increased the depleted GSH level in t-BHP-treated HepG2 cells due to the high phenolic and flavonoid contents contained in the powders. Moreover, the flavonoid narirutin in particular manifestly increased the depleted GSH level in t-BHP-treated HepG2 cells. Chen et al. (49) have previously reported that hesperidin markedly reversed the depletion of GSH level in t-BHP-treated HepG2 cells, which is similar to our results. The protection provided against GSH depletion was probably the most relevant effect of the orange and tomato juice powders, making them effective cellular reducing agents and thereby leading to the detoxification of xenobiotics.
The induction of the phase II enzyme system is an important event in the cellular stress response, during which a diverse array of electrophilic and oxidative toxicants can be eliminated or inactivated before they damage critical cellular macromolecules (2). Antioxidant agents can either scavenge ROS or stimulate the detoxification mechanism within cells, resulting in removal of ROS. HO-1 is a key enzyme of the antioxidant defense system. Increased HO-1 activity leads to enhanced protection against free radicals produced by intrinsic or extrinsic stimuli. HO-1 is the inducible form of HO that catalyzes the conversion of heme into biliverdin, carbon monoxide, and free iron as a rate-limiting enzyme (52). Previous studies have demonstrated the potent antioxidant and cytoprotective activities of heme-derived metabolites produced by HO-1 (53). Therefore, enhanced activity of HO-1 may protect HepG2 cells against possible oxidative damage. The cytoprotective properties of antioxidants have been partially attributed to their ability to induce cytoprotective enzymes. Among the various cytoprotective enzymes, HO-1 expression has been considered as an adaptive and beneficial response to oxidative stress in a wide variety of cells (54). The diverse nature of the stimuli that can induce HO-1 suggests that the molecular mechanisms that regulate HO-1 are complex. Our results showed that unshiu mikan, sweet orange, and boiled tomato juice powders and the flavonoids hesperidin, rutin, and narirutin increased HO-1 protein expression level in HepG2 cells (Fig. 6). The increase in HO-1 expression by unshiu mikan, sweet orange, and boiled tomato juice powders and by the flavonoids investigated conferred cytoprotection against t-BHP-induced oxidative stress. These results suggest that anti-oxidant gene expression induced by orange and tomato juice powders and the flavonoids hesperidin, rutin, and narirutin serves as an important mechanism for the cytoprotective effects of these juice powders. There are many reports showing that HO-1 is induced by various phytochemicals, but the present result is the first demonstration that juice powders of oranges and boiled tomatoes, as well as flavonoids hesperidin, rutin, and narirutin, are potent inducers of HO-1 expression in HepG2 cells.
Many studies have shown that naturally occurring compounds, such as polyphenolic compounds, exert remarkable biological activity. The peel and pulp of citrus fruits and tomatoes contain phenolic and flavonoid compounds. Although it was previously reported that orange peel and cherry tomato contain significant levels of polyphenolic compounds (49,55), this study is the first to measure the total phenolic and flavonoid contents of oranges and tomato juice powders. The levels of polyphenolic compounds in unshiu mikan, sweet orange, and boiled tomato were 7.74, 5.48, and 9.66 mg/g, respectively, whereas the flavonoid contents were 1.83, 1.0, and 0.90 mg/g. We suggest that the levels of polyphenolic compounds in orange and tomato juice powders may contribute to their cytoprotective effects on t-BHP-treated HepG2 cells. In addition, the HPLC analyses of orange juice powders identified hesperidin and narirutin as the major compounds contained in the fruits, while rutin was identified as the main compound in tomatoes. Hesperidin and narirutin are flavanone O-glycosides, whereas rutin is a flavone O-glycoside. Flavanones and flavones have been reported to possess a wide range of biological and pharmacological activities. For instance, hesperidin, a flavanone glycoside, has been reported to have hepatoprotective, antioxidant, anticarcinogenic, antihypotensive, antimicrobial, anti-inflammatory, diuretic, analgesic, and hypolipidemic activities, as well as to improve vascular integrity and decrease capillary permeability (49,56,57). Narirutin, a flavanone, also has a wide range of therapeutic properties, including anti-adipogenic (58), anti-inflammatory, anti-allergic (59), antioxidant, and hepatoprotective activities (60). Rutin, a flavone, has been reported to possess a wide range of biological activities such as antibacterial (61), anti-oxidant (62), and antiproliferative activities (63). In the present study, hesperidin, narirutin, and rutin significantly inhibited oxidative stress in HepG2 cells induced by t-BHP. These activities may be, in part, responsible for the ameliorating effects of orange and tomato juice powders on the oxidative damage in HepG2 cells induced by t-BHP. In addition, other than hesperidin, narirutin, and rutin, orange and tomato contain other bioactive compounds such as polymethoxyflavones, phenolic acid, limonoids, flavonoids, and fiber (15,22,49). In the present study, HPLC analysis indicated the presence of other compounds in the juice powders of orange and tomato, suggesting that other uncharacterized bioactive compounds are present in these juice powders. Therefore, the flavonoids together with additional unidentified compounds may exhibit synergistic effects, leading to the protective effect of orange and tomato juice powders against oxidative stress in t-BHP-induced HepG2 cells.
