Abstract
The bone marrow microenvironment is characterized by its multicellular nature, and perhaps less obviously by the high mobility of multiple transient and stationary cell lineages present in this environment. The trafficking of hematopoietic and mesenchymal cells between the bone marrow and blood compartments is regulated by a number of bone marrow-derived factors. It is suspected that transformed metastatic cells “hijack” these processes to engraft into the skeleton and eventually cause the skeletal complications associated with metastatic disease. In this short review, experimental and association data supporting the contribution of a less recognized cell type of the bone marrow – the nerves of the sympathetic nervous system – to early events of the breast cancer bone metastatic process, are summarized.
Keywords: Breast cancer, Bone metastasis, Bone microenvironment, Sympathetic nervous system, Adrenergic receptor, Beta-blockers
Metastatic cancer cells circulate through every organ via blood vessels, and can infiltrate some more than others to eventually cause lethal pathological dysfunction. Metastasis to distant organs, including liver, brain and the skeleton is nowadays the main cause of death in patients treated for breast or prostate cancer. Metastasis to the skeleton is very common, as 73% of patients with terminal breast and prostate cancers have evidence of metastatic bone disease [1], [2]. This leads to predominantly lytic lesions causing pain, hypercalcemia, fractures, and spinal cord/nerve compression. Metastasis may remain confined to the skeleton, and eventual cause of mortality is almost entirely due to skeletal complications and their treatments. The 5-year survival rate of women diagnosed with bone metastasis is 22% (National Cancer Institute's SEER database).
There are multiple reasons why the skeleton is a preferential organ for metastasis. First, it is a large and well-vascularized tissue, with a fenestrated sinusoidal vasculature that may favor circulating cancer cell arrest and establishment into the skeleton, especially for cancer cells metastasizing as cluster. Host cells residing within the bone marrow environment also secrete a number of cytokines and extracellular matrix proteins that promote the homing or retention of metastatic cancer cells within the skeleton, as well as their survival, dormancy and drug resistance [3].
Skeletal metastasis in women with advanced breast cancer often leads to death within a couple of years. Though the above mentioned skeletal complications and their treatment contribute to this high mortality rate, additional mechanisms are likely to be involved. The fact that the skeleton is an endocrine organ that can impact the function of other organs, including the pancreas [4], [5], the gonads [6] and muscles [7] for instance suggests that skeletal metastasis, especially