Abstract
The skeleton represents a common site of metastases for osteotropic cancers such as prostate and breast tumors and novel therapeutic targets and new markers for the monitoring of bone lesions are urgently needed. The formation of bone metastases is a complex process that starts at the level of the confined tumor and that is characterized by a dynamic crosstalk between the primary cancer and the future metastatic site, the bone. Factors released by the primary tumor contribute to prepare a fertile “soil”, where a “pre-metastatic niche” is established prior to future colonization by cancer cells. When the primary cancer progress from the confined disease to its invasive phase, tumor cells will acquire an invasive phenotype, enter into the circulation and colonize the previously prepared site where they will establish a “metastatic niche”. Among the variety of molecules that participate in the metastatic cascade, microRNAs are a class of small non-coding RNA that play an important role in the development of metastatic bone lesions. Many studies have addressed the role of small non-coding RNAs (miRs) in metastasis in osteotropic cancers and have highlighted the role of miRs as oncogenes (oncomiRs) or tumor suppressor miRs.
In this review we present describe the role of miRs in the processing of the supportive bone microenvironment prior and after the bone colonization by cancer cells. Finally, future therapeutic strategies and perspectives are also discussed.
1. Introduction
The skeleton represents a common site of metastases for prostate and breast cancer with approximately 70% of the patients dying of these cancers showing evidence of metastatic bone disease at autopsy. In addition, carcinomas of thyroid, kidney and lung cancer metastasize to bone, albeit at lower frequency (30% to 40% of the cancer deaths at autopsy) [1]. One of the major challenges in oncological research is the identification of new therapeutic targets and the discovery of new markers for the monitoring the development of bone lesions, particularly at the early stage and for their treatment.
The notion that molecular factors might be involved in the specific bone tropism of some cancer cells was for the first time postulated by Sir Stephen Paget who introduced the “Seed and Soil” hypothesis in which he compared the bone metastatic breast cancer cells to the seed of plants, capable of growing only in a fertile soil, the bone marrow [2]. Even more, tumor cells localized at the primary site are known to prepare this fertile soil for future tissue/organ colonization through the establishment of the so called “pre-metastatic niche” [3]. The formation of bone metastasis is characterized by a complex number of sequential events, that occur at the level of the primary tumor, when the cancer progress from the confined disease to its invasive phase. For skeletal metastasis to occur, osteotropic tumor cells must acquire an invasive phenotype by various modes of migration, either as single cells or as multicellular cluster also known as collective migration [4]. For instance, several carcinomas may undergo the so-called epithelial-to-mesenchymal transition (EMT) and acquire invasive characteristics. These cells switch from a sessile/epithelial to an invasive/mesenchymal phenotype, invade the extracellular matrix and the surrounding stroma and, subsequently, may enter the lymphatic or blood circulation. In aggressive cancer, many cancer cells are shed into the circulation every day, now referred to as circulating tumor cells (CTCs), but the efficacy of CTCs to successfully colonize distant tissues and develop into clinically overt metastases is relatively low. Once that these CTCs have colonized the metastatic site (e.g. the bone/bone marrow), they are referred to as disseminated tumor cells (DTCs). DTCs may remain dormant for years [5] and, due to largely unknown mechanisms, develop into macrometastases. The reciprocal interaction between cancer cells and the tissue-specific stroma is critical for primary and metastatic tumor growth progression. Prostate and mammary cancer cells metastasize to bone, where they induce either an osteoblastic or osteolytic response respectively. These opposite stromal responses suggest that different types of cancers adopt distinct strategies to hijack the bone marrow/bone stroma for their growth support (‘metastatic niche’). However, the molecular signals underlying these divergent responses are largely elusive [6].
In the past decade, preclinical studies have addressed the putative role of non-coding RNAs in tumorigenesis, metastasis and therapy response in osteotropic cancers. Besides long non-coding RNAs, microRNAs (miRs) represent a class of small non-coding RNAs, (18–25 nucleotides long), that regulate protein abundance by promoting mRNA degradation or translational repression [7], thus acting as oncogenes (oncomiRs) or tumor suppressor miRs. Several miRs have been identified as key molecules in tumorigenesis, bone tropism and the development of metastatic bone disease [8].
In this review, we provide a concise description on the role of miRs in bone metastases. In particular, we will focus on the processing of the supportive microenvironment in bone marrow prior and after the bone colonization mediated by specific miRs. Finally, future therapeutic perspectives are also discussed.