CONCLUSION
In the present study, our results indicate that the juice powders of orange, unshiu mikan, and boiled tomato, which showed no significant toxicity when used at concentrations up to 200 μg/mL, could serve as candidates with strong hepatoprotective effects against t-BHP-induced hepatoma cell damage. A possible mechanism for this protection is the significant scavenging of ROS in t-BHP-induced HepG2 cells. The decrease in ROS content appeared in parallel with up-regulation of GSH level and antioxidant enzyme activity. Moreover, the direct scavenging of ROS by juice powders may increase the expression level of HO-1. The protective effects of juice powders and their major flavonoids in 200 μM t-BHP-induced HepG2 cells significantly inhibited the cytotoxic effects and also may be associated with positive regulation of GSH levels and decrease in ROS production, thereby preventing cellular damage and the resultant increase in HO-1 activity. Therefore, these fruit juice powders could be advantageous as sources of bioactive compounds for the prevention of oxidative injury in hepatoma cells.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2012R1A6A1028677).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Breimer LH. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: the role of DNA base damage. Mol Carcinog. 1990;3:188–197. doi: 10.1002/mc.2940030405. [DOI] [PubMed] [Google Scholar]
- 2.Rushmore TH, Kong ANT. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab. 2002;3:481–490. doi: 10.2174/1389200023337171. [DOI] [PubMed] [Google Scholar]
- 3.Kapitulnik J. Bilirubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol Pharmacol. 2004;66:773–779. doi: 10.1124/mol.104.002832. [DOI] [PubMed] [Google Scholar]
- 4.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response element pathway. Curr Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 5.Morse D, Choi AMK. Heme oxygenase-1: the “emerging molecule” has arrived. Am J Respir Cell Mol Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 6.Sies H. Glutathione and its role in cellular functions. Free Radic Biol Med. 1999;27:916–921. doi: 10.1016/S0891-5849(99)00177-X. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Liu H, Liu RM. Gender difference in glutathione metabolism during aging in mice. Exp Gerontol. 2003;38:507–517. doi: 10.1016/S0531-5565(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida K, Hirokawa J, Tagami S, Kawakami Y, Urata Y, Kondo T. Weakened cellular scavenging activity against oxidative stress in diabetes mellitus: regulation of glutathione synthesis and efflux. Diabetologia. 1995;38:201–210. doi: 10.1007/BF00400095. [DOI] [PubMed] [Google Scholar]
- 9.Tripoli E, La Guardia M, Giammanco S, Di Majo D, Giammanco M. Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 2007;104:466–479. doi: 10.1016/j.foodchem.2006.11.054. [DOI] [Google Scholar]
- 10.Niu LY, Wu JH, Liao XJ, Chen F, Wang ZF, Zhao GH, Hu XS. Physicochemical characteristics of orange juice samples from seven cultivars. Agric Sci China. 2008;7:41–47. doi: 10.1016/S1671-2927(08)60020-6. [DOI] [Google Scholar]
- 11.Cesar TB, Aptekmann NP, Araujo MP, Vinagre CC, Maranhão RC. Orange juice decreases low-density lipoprotein cholesterol in hypercholesterolemic subjects and improves lipid transfer to high-density lipoprotein in normal and hypercholesterolemic subjects. Nutr Res. 2010;30:689–694. doi: 10.1016/j.nutres.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Etebu E, Nwauzoma AB. A review on sweet orange (Citrus sinensis L Osbeck): health, diseases and management. Am J Res Commun. 2014;2:33–70. [Google Scholar]
- 13.Kang SI, Shin HS, Kim HM, Hong YS, Yoon SA, Kang SW, Kim JH, Kim MH, Ko HC, Kim SJ. Immature Citrus sunki peel extract exhibits antiobesity effects by β-oxidation and lipolysis in high-fat diet-induced obese mice. Biol Pharm Bull. 2012;35:223–230. doi: 10.1248/bpb.35.223. [DOI] [PubMed] [Google Scholar]
- 14.Park HJ, Jung UJ, Cho SJ, Jung HK, Shim S, Choi MS. Citrus unshiu peel extract ameliorates hyperglycemia and hepatic steatosis by altering inflammation and hepatic glucose- and lipid-regulating enzymes in db/db mice. J Nutr Biochem. 2013;24:419–427. doi: 10.1016/j.jnutbio.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Wang J, Gu S, Liu Z, Zhang Y, Zhang X. Simultaneous determination of flavonoids in different parts of Citrus reticulata ‘Chachi’ fruit by high performance liquid chromatography-photodiode array detection. Molecules. 2010;15:5378–5388. doi: 10.3390/molecules15085378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kootstra A. Protection from UV-B-induced DNA damage by flavonoids. Plant Mol Biol. 1994;26:771–774. doi: 10.1007/BF00013762. [DOI] [PubMed] [Google Scholar]
- 17.Lin N, Sato T, Takayama Y, Mimaki Y, Sashida Y, Yano M, Ito A. Novel anti-inflammatory actions of nobiletin, a citrus polymethoxy flavonoid, on human synovial fibroblasts and mouse macrophages. Biochem Pharmacol. 2003;65:2065–2071. doi: 10.1016/S0006-2952(03)00203-X. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi S, Tanabe S. Evaluation of the anti-allergic activity of Citrus unshiu using rat basophilic leukemia RBL-2H3 cells as well as basophils of patients with seasonal allergic rhinitis to pollen. Int J Mol Med. 2006;17:511–515. [PubMed] [Google Scholar]
- 19.Di Majo D, Giammanco M, La Guardia M, Tripoli E, Giammanco S, Finotti E. Flavanones in Citrus fruit: structure-antioxidant activity relationships. Food Res Int. 2005;38:1161–1166. doi: 10.1016/j.foodres.2005.05.001. [DOI] [Google Scholar]
- 20.Bracke M, Vyncke B, Opdenakker G, Foidart JM, De Pestel G, Mareel M. Effect of catechins and citrus flavonoids on invasion in vitro. Clin Exp Metastasis. 1991;9:13–25. doi: 10.1007/BF01831706. [DOI] [PubMed] [Google Scholar]
- 21.Manach C, Regerat F, Texier O, Agullo G, Demigne C, Remesy C. Bioavailability, metabolism and physiological impact of 4-oxo-flavonoids. Nutr Res. 1996;16:517–544. doi: 10.1016/0271-5317(96)00032-2. [DOI] [Google Scholar]
- 22.Rosales MA, Cervilla LM, Sánchez-Rodríguez E, Rubio-Wilhelmi Mdel M, Blasco B, Ríos JJ, Soriano T, Castilla N, Romero L, Ruiz JM. The effect of environmental conditions on nutritional quality of cherry tomato fruits: evaluation of two experimental Mediterranean greenhouses. J Sci Food Agric. 2011;91:152–162. doi: 10.1002/jsfa.4166. [DOI] [PubMed] [Google Scholar]
- 23.Raiola A, Rigano MM, Calafiore R, Frusciante L, Barone A. Enhancing the health-promoting effects of tomato fruit for biofortified food. Mediators Inflammation. 2014;2014:139873. doi: 10.1155/2014/139873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 25.Lebel CP, Bondy SC. Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochem Int. 1990;17:435–440. doi: 10.1016/0197-0186(90)90025-O. [DOI] [PubMed] [Google Scholar]
- 26.Choi JS, Han YR, Byeon JS, Choung SY, Sohn HS, Jung HA. Protective effect of fucosterol isolated from the edible brown algae, Ecklonia stolonifera and Eisenia bicyclis, on tert-butyl hydroperoxide- and tacrine-induced HepG2 cell injury. J Pharm Pharmacol. 2015;67:1170–1178. doi: 10.1111/jphp.12404. [DOI] [PubMed] [Google Scholar]
- 27.Doll R. An overview of the epidemiological evidence linking diet and cancer. Proc Nutr Soc. 1990;49:119–131. doi: 10.1079/PNS19900018. [DOI] [PubMed] [Google Scholar]
- 28.Willett WC. Diet and health: what should we eat? Science. 1994;264:532–537. doi: 10.1126/science.8160011. [DOI] [PubMed] [Google Scholar]
- 29.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113:71–88. doi: 10.1016/S0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 30.Aschoff JK, Kaufmann S, Kalkan O, Neidhart S, Carle R, Schweiggert RM. In vitro bioaccessibility of carotenoids, flavonoids, and vitamin C from differently processed oranges and orange juices [Citrus sinensis (L.) Osbeck] J Agric Food Chem. 2015;63:578–587. doi: 10.1021/jf505297t. [DOI] [PubMed] [Google Scholar]
- 31.Aptekmann NP, Cesar TB. Long-term orange juice consumption is associated with low LDL-cholesterol and apolipoprotein B in normal and moderately hypercholesterolemic subjects. Lipids Health Dis. 2013;12:119. doi: 10.1186/1476-511X-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foroudi S, Potter AS, Stamatikos A, Patil BS, Deyhim F. Drinking orange juice increases total antioxidant status and decreases lipid peroxidation in adults. J Med Food. 2014;17:612–617. doi: 10.1089/jmf.2013.0034. [DOI] [PubMed] [Google Scholar]
- 33.Ghanim H, Sia CL, Upadhyay M, Korzeniewski K, Viswanathan P, Abuaysheh S, Mohanty P, Dandona P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am J Clin Nutr. 2010;91:940–949. doi: 10.3945/ajcn.2009.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 35.Crozier A, Lean MEJ, McDonald MS, Black C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J Agric Food Chem. 1997;45:590–595. doi: 10.1021/jf960339y. [DOI] [Google Scholar]
- 36.Stewart AJ, Bozonnet S, Mullen W, Jenkins GI, Lean MEJ, Crozier A. Occurrence of flavonols in tomatoes and tomato-based products. J Agric Food Chem. 2000;48:2663–2669. doi: 10.1021/jf000070p. [DOI] [PubMed] [Google Scholar]
- 37.Raiola A, Giudice RD, Monti DM, Tenore GC, Barone A, Rigano MM. Bioactive compound content and cytotoxic effect on human cancer cells of fresh and processed yellow tomatoes. Molecules. 2016;21:33. doi: 10.3390/molecules21010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cenariu D, Pintea A, Fischer-Fodor E, Bucak MN, Cenariu M, Crişan G. Cytotoxic potential of antioxidants from tomatoes on tumoral cells. Bull Univ Agric Sci Vet Med Cluj-Napoca Vet Med. 2015;72:401–405. [Google Scholar]
- 39.Chen RY, Wu JJ, Tsai MJ, Liu MS. Effect of storage and thermal treatment on the antioxidant activity of tomato fruits. J Chin Agric Chem Soc. 2000;38:353–360. [Google Scholar]
- 40.Wang H, Cao G, Prior RL. Total antioxidant capacity of fruits. J Agric Food Chem. 1996;44:701–705. doi: 10.1021/jf950579y. [DOI] [Google Scholar]
- 41.Chang CH, Lin HY, Chang CY, Liu YC. Comparisons on the antioxidant properties of fresh, freeze-dried and hotair-dried tomatoes. J Food Eng. 2006;77:478–485. doi: 10.1016/j.jfoodeng.2005.06.061. [DOI] [Google Scholar]
- 42.Yen GC, Yeh CT, Chen YJ. Protective Effect of Mesona procumbens against tert-butyl hydroperoxide-induced acute hepatic damage in rats. J Agric Food Chem. 2004;52:4121–4127. doi: 10.1021/jf049840d. [DOI] [PubMed] [Google Scholar]
- 43.García-Alonso J, Ros G, Periago MJ. Antiproliferative and cytoprotective activities of a phenolic-rich juice in HepG2 cells. Food Res Int. 2006;39:982–991. doi: 10.1016/j.foodres.2006.07.001. [DOI] [Google Scholar]
- 44.Williams GM, Jeffrey AM. Oxidative DNA damage: endogenous and chemically induced. Regul Toxicol Pharmacol. 2000;32:283–292. doi: 10.1006/rtph.2000.1433. [DOI] [PubMed] [Google Scholar]
- 45.Minotti G, Borrello S, Palombini G, Galeotti T. Cytochrome P-450 deficiency and resistance to t-butyl hydroperoxide of hepatoma microsomal lipid peroxidation. Biochim Biophys Acta. 1986;876:220–225. doi: 10.1016/0005-2760(86)90277-8. [DOI] [PubMed] [Google Scholar]
- 46.Luper S. A review of plants used in the treatment of liver disease: part 1. Altern Med Rev. 1998;3:410–421. [PubMed] [Google Scholar]
- 47.Alia M, Romos S, Mateos R, Bravo L, Goya L. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2) J Biochem Mol Toxicol. 2005;19:119–128. doi: 10.1002/jbt.20061. [DOI] [PubMed] [Google Scholar]
- 48.Kaliora AC, Dedoussis GV, Schmidt H. Dietary antioxidants in preventing atherogenesis. Atherosclerosis. 2006;187:1–17. doi: 10.1016/j.atherosclerosis.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Chen ZT, Chu HL, Chyau CC, Chu CC, Duh PD. Protective effects of sweet orange (Citrus sinensis) peel and their bioactive compounds on oxidative stress. Food Chem. 2012;135:2119–2127. doi: 10.1016/j.foodchem.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 50.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/S0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 51.Yu HM, Wang BS, Chu HL, Chang LW, Yen WJ, Lin CJ, Duh PD. Napiergrass (Pennisetum purpureum S.) protects oxidative damage of biomolecules and modulates antioxidant enzyme activity. Food Chem. 2007;105:1364–1374. doi: 10.1016/j.foodchem.2007.05.003. [DOI] [Google Scholar]
- 52.Choi AM, Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 53.Ryter SW, Otterbein LE, Morse D, Choi AM. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002;234–235:249–263. doi: 10.1023/A:1015957026924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qaisiya M, Coda Zabetta CD, Bellarosa C, Tiribelli C. Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway. Cell Signal. 2014;26:512–520. doi: 10.1016/j.cellsig.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 55.Rizzo V, Clifford MN, Brown JE, Siracusa L, Muratore G. Effects of processing on the polyphenol and phenolic acid content and antioxidant capacity of semi-dried cherry tomatoes (Lycopersicon esculentum M.) J Sci Food Agric. 2016;96:2040–2046. doi: 10.1002/jsfa.7315. [DOI] [PubMed] [Google Scholar]
- 56.Garg A, Garg S, Zaneveld LJ, Singla AK. Chemistry and pharmacology of the citrus bioflavonoid hesperidin. Phytother Res. 2001;15:655–669. doi: 10.1002/ptr.1074. [DOI] [PubMed] [Google Scholar]
- 57.Emim JA, Oliveira AB, Lapa AJ. Pharmacological evaluation of the anti-inflammatory activity of a citrus bioflavonoid, hesperidin, and the isoflavonoids, duartin and claussequinone, in rats and mice. J Pharm Pharmacol. 1994;46:118–122. doi: 10.1111/j.2042-7158.1994.tb03753.x. [DOI] [PubMed] [Google Scholar]
- 58.Lim H, Yeo E, Song E, Chang YH, Han BK, Choi HJ, Hwang J. Bioconversion of Citrus unshiu peel extracts with cytolase suppresses adipogenic activity in 3T3-L1 cells. Nutr Res Pract. 2015;9:599–605. doi: 10.4162/nrp.2015.9.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Funaguchi N, Ohno Y, La BL, Asai T, Yuhgetsu H, Sawada M, Takemura G, Minatoguchi S, Fujiwara T, Fujiwara H. Narirutin inhibits airway inflammation in an allergic mouse model. Clin Exp Pharmacol Physiol. 2007;34:766–770. doi: 10.1111/j.1440-1681.2007.04636.x. [DOI] [PubMed] [Google Scholar]
- 60.Park HY, Ha SK, Eom H, Choi I. Narirutin fraction from citrus peels attenuates alcoholic liver disease in mice. Food Chem Toxicol. 2013;55:637–644. doi: 10.1016/j.fct.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 61.Arima H, Ashida H, Danno G. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci Biotechnol Biochem. 2002;66:1009–1014. doi: 10.1271/bbb.66.1009. [DOI] [PubMed] [Google Scholar]
- 62.Kessler M, Ubeaud G, Jung L. Anti- and pro-oxidant activity of rutin and quercetin derivatives. J Pharm Pharmacol. 2003;55:131–142. doi: 10.1211/002235702559. [DOI] [PubMed] [Google Scholar]
- 63.Baldisserotto A, Vertuani S, Bino A, De Lucia D, Lampronti I, Milani R, Gambari R, Manfredini S. Design, synthesis and biological activity of a novel Rutin analogue with improved lipid soluble properties. Bioorg Med Chem. 2015;23:264–271. doi: 10.1016/j.bmc.2014.10.023. [DOI] [PubMed] [Google Scholar]