Abstract
A new type of doenjang was manufactured by mixing soaked soybean, koji (Rhizopus oryzae), cheonggukjang (Bacillus amyloliquefaciens MJ1-4 and B. amyloliquefaciens EMD17), and Pichia farinosa SY80 as a yeast, salt, and water, followed by fermentation with koji that was made by fermenting whole wheat with R. oryzae. The mixed culture doenjang was designed to have a more palatable flavor and stronger biological activities than the conventional product. The extract of mixed culture doenjang showed higher antioxidant activity than the commercial doenjang as evaluated by the ferric reducing antioxidant power assay although it was not significantly different from the commercial product in 2,2-diphenyl-1-picrylhydrazyl and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging activities. Further, the mixed culture doenjang reduced intracellular reactive oxygen species levels and protected cells from glutamate-induced cytotoxicity more efficiently in human hippocampal HT22 neuroblastoma cells than the commercial doenjang. In conclusion, a newly-developed mixed culture doenjang had a strong antioxidant activity in vitro and cultured cell model systems, exhibited a potential to prevent oxidative stress-associated disorders although animal and clinical studies are needed to confirm its in vivo efficacy.
Keywords: Rhizopus ozyzae, doenjang, antioxidant, neuroprotective effect
INTRODUCTION
In Korea, soybean has been processed in various ways such as tofu, soymilk, soy sprout, and other fermented products. In particular, doenjang, ganjang, and gochujang are major traditional fermented soy foods, with annual production of 91,449, 116,458, and 140,832 metric ton, respectively, in 2012 (1). The main ingredient in doenjang is meju, which is normally made by fermenting cooked soybean with airborne fungi or artificial inoculation with Aspergillus oryzae or Aspergillus sojae.
It has been reported that doenjang has several health benefits including anticarcinogenic, antiobesity, anti-inflammatory, antioxidant, and anti-melanogenesis actions (2–5). The major components that contribute to the health benefits of doenjang are isoflavones and their derivatives, peptides, and undigested protein (6–8). A commercial doenjang could be further improved in terms of favor, quality, and bioactive function. Therefore, we developed a novel formula for mixed culture doenjang consisting of 2,000 g soaked soybean, 1,080 g koji, 300 g cheonggukjang, 120 g yeast, 500 g salt, and 1,000 g water. Yeast (Pichia farinosa SY80) and cheonggukjang containing Bacillus amyloliquefaciens MJ1-4 and B. amyloliquefaciens EMD17 were added to improve flavor and to confer antimicrobial and antioxidant activities. Our previous study showed that fermentation of soybean with B. amyloliquefaciens MJ1-4 and B. amyloliquefaciens EMD17 significantly increased fibrinolytic, anti-microbial, and antioxidant activity (9), and these Bacillus strains were utilized for manufacturing cheonggukjang that was used in preparing the mixed culture doenjang.
Oxidative stress is associated with numerous diseases including cancer, atherosclerosis, Parkinson’s disease, and Alzheimer’s disease (AD). The brain is particularly vulnerable to oxidative damage and has high oxygen consumption. About 20% of all oxygen and 25% of all glucose are consumed by cerebral functions. The brain also contains a relatively high content of polyunsaturated fatty acids (PUFA) that are more sensitive to oxidation (10). On the other hand, levels of antioxidant defense in the brain are modest, which render neurons especially sensitive to a disturbance in the balance between antioxidants and production of ROS. Moreover, the brain also has a high content of redox active metals, which can promote the formation of reactive oxygen species (ROS) and has been linked to AD pathology.
Therefore, numerous attempts have been made to treat or prevent AD using antioxidants. Although most such trials failed, antioxidant compounds still remain as promising candidates for controlling AD.
While soybean contains a variety of bioactive components, fungal fermentation could lead to enhanced biological activity by converting glycoside forms of bioactive compounds into aglycones that are more bioavailable forms (11). Thus this study attempted to develop a new mixed culture doenjang with higher antioxidant and neuroprotective activities than the commercial product while keeping good sensory properties.
MATERIALS AND METHODS
Materials
All reagents used were of ACS grade, and were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All cell culture reagents and fetal bovine serum (FBS) were obtained from Welgene (Daegu, Korea) and Gibco BRL (Grand Island, NY, USA). 2′,7′-dichlorofluorescein diacetate (DCFDA) used as a molecular dye was purchased from Invitrogen (Carlsbad, CA, USA).
Preparation of doenjang extract
Mixed culture doenjang was prepared by mixing soaked soybean (2,000 g), koji (1,080 g), cheonggukjang (300 g), yeast (120 g), salt (500 g), and water (1,000 g). An aliquot was stored in a deep freezer for the study while the rest of it was subjected to fermentation for 6 weeks at 25±3°C. For koij preparation, whole wheat was soaked in water for 6 h, autoclaved at 121°C for 30 min, cooled down to room temperature, followed by inoculation with Rhizopus oryzae (5%, w/w) and incubated for 5 days 25± 1°C. Cheonggukjang containing B. amyloliquefaciens MJ1-4 and B. amyloliquefaciens EMD17 strains was used for its antioxidative, anti-bacterial, and fibrinolytic activities. Commercial doenjang was obtained from Monggo Foods Co., Ltd. (Changwon, Korea). Freeze-dried doenjang samples were extracted with 10 volumes of 80% (v/v) ethanol, filtered, and concentrated to a final concentration of 10 mg/mL, and filtered through 0.2 μm sterile syringe filter before assays.
FRAP assay
The ferric reducing antioxidant power (FRAP) of doenjang extract was determined as described previously (12). Briefly, 30 μL of H2O, 30 μL of ferrous sulfate as standard, or samples were incubated at room temperature with 1 mL of FRAP reagent [300 mmol/L acetate buffer (pH 6.3), 10 mmol/L 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ) solution, 20 mmol/L FeCl3 solution, and H2O], and the absorbance was recorded after 4 min. FRAP values of unknowns were calculated on the basis of standard curves established using standards at 0.1~1.0 mmol/L.
DPPH radical scavenging assay
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity of doenjang extract was evaluated as previously described (13). Briefly, 50 μL of sample solution [or dimethyl sulfoxide (DMSO)] was added to 200 μL of 200 μM DPPH radical solution, which was freshly made. After 30 min of incubation at room temperature, the absorbance at 515 nm was measured.
A synthetic antioxidant reagent, L-ascorbic acid (AA), was used as a positive control, and all tests were carried out in triplicate.
ABTS radical cation decolorization assay
The 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) solution was prepared by reacting 5 mL of 7 mM ABTS and 80 μL of 2.45 mM potassium persulfate, and the mixture was shaken in the dark at room temperature for 12 h before use (14). It was diluted with ethanol so that its absorbance was adjusted to 0.7 at 734 nm. ABTS (1 mL) was added to glass test tubes containing 50 μL of samples and mixed by a vortex mixer for 30 s. The absorbance was measured at 734 nm after 5 min. The percentage of radical scavenging activity was calculated by comparing the absorbance values of the control without samples. All determinations were performed 3 times.
Determination of total phenolic and flavonoid contents
Total phenolics were determined using the Folin-Ciocalteu reagent (15). Briefly, 100 μL of extract was mixed with 50 μL of sodium bicarbonate solution (10%, w/v), followed by the addition of 15 μL of Folin-Ciocalteu reagent (previously diluted 5-fold with distilled water). After 5 min at room temperature, the sample mixture was transferred to a 96-well microplate, and the absorbance at 593 nm was measured using a microplate reader. Results are expressed as gallic acid equivalents.
Total flavonoid content was determined by aluminum chloride using a colorimetric method previously described with slight modifications (16). Briefly, 25 μL of the sample was mixed with 75 μL of 95% methanol in a 96-well microplate. Then, 5 μL of 10% AlCl3·6H2O, 1 M potassium acetate, and 14 μL of distilled water were added, and the mixture was incubated for 40 min at room temperature. The absorbance readings were obtained at 415 nm with a microplate reader (Sunrise, Tecan Group Ltd., Männedorf, Switzerland). The total flavonoid content of the samples was extrapolated from standard curves established with quercetin at 0~50 μg/mL.
Determination of intracellular ROS levels in HT22 cells
Intracellular levels of ROS were quantified by the 2,7-dichlorofluorescein (DCF) assay according to Wang and Joseph (17), with slight modifications. A mouse hippocampal cell line (HT22 cells) was obtained from Professor Dong Seok Lee and Kyung Sik Song (Kyungpook National University, Daegu, Korea). For routine maintenance, cells were grown in α-minimum essential media (Gibco BRL) supplemented with 10% heat-inactivated FBS at 37°C in an atmosphere of 5% CO2/95% air under saturating humidity and passaged every other day (1:4 split ratio) by trypsinization with 0.25% trypsin/0.02% ethylenediaminetetraacetic acid sodium salt solution (Thermo Fisher Scientific, Waltham, MA, USA).
The cells (2,000 cells per well) were seeded into a black-bottom 96-well plate and cultivated for 24 h. After cell attachment, the plates were washed with phosphate buffered saline (PBS) and treated with increasing concentrations of sample extracts suspended in 10% FBS containing media for 12 h in the absence or presence of 5 mM glutamate. The cells treated with samples were washed with PBS and incubated for 30 min with DCFDA dissolved in DMSO (final concentration 50 μM). Fluorescence was measured at 0 and 40 min using an excitation wavelength of 485 nm and emission of 535 nm in a fluorescence microplate reader (Infinite 200, Tecan Group Ltd.). Most of the steps, including incubation of reaction mixture containing dye and oxidant, washing, and fluorimetric determination, were performed in the dark. The intensity of fluorescence was calculated as [(F40 min−F0 min)/F0 min]×100 as described elsewhere (17). Results are expressed as the relative intensity of fluorescence (in % of negative control).
Neuroprotective activity assay
HT22 mouse hippocampal neuronal cells were routinely maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS and 100 units/mL penicillin and 100 μg/mL streptomycin in a humidified CO2 incubator (MCO-19-AIC, Sanyo, Osaka, Japan) at 37°C and 5% CO2/95% air.
The cells were seeded in a 96-well culture dish in DMEM supplemented with 10% FBS at a density of 2×103 cells per well for HT22. The next day, various doses of samples and 5 mM of glutamate were added to the cells (18). After culturing cells for 24 h, the cell survival rate was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay (18). Cell viability is presented as the percentage relative to the untreated control.
Statistical analysis
Statistical significance of the data was tested by analysis of variance, followed by Student’s t-test (for independent samples), using SPSS Statistics software version 22 (SPSS Inc., Chicago, IL, USA). Data are presented as mean±standard error (SE).
RESULTS
Ferric reducing capacity of extracts from mixed culture and commercial doenjang
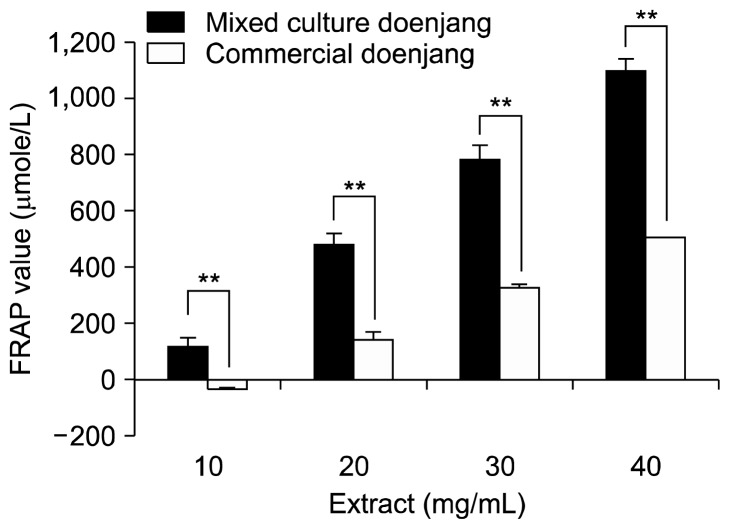
When the Fe3+-TPTZ complex is reduced to the Fe2+ form by an antioxidant under acidic conditions, an intense blue color with an absorption maximum develops at 593 nm. Therefore, the antioxidant effect (reducing ability) can be evaluated by monitoring the formation of a Fe2+-TPTZ complex using a spectrophotometer. The ferric reducing capacity of extracts from both newly-developed and commercial doenjang was dose-dependently increased. Furthermore, the mixed culture doenjang extract was found to have a higher FRAP value than the commercial doenjang (Fig. 1).
Fig. 1.
Ferric reducing antioxidant power (FRAP) values of mixed culture and commercial doenjang extracts. Sample extracts were prepared by extracting freeze-dried doenjang samples with 10 volumes of 80% (v/v) ethanol, removing solvent by rotary evaporation and nitrogen flushing, and then redissolving the extract in the solvent at the concentration of 10 mg/mL. Significantly different from each other by Student’s t-test at **P<0.01.
Radical scavenging activity of extracts from mixed culture and commercial doenjang
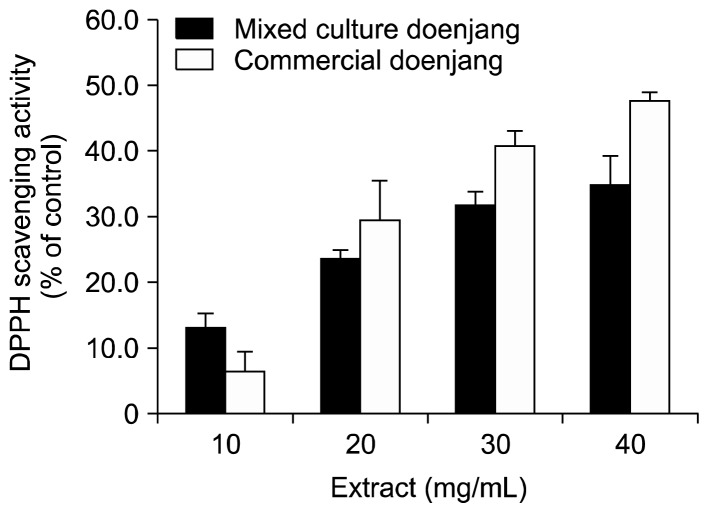
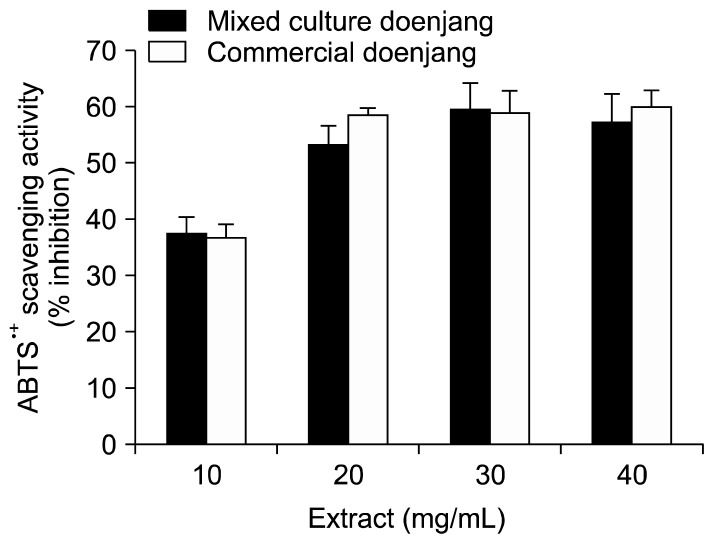
The aqueous ethanolic extracts of mixed culture and commercial doenjang showed scavenging activities from DPPH and ABTS+ radicals in dose-dependent manners (Fig. 2 and 3). However, there was no significant difference in radical scavenging activity of the extracts from the 2 different types of doenjang.
Fig. 2.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activities of mixed culture and commercial doenjang extracts. Refer to the legend under Fig. 1 for sample preparation for the assay.
Fig. 3.
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging activities of mixed culture and commercial doenjang extracts. Refer to the legend under Fig. 1 for sample preparation for the assay.
Inhibition of glutamate-induced ROS production by doenjang extract
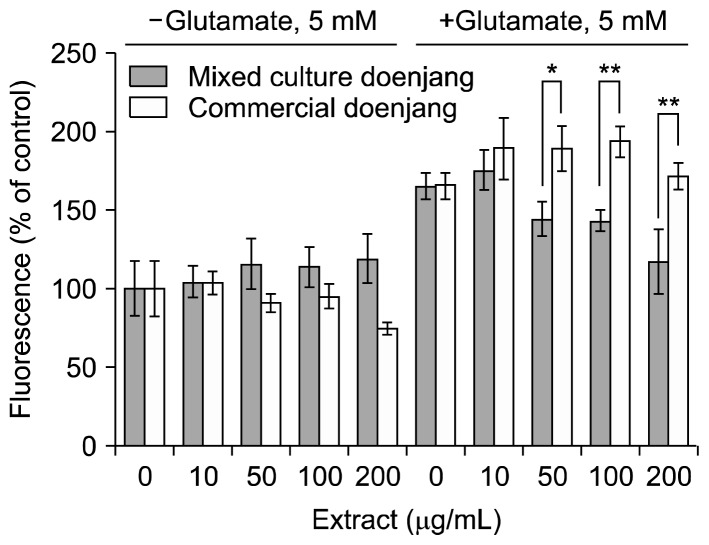
In the absence of glutamate treatment, intracellular ROS levels were not significantly different in HT22 cells treated with either mixed-culture or commercial doenjang extract although it was lower in the cells treated with 200 μg/mL extract from commercial doenjang than the mixed culture doenjang. Treatment with 5 mM glutamate caused a significant increase of intracellular ROS levels that was suppressed by simultaneous treatment with the mixed culture doenjang extract but not by the commercial doenjang extract (Fig. 4).
Fig. 4.
Effect of mixed culture and commercial doenjang extracts on glutamate-induced reactive oxygen species (ROS) production in HT22 cells. HT22 cells were incubated with doenjang extracts (0, 30, 50, 100, and 200 μg/mL) in the absence or presence of 5 mM glutamate for 12 h. ROS production was monitored by incubating with 2′,7′-dichlorofluorescein diacetate for 30 min and measuring fluorescence at an excitation of 485 nm and emission of 535 nm. Fluorescence values are presented as the percentage relative to the untreated control (neither treated with glutamate nor doenjang extract). Significantly different from each other by Student’s t-test at *P<0.05 and **P<0.01.
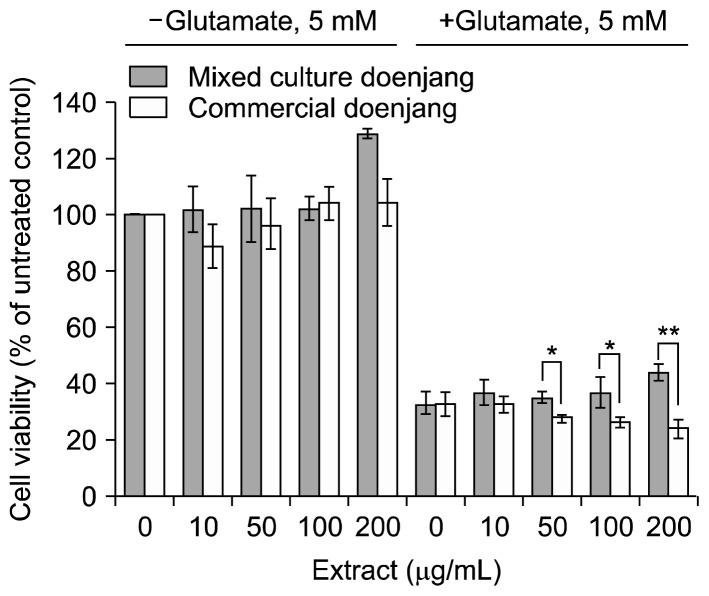
Protective effects of doenjang extract from glutamate-induced cytotoxicity in HT22 cells
Mouse hippocampal HT22 cells treated with glutamate (5 mM), were killed by 60~70%. However, addition of mixed culture doenjang extract into the culture attenuated glutamate-induced cytotoxicity in a dose-dependent manner (Fig. 5). However, commercial doenjang extract did not recover glutamate-induced cytotoxicity and even further amplified growth inhibition caused by glutamate.
Fig. 5.
Protective effect of mixed culture and commercial doenjang extracts against glutamate-induced cytotoxicity in HT22 cells. HT22 cells were seeded in a 96-well culture dish in DMEM supplemented with 10% fetal bovine serum at a density of 2,000 cells per well. The next day, various doses of samples (0, 10, 50, 100, and 200 μg/mL) and 5 mM of glutamate were added to the cells. After culturing cells for 24 h, the cell survival rate was determined by using MTT assay. Cell viability is presented as the percentage relative to the untreated control (neither treated with glutamate nor doenjang extract). Significantly different from each other by Student’s t-test at *P<0.05 and **P<0.01.
DISCUSSION
Doenjang, one of the most consumed traditional seasonings and condiments in Korea, is usually made from soybean fermented with fungi such as Aspergillus oryzae and salt. Doenjang is coarser in texture, like a chunky peanut butter, and it is an excellent sources of free amino acids, organic acids, minerals, and vitamins (19). In particular, the taste of doenjang was found to be influenced by free amino acids, sugars, and organic acids (oxalic, succinic, fumaric, and citric acids), whereas the flavor was associated with acetic acid, ethyl alcohol, benzaldehyde, ethyl acetate, ethyl 2-methyl butanoate, 2,5-dimethylpyrazine, tetramethylpyrazine, isovaleric acid, 2-methylbenzaldehyde, tetramethylpyrazine, benzaldehyde, ethyl alcohol, ethyl caprylate, furfural, and butanoic acid (20).
As yeast was reported to improve the flavor of doenjang while B. amyloliquefaciens MJ1-4 and B. amyloliquefaciens EMD17 had anti-microbial, antioxidant, and fibrinolytic activities, we prepared the mixed culture doenjang using these Bacillus strains, yeast, and R. oryzae. In particular, doenjang manufactured with R. oryzae was reported to contain high levels of chitooligosaccharides (171 μg/g) due to autolysis of fungal cell walls consisting of chitin during aging whereas doenjang made with koji of A. oryzae had low level of chitooligosacchardes (0~37.2 μg/g) (21).
Overall sensory properties of the newly-developed product were superior to commercial brands of doenjang (data not shown). Furthermore, it showed stronger antioxidant activity as measured by FRAP, compared to commercial doenjang, consistent with our previous data that the manufacturing of cheonggukjang using B. amyloliquefaciens MJ1-4 enhanced the levels of total phenolics and isoflavone aglycones as well as antioxidant activity (9). However, there was no significant difference in DPPH and ABTS radical scavenging activities between mixed culture and commercial doenjang. That is, the mixed culture doenjang had stronger ferric ion-reducing activity than commercial doenjang, but both kinds of doenjang showed similar potential to scavenge radicals from DPPH and ABTS. Although the FRAP assay was reported to be similar to the results obtained from the DPPH and ABTS radical scavenging assays, the antioxidant activities of the doenjang samples tested in the study showed discrepancies depending on the assays used, maybe due to mechanistic differences (22).
In addition, the mixed culture doenjang with cheonggukjang prepared by fermenting it with B. amyloliquefaciens MJ1-4 showed higher amounts of total phenolics than the commercial doenjang (Table 1), suggesting that isoflavone glycosides of soybean were extensively metabolized into aglycones during fermentation and/or the aging process of mixed culture doenjang. Our previous study showed that B. amyloliquefaciens MJ1-4 was capable of producing β-glycosidase in soybeans and was accompanied by rapid conversion of isoflavone glycosides into aglycones in the process of preparing cheonggukjang (9).
Table 1.
Total phenolics and flavonoids contents of mixed culture and commercial doenjang
| Samples | ||
|---|---|---|
|
|
||
| Commercial doenjang | Mixed culture doenjang | |
| Total phenolics (GAE mg/g)1) | 10.9±0.2 | 10.8±0.3 |
| Total flavonoids (μg/g) | 273.2±23.2 | 525.9±79.4 |
Data represent mean±SD (n=3).
Gallic acid equivalents.
It is widely recognized that the aglycone forms of isoflavones had higher in vivo and ex vivo antioxidant capacity than their glycosides (23), consistent with our finding that the mixed culture doenjang with higher flavonoids inhibited intracellular ROS production and glutamate-induced cytotoxicity in mouse hippocampal HT22 cells more efficiently than the commercial counterpart.
Several studies have shown that soy isoflavones have neuroprotective effects in various models including rat primary cortical neuron, hippocampus, and SH-SY5Y challenged with β-amyloid, glutamate, thapsigargin, and tert-butylhydroperoxide (24). In particular, genistein was reported to attenuate learning and memory deficits in amyloid β(1-40)-treated rat model of AD (25).
Meanwhile, soy isoflavone aglycones are found to be significantly more bioavailable than their glycosides in animal models, and thereby have increased potential to exert antioxidant action and health benefits including neuroprotection.
In conclusion, the mixed culture doenjang developed in the study had higher antioxidant and neuroprotective activities than the commercial product, and warrants further study to confirm its preventive effects against ROS-mediated chronic diseases including cancer, cardiovascular disease, cataract, age-related macular degeneration, and aging in general.
ACKNOWLEGEMENTS
This research was supported by the Kyungpook National University Bokhyeon Research Fund, 2015.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.MFDS. 2014 Production of food and food additives. Ministry of Food and Drug Sarefy; Osong, Chungbuk, Korea: 2014. p. 86. [Google Scholar]
- 2.Lee DE, Lee KW, Byun S, Jung SK, Song N, Lim SH, Heo YS, Kim JE, Kang NJ, Kim BY, Bowden GT, Bode AM, Lee HJ, Dong Z. 7,3′,4′-Trihydroxyisoflavone, a metabolite of the soy isoflavone daidzein, suppresses ultraviolet B-induced skin cancer by targeting Cot and MKK4. J Biol Chem. 2011;286:14246–14256. doi: 10.1074/jbc.M110.147348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nam YR, Won SB, Chung YS, Kwak CS, Kwon YH. Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet. Nutr Res Pract. 2015;9:235–241. doi: 10.4162/nrp.2015.9.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong JK, Chang HK, Park KY. Doenjang prepared with mixed starter cultures attenuates azoxymethane and dextran sulfate sodium-induced colitis-associated colon carcinogenesis in mice. J Carcinog. 2014;13:9. doi: 10.4103/1477-3163.137699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cha YS, Park Y, Lee M, Chae SW, Park K, Kim Y, Lee HS. Doenjang, a Korean fermented soy food, exerts antiobesity and antioxidative activities in overweight subjects with the PPAR-γ2 C1431T polymorphism: 12-week, double-blind randomized clinical trial. J Med Food. 2014;17:119–127. doi: 10.1089/jmf.2013.2877. [DOI] [PubMed] [Google Scholar]
- 6.Hwang KM, Jung KO, Song CH, Park KY. Increased antimutagenic and anticlastogenic effects of doenjang (Korean fermented soybean paste) prepared with bamboo salt. J Med Food. 2008;11:717–722. doi: 10.1089/jmf.2007.0151. [DOI] [PubMed] [Google Scholar]
- 7.Park KY, Jung KO, Rhee SH, Choi YH. Antimutagenic effects of doenjang (Korean fermented soypaste) and its active compounds. Mutat Res Fundam Mol Mech Mutagen. 2003;523–524:43–53. doi: 10.1016/S0027-5107(02)00320-2. [DOI] [PubMed] [Google Scholar]
- 8.Lovati MR, Manzoni C, Gianazza E, Arnoldi A, Kurowska E, Carroll KK, Sirtori CR. Soy protein peptides regulate cholesterol homeostasis in Hep G2 cells. J Nutr. 2000;130:2543–2549. doi: 10.1093/jn/130.10.2543. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Hwang CE, Lee CK, Lee JH, Kim GM, Jeong SH, Shin JH, Kim JS, Cho KM. Characteristics and antioxidant effect of garlic in the fermentation of cheonggukjang by Bacillus amyloliquefaciens MJ1-4. J Microbiol Biotechnol. 2014;24:959–968. doi: 10.4014/jmb.1310.10065. [DOI] [PubMed] [Google Scholar]
- 10.Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annu Rev Cell Dev Biol. 2005;21:633–657. doi: 10.1146/annurev.cellbio.21.122303.120624. [DOI] [PubMed] [Google Scholar]
- 11.Nam DH, Kim HJ, Lim JS, Kim KH, Park CS, Kim JH, Lim J, Kwon DY, Kim IH, Kim JS. Simultaneous enhancement of free isoflavone content and antioxidant potential of soybean by fermentation with Aspergillus oryzae. J Food Sci. 2011;76:H194–H200. doi: 10.1111/j.1750-3841.2011.02350.x. [DOI] [PubMed] [Google Scholar]
- 12.Benzie IFF. An automated, specific, spectrophotometric method for measuring ascorbic acid in plasma (EFTSA) Clin Biochem. 1996;29:111–116. doi: 10.1016/0009-9120(95)02013-6. [DOI] [PubMed] [Google Scholar]
- 13.Katsube T, Tabata H, Ohta Y, Yamasaki Y, Anuurad E, Shiwaku K, Yamane Y. Screening for antioxidant activity in edible plant products: comparison of low-density lipoprotein oxidation assay, DPPH radical scavenging assay, and Folin-Ciocalteu assay. J Agric Food Chem. 2004;52:2391–2396. doi: 10.1021/jf035372g. [DOI] [PubMed] [Google Scholar]
- 14.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 15.Kuhnen S, Moacyr JR, Mayer JK, Navarro BB, Trevisan R, Honorato LA, Maraschin M, Pinheiro Machado Filho LC. Phenolic content and ferric reducing-antioxidant power of cow’s milk produced in different pasture-based production systems in southern Brazil. J Sci Food Agric. 2014;94:3110–3117. doi: 10.1002/jsfa.6654. [DOI] [PubMed] [Google Scholar]
- 16.Chung HS, Chang LC, Lee SK, Shamon LA, van Breemen RB, Mehta RG, Farnsworth NR, Pezzuto JM, Kinghorn AD. Flavonoid constituents of Chorizanthe diffusa with potential cancer chemopreventive activity. J Agric Food Chem. 1999;47:36–41. doi: 10.1021/jf980784o. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/S0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 18.Behl C, Widmann M, Trapp T, Holsboer F. 17-β Estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun. 1995;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- 19.Yang SH, Choi MR, Kim JK, Chung YG. Optimization of the taste components composition in traditional Korean soybean paste. J Korean Soc Food Nutr. 1992;21:449–453. [Google Scholar]
- 20.Lee JE, Kang SH, Kim HR, Lim SI. Volatile compounds analysis of certified traditional Doenjang. J Korean Soc Food Sci Nutr. 2015;44:944–950. doi: 10.3746/jkfn.2015.44.6.944. [DOI] [Google Scholar]
- 21.Eum BW, Kwak BY, Kim SY, Shon DH, Lee KH. Enhancement of chitooligosaccharides in doenjang (soybean paste) and kanjang (soy sauce) using Bacillus subtilis koji and Rhizopus oryzae koji. Korean J Food Sci Technol. 2003;35:291–296. [Google Scholar]
- 22.Moon JK, Shibamoto T. Antioxidant assays for plant and food components. J Agric Food Chem. 2009;57:1655–1666. doi: 10.1021/jf803537k. [DOI] [PubMed] [Google Scholar]
- 23.Lee CH, Yang L, Xu JZ, Yeung SYV, Huang Y, Chen ZY. Relative antioxidant activity of soybean isoflavones and their glycosides. Food Chem. 2005;90:735–741. doi: 10.1016/j.foodchem.2004.04.034. [DOI] [Google Scholar]
- 24.Lee YB, Lee HJ, Sohn HS. Soy isoflavones and cognitive function. J Nutr Biochem. 2005;16:641–649. doi: 10.1016/j.jnutbio.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Bagheri M, Joghataei MT, Mohseni S, Roghani M. Genistein ameliorates learning and memory deficits in amyloid β(1-40) rat model of Alzheimer’s disease. Neurobiol Learn Mem. 2011;95:270–276. doi: 10.1016/j.nlm.2010.12.001. [DOI] [PubMed] [Google Scholar]