2. Osteotropism and miRs
The dissemination of prostate and breast cancer metastatic cells specifically to the skeleton is defined as osteotropism and is determined by multiple factors expressed by the tumor cells and the bone microenvironment. The interactions between cancer cells and the endothelium of the bone marrow vasculature is one of the key processes which precedes extravasation from the blood vessels and suggested to underlie the bone-specific dissemination [9]. During this process, surface molecules expressed on cancer cells such as the chemokine (C-X-C motif) receptor (CXCR) 4 (CXCR4), αvβ3 integrin, CD44 and RANK are directly involved in homing to bone [10]. Briefly, the interaction between CXCR4 and its ligand stromal derived factor 1 (SFD1, also known as CXCL12) is critically important in the formation of prostate and breast cancer bone metastasis. CXCR4 is significantly elevated in breast carcinoma compared to normal tissue and miR-218 has been shown to up-regulate CXCR4 [11]. Moreover, CXCR4 signaling induces expression of matrix metallopeptidase (MMP) 9 and MMP13 in tumor cells. Interestingly, miR-218 has also been shown to reduce MMP9 expression [12] and appears to be a crucial regulator of osteomimicry in breast cancer, a process which regulate the expression of osteoblast specific genes by tumor cells, that may facilitate the growth of metastatic cells in the bone microenvironment [13]. MMP13 is, on the other hand, regulated by miR-126, which has been shown to reduce the formation of breast cancer bone metastases [14] and has also been associated with prostate cancer metastases [15]. Osteotropic breast cancer cells express vascular-endothelial molecule-1 (VCAM1) that is also targeted by miR-126 [16]. VCAM1 binds α4β7 and α4β1 integrins on osteoclast progenitors that, in turn, can induce excess osteoclastogenesis and subsequently lead to radiologically-evident osteolytic lesions. Furthermore other members of the integrin family, αv integrins, have been shown to be required for the maintenance of cancer stem cell properties and to be involved in bone colonization, angiogenesis by activated endothelial cells and osteoclastic bone resorption [17]. CXCL12 has been shown to induce the activation of integrin αvβ3, that mediates multiple cell-extracellular matrix interactions during tumor progression and skeletal metastasis, including stromal processes like osteoclastic bone resorption and angiogenesis. Recently, our group demonstrated that miR-25 is strongly decreased in the highly osteotropic cancer stem/progenitor subpopulation of human prostate cancer cells and directly regulates integrin-αv expression [18]. Overexpression of miR-25 reduces the metastatic dissemination, thus supporting the notion that miR-25 is a key regulator of cancer stemness and in the formation of distant bone metastases.
3. miRs and the ‘pre-metastatic’ bone niches
As described above, the reciprocal interaction between cancer cells and the tissue-specific stroma is critical for primary and metastatic tumor growth progression. In organ-confined primary tumors soluble factors and extracellular vesicles (e.g. exosomes) can be released in the circulation. These factors may contribute to the conditioning of the future, distant metastatic sites, the so-called ‘pre-metastatic niche’ [3]. During this process, hematopoietic progenitor cells (HPCs) expressing VEGF receptor 1 (VEGFR1) are recruited to metastatic target organs by specific factors released by the primary tumor. Among these factors, LOXL enzymes, VEGFA, VEGFC, TNFα and TGF-β produced by the primary tumor stimulate inflammation, attachment, differentiation and recruitment of, for example, immunosuppressive myeloid cells. Recently, miR-26a and miR-29 have been shown to decrease LOXL2 [19] which suggest a tumor suppressive role for these two miRs. Additionally, miR-29 has been proven to inhibit cell migration in prostate cancer [20]. Interestingly, it has been proposed that extracellular vesicles and exosomes released from the primary tumor represent a mechanism of communication between the primary cancer cells and the metastatic sites during the induction of the “pre-metastatic niche”, recently reviewed in [21]. Additionally, extracellular vesicles released from bone marrow mesenchymal stem/stromal cells (MSCs) have been shown to transport tumor supportive miRs [22]. Together these observations reinforce the notion that bi-directional interactions tumor-bone stroma participate in the establishment of bone metastases.
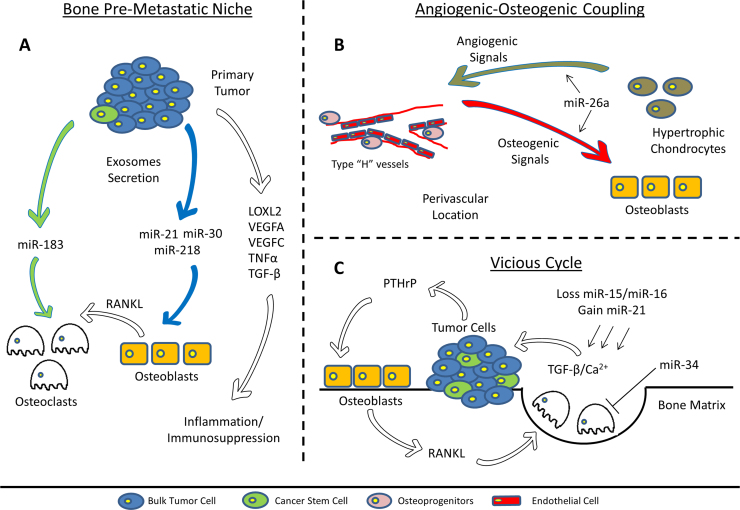
The miR signature of exosomes isolated from bulk prostate cancer cells (Fig. 1A, blue cells) and from their cancer stem cell compartment (Fig. 1A, green cells), display high levels of miR-21, miR-30 and miR-218 [23] (Fig. 1A, blue arrow). miR-21 and miR-30 have been identified as key regulators of osteoblast differentiation and have been included in a panel of microRNA biomarkers designated as “OstemiR” [24]. Therefore, high levels of these miRs may lead to increased bone remodeling “at a distance” and may facilitate subsequent metastatic colonization and cancer cell growth in the bone marrow microenvironment. Moreover, miR-21 increased expression of MMP2, MMP9 and MMP13, inducing extracellular matrix (ECM) remodeling and facilitating EMT. In the same study, CSC-derived exosomes (Fig. 1A, green arrow), were found to contain high amounts of miR-183 (Fig. 1). Furthermore, miR-183 has been shown to increase osteoclastogenesis by repressing heme oxygenase-1 (HO-1) [25]. It appears, therefore, that exosomal miR-183 may represent a supportive factor in the conditioning of the bone microenvironment by highly metastatic cells and reinforce their role in the conditioning and formation of the ‘receptive pre-metastatic niches’.
Fig. 1.
Schematic representation of the involvement of miRs in tumor progression and bone metastasis.
4. miRs and metastatic bone niches
In primary and metastatic cancers, tumor cells interact with different cell types that constitute the bone/bone marrow stroma such as osteoblast and osteoclasts, tumor-associated macrophages (TAMs), bone marrow stromal fibroblasts, endothelial cells, pericytes, MSCs and immune cells like myeloid-derived suppressor cells (MDSCs) [26]. Multiple miRs have been associated with the interaction between tumor cells and stromal cells, reviewed in [27]. miR-511-3p reduced the pro-tumoral activity of TAMs [28]; up-regulation of miR-31 and miR-214 while inhibition of miR-155, abrogated the CAF phenotype [29]. Tumor cells produce several factors that “activate” the surrounding stromal cells and induce remodeling of extracellular matrices. These factors include fibroblast growth factor 2 (FGF2), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), interleukins colony-stimulating factors, the transforming growth factor (TGF-β) superfamily and proteolytic enzymes that remodel ECM, thus enabling cell migration [6], [30]. Interestingly, TGF-β-induced factor 2 (Tgif2) induces osteoclastogenesis due to the down regulation of miR-34a expression in osteoclasts and administration of miR-34a-carrying nanoparticles in mice prevented bone metastasis of human breast cancer cell [31]. Tumor cells with stem cell-like characteristics, that survive in the circulation, seem to preferably extravasate at those distant sites where “pre-metastatic niches” have been established, i.e. a distant microenvironment that facilitates metastatic colonization.
Typically, the bone metastatic niches are anatomically localized at perivascular locations and endosteal bone surfaces, although it cannot be excluded that these may represent the same entity [32]. CTCs and DTCs may, establish a metastatic niche through competition with hematopoietic stem cells (HSCs) at the level of the endosteal niche [33]. DTCs can survive in the bone microenvironment as non-proliferating (dormant) cells that originate micrometastases and the perivascular niche has been shown to regulate tumor cell dormancy [34]. Recently, high levels of miR-23b has been found in exosomes isolated from bone marrow mesenchymal stem cells and miR-23b was shown to promote dormancy in metastatic breast cancer cells [35]. The mechanisms that induce exit from dormancy are largely unknown and have remained largely elusive. It was found that the cytoskeletal reorganization in dormant cells mediated by a collagen-I enriched fibrotic environment contributes to exit from dormancy [36]. Once disseminated cancer cells ‘awake’, they can induce a local inflammatory environment, followed by vascular and bone remodeling and progression to a distant overt bone metastasis. Recently, it was revealed that the molecular signature of the stroma response in prostate cancer-induced osteoblastic bone metastasis highlights the amplification of hematopoietic and prostate epithelial stem cell niche [6]. This observation further supports the notion that expansion of such perivascular metastatic niches in osteoblastic metastases may occur via the induction of angiogenesis (Fig. 1B, light-brown arrow) that, in turn, leads to osteoinduction (Fig. 1B, orange arrow). Indeed, it was recently demonstrated that a specific type of vessels, namely “Type H vessels” orchestrate the coupling of angiogenesis and osteogenesis [37]. In this respect, miR-26a may positively regulate the angiogenesis-osteogenesis coupling [38] (Fig. 1B). Interestingly, the above-mentioned miR-26a is considered a tumor-suppressor miR and low miR-26a levels have been detected in breast and prostate cancer tissues [39], [40]. Together, these data suggest that miR-26a might function in several steps during tumor progression. Moreover, low miR-26a levels would have a negative impact on the angiogenesis-osteogenesis coupling, thus de-regulating this mechanism and facilitating the formation of bone metastases.
5. miRs and deregulation of bone remodeling
Bone remodeling can be affected by osteotropic cancers at a distance (e.g. pre-metastatic niches) or in close proximity. It has been hypothesized that osteolytic cancer cells produce various osteolytic factors (e.g. PTHrP) that stimulate osteoblasts to secrete RANKL and stimulate osteoclastic development and subsequent bone resorption (Fig. 1C, white arrows). During this process, many factors such as TGF-β, IGF-1 and calcium are released from the mineralized matrix to further feed cancer cell growth, thus perpetuating this “vicious cycle” [41]. In addition to the stimulation of osteoclast activity, osteolytic cancer cells can produce factors (DKK1, Noggin, Sclerostin) that modulate Wnt and BMP signaling pathways leading to osteoblast suppression and vice versa for osteoinductive cancers. The involvement of miRs in the process of bone remodeling have been extensively studied and the outcome of these studies were reviewed recently [42]. Among the variety of signaling pathways that orchestrate the balance between osteoclastic- and osteoblastic-activity, TGF-β, Wnt and Notch signaling are the three fundamental networks involved in the maintenance and expansion of osteoprogenitor cells and differentiation towards osteoblasts. Recently, we have identified a signature of 30 validated miRs linked to EMT that are linked to key genes of TGF-β, Wnt and Notch signaling pathways [43]. Strikingly, this signature contains multiple miRs that have also been directly linked to the formation of bone metastasis and to the interference with normal osteoblast and osteoclast activity. In particular, loss of miR-15 and miR-16 and gain of miR-21 in prostate cancer cells has been shown to activate TGF-β signaling and promote bone marrow colonization and osteolysis in prostate cancer [44] (Fig. 1C). Additionally, miR-16, together with miR-378, positively correlate with detection of bone metastases, which makes them interesting targets as biomarkers [45]. Moreover miR-34a, that downregulates Notch signaling, inhibits osteoclastogenesis and reduces bone metastasis in breast cancer [31]. Additionally, miR-155 can block the cytostatic signaling of TGF-β, modulate osteogenic differentiation [46], and promote invasion and metastasis in breast cancer [47].
6. miRs and potential treatment of bone metastases
Non-coding RNAs/miRs may have clear diagnostic and prognostic value and can be employed as predictors of therapy response and biomarkers [21]. However, the applicability of miRs as therapeutic agents in oncology is still in its infancy due a number of challenges (specificity, drug delivery etc.). The principle of therapeutic delivery of siRNA or miR sequences to selected cells in order to modulated expression of key metastasis oncogenes, has remained a major impediment. Several limitations of this approach exist. On one hand, a drug carrier or nanodrug delivery system should be coupled to the administration of these small molecules, on the other hand, some siRNA sequences might also evoke an immune response by activating the Toll-Like Receptor (TLR) pathway [48]. Improvement of nanodrug delivery systems and lipid-based nanoparticles, may pave the way to clinical translation. Only few miRs seem to prevent metastatic bone disease in vivo. These include miR-141 and miR-219, that have been shown to decrease osteolytic breast cancer bone metastases presumably via inhibition of osteoclast activity [49]. Two other miRs, miR-203 and miR-135, were found to reduce breast cancer bone metastases via targeting of Runx2 [50]. Currently, only one candidate miR is studied in clinical trials in liver cancer and other selected solid tumors: MRX34 (Mirna therapeutics, Inc.; ClinicalTrials.gov Identifier: NCT01829971). MRX34 is a liposomal formulation of a miR-34 mimic employed to restore this tumor suppressor miR. Interestingly, miR-34 is downregulated in metastatic breast cancer and it protects breast cancer induced osteolytic disease [51]. Taken together, the employment of miRs as therapeutic agents remains challenging and is still strictly dependent on the identification of a specific targeting strategy to selectively target highly metastatic cells.
7. Outstanding questions
-
1.
Should modulation of miRs be achieved at the primary tumor and/or at future metastatic bone sites (‘premetastatic niches’) in order to prevent skeletal metastasis?
-
2.
Tumor-derived exosomes contain miRs (and proteins) that mediate the cross-talk between the primary tumor and the future bone metastatic niches. Vice versa, the supportive cellular microenvironment at the primary and distant sites may also shed non-coding RNAs via exosomes that may influence the behavior of cancer cells. Should we target exosomes or their miRs contained within these exosomes?
-
3.
Which drug delivery strategy should be developed to achieve a feasible therapeutic targeting of therapeutic miR mimics?
-
4.
miRs regulate a multitude of genes. Even in presence of a highly selective drug delivery strategy, can therapeutic miRs or antagomiRs achieve anti-tumor responses with acceptable side effects?
-
5.
One of the clinical challenges is the lack of biomarkers in micrometastatic disease. Can miR signatures be used for the assessment of micrometastatic bone disease?
-
6.
Can we exploit autologous exosomes or extracellular vesicles structures for drug or miR delivery?
Conflict of interest
The authors disclose no conflict of interest.
Acknowledgments
This work was supported by the Dutch Cancer Society UL2015-7599 KWF (EZ).
References
- 1.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin. Cancer Res. 2006;12(20 Pt 2):6243s–6249ss. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastas. Rev. 1989;8(2):98–101. [PubMed] [Google Scholar]
- 3.Kaplan R.N., Rafii S., Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res. 2006;66(23):11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haeger A., Wolf K., Zegers M.M., Friedl P. Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 2015;25(9):556–566. doi: 10.1016/j.tcb.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Brabletz T. To differentiate or not--routes towards metastasis. Nat. Rev. Cancer. 2012;12(6):425–436. doi: 10.1038/nrc3265. [DOI] [PubMed] [Google Scholar]
- 6.Ozdemir B.C., Hensel J., Secondini C., Wetterwald A., Schwaninger R., Fleischmann A. The molecular signature of the stroma response in prostate cancer-induced osteoblastic bone metastasis highlights expansion of hematopoietic and prostate epithelial stem cell niches. PLoS One. 2014;9(12):e114530. doi: 10.1371/journal.pone.0114530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Browne G., Taipaleenmaki H., Stein G.S., Stein J.L., Lian J.B. MicroRNAs in the control of metastatic bone disease. Trends Endocrinol. Metab. 2014;25(6):320–327. doi: 10.1016/j.tem.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrett J.M., Mangold K.A., Jilling T., Kaul K.L. Bi-directional interactions of prostate cancer cells and bone marrow endothelial cells in three-dimensional culture. Prostate. 2005;64(1):75–82. doi: 10.1002/pros.20206. [DOI] [PubMed] [Google Scholar]
- 10.Rahim F., Hajizamani S., Mortaz E., Ahmadzadeh A., Shahjahani M., Shahrabi S. Molecular regulation of bone marrow metastasis in prostate and breast cancer. Bone Marrow Res. 2014;2014:405920. doi: 10.1155/2014/405920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tie J., Pan Y., Zhao L., Wu K., Liu J., Sun S. MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS Genet. 2010;6(3):e1000879. doi: 10.1371/journal.pgen.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin J., Cai L., Liu Z.M., Zhou X.S. miRNA-218 inhibits osteosarcoma cell migration and invasion by down-regulating of TIAM1, MMP2 and MMP9. Asian PAC J. Cancer Prev. 2013;14(6):3681–3684. doi: 10.7314/apjcp.2013.14.6.3681. [DOI] [PubMed] [Google Scholar]
- 13.Hassan M.Q., Maeda Y., Taipaleenmaki H., Zhang W., Jafferji M., Gordon J.A. miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J. Biol. Chem. 2012;287(50):42084–42092. doi: 10.1074/jbc.M112.377515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavazoie S.F., Alarcon C., Oskarsson T., Padua D., Wang Q., Bos P.D. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451(7175):147–152. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watahiki A., Wang Y., Morris J., Dennis K., O’Dwyer H.M., Gleave M. MicroRNAs associated with metastatic prostate cancer. PLoS One. 2011;6(9):e24950. doi: 10.1371/journal.pone.0024950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris T.A., Yamakuchi M., Ferlito M., Mendell J.T., Lowenstein C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA. 2008;105(5):1516–1521. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Horst G., van den Hoogen C., Buijs J.T., Cheung H., Bloys H., Pelger R.C. Targeting of alpha(v)-integrins in stem/progenitor cells and supportive microenvironment impairs bone metastasis in human prostate cancer. Neoplasia. 2011;13(6):516–525. doi: 10.1593/neo.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoni E., van der Horst G., van de Merbel A.F., Chen L., Rane J.K., Pelger R.C. miR-25 Modulates Invasiveness and Dissemination of Human Prostate Cancer Cells via Regulation of alphav- and alpha6-Integrin Expression. Cancer Res. 2015;75(11):2326–2336. doi: 10.1158/0008-5472.CAN-14-2155. [DOI] [PubMed] [Google Scholar]
- 19.Wong C.C., Tse A.P., Huang Y.P., Zhu Y.T., Chiu D.K., Lai R.K. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology. 2014;60(5):1645–1658. doi: 10.1002/hep.27320. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa R., Goto Y., Kojima S., Enokida H., Chiyomaru T., Kinoshita T. Tumor-suppressive microRNA-29s inhibit cancer cell migration and invasion via targeting LAMC1 in prostate cancer. Int. J. Oncol. 2014;45(1):401–410. doi: 10.3892/ijo.2014.2437. [DOI] [PubMed] [Google Scholar]
- 21.Junker K., Heinzelmann J., Beckham C., Ochiya T., Jenster G. Extracellular Vesicles and their role in urologic malignancies. Eur. Urol. 2016 doi: 10.1016/j.eururo.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 22.Vallabhaneni K.C., Penfornis P., Dhule S., Guillonneau F., Adams K.V., Mo Y.Y. Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites. Oncotarget. 2015;6(7):4953–4967. doi: 10.18632/oncotarget.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanchez C.A., Andahur E.I., Valenzuela R., Castellon E.A., Fulla J.A., Ramos C.G. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2015 doi: 10.18632/oncotarget.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eguchi T., Watanabe K., Hara E.S., Ono M., Kuboki T., Calderwood S.K. OstemiR: a novel panel of microRNA biomarkers in osteoblastic and osteocytic differentiation from mesencymal stem cells. PLoS One. 2013;8(3):e58796. doi: 10.1371/journal.pone.0058796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ke K., Sul O.J., Rajasekaran M., Choi H.S. MicroRNA-183 increases osteoclastogenesis by repressing heme oxygenase-1. Bone. 2015;81:237–246. doi: 10.1016/j.bone.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Pietras K., Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp. Cell Res. 2010;316(8):1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 27.Ell B., Kang Y. MicroRNAs as regulators of tumor-associated stromal cells. Oncotarget. 2013;4(12):2166–2167. doi: 10.18632/oncotarget.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Squadrito M.L., Pucci F., Magri L., Moi D., Gilfillan G.D., Ranghetti A. miR-511-3p modulates genetic programs of tumor-associated macrophages. Cell Rep. 2012;1(2):141–154. doi: 10.1016/j.celrep.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Mitra A.K., Zillhardt M., Hua Y., Tiwari P., Murmann A.E., Peter M.E. MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov. 2012;2(12):1100–1108. doi: 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller M.M., Fusenig N.E. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat. Rev. Cancer. 2004;4(11):839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 31.Krzeszinski J.Y., Wei W., Huynh H., Jin Z., Wang X., Chang T.C. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature. 2014;512(7515):431–435. doi: 10.1038/nature13375. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Hensel J., Thalmann G.N. Biology of bone metastases in prostate cancer. Urology. 2016 doi: 10.1016/j.urology.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 33.Shiozawa Y., Pedersen E.A., Havens A.M., Jung Y., Mishra A., Joseph J. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Investig. 2011;121(4):1298–1312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghajar C.M., Peinado H., Mori H., Matei I.R., Evason K.J., Brazier H. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013;15(7):807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ono M., Kosaka N., Tominaga N., Yoshioka Y., Takeshita F., Takahashi R.U. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci. Signal. 2014;7(332):ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- 36.Barkan D., El Touny L.H., Michalowski A.M., Smith J.A., Chu I., Davis A.S. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70(14):5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y., Fan L., Liu S., Liu W., Zhang H., Zhou T. The promotion of bone regeneration through positive regulation of angiogenic-osteogenic coupling using microRNA-26a. Biomaterials. 2013;34(21):5048–5058. doi: 10.1016/j.biomaterials.2013.03.052. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X.X., Yuan Q.Z., Mu D.P., Sun D.W., Bo Q.A., Pan G.Z. MicroRNA-26a inhibits proliferation by targeting high mobility group AT-hook 1 in breast cancer. Int. J. Clin. Exp. Pathol. 2015;8(1):368–373. [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao S., Ye X., Xiao L., Lian X., Feng Y., Li F. MiR-26a inhibits prostate cancer progression by repression of Wnt5a. Tumour Biol. 2014;35(10):9725–9733. doi: 10.1007/s13277-014-2206-4. [DOI] [PubMed] [Google Scholar]
- 41.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2(8):584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 42.Jing D., Hao J., Shen Y., Tang G., Li M.L., Huang S.H. The role of microRNAs in bone remodeling. Int. J. Oral. Sci. 2015;7(3):131–143. doi: 10.1038/ijos.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zoni E., van der Pluijm G., Gray P.C., Kruithof-de Julio M. Epithelial Plasticity in Cancer: Unmasking a MicroRNA Network for TGF-beta-, Notch-, and Wnt-Mediated EMT. J. Oncol. 2015;2015:198967. doi: 10.1155/2015/198967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonci D., Coppola V., Patrizii M., Addario A., Cannistraci A., Francescangeli F. A microRNA code for prostate cancer metastasis. Oncogene. 2015 doi: 10.1038/onc.2015.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waning D.L., Mohammad K.S., Guise T.A. Cancer-associated osteoclast differentiation takes a good look in the miR(NA)ror. Cancer Cell. 2013;24(4):407–409. doi: 10.1016/j.ccr.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu T., Xie M., Wang X., Jiang X., Li J., Huang H. miR-155 modulates TNF-alpha-inhibited osteogenic differentiation by targeting SOCS1 expression. Bone. 2012;51(3):498–505. doi: 10.1016/j.bone.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Johansson J., Berg T., Kurzejamska E., Pang M.F., Tabor V., Jansson M. MiR-155-mediated loss of C/EBPbeta shifts the TGF-beta response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer. Oncogene. 2013;32(50):5614–5624. doi: 10.1038/onc.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conde J., Artzi N. Are RNAi and miRNA therapeutics truly dead? Trends Biotechnol. 2015;33(3):141–144. doi: 10.1016/j.tibtech.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 49.Ell B., Mercatali L., Ibrahim T., Campbell N., Schwarzenbach H., Pantel K. Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell. 2013;24(4):542–556. doi: 10.1016/j.ccr.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taipaleenmaki H., Browne G., Akech J., Zustin J., van Wijnen A.J., Stein J.L. Targeting of Runx2 by miR-135 and miR-203 impairs progression of breast cancer and metastatic bone disease. Cancer Res. 2015;75(7):1433–1444. doi: 10.1158/0008-5472.CAN-14-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaboli P.J., Rahmat A., Ismail P., Ling K.H. MicroRNA-based therapy and breast cancer: A comprehensive review of novel therapeutic strategies from diagnosis to treatment. Pharmacol. Res. 2015;97:104–121. doi: 10.1016/j.phrs.2015.04.015. [DOI] [PubMed] [Google Scholar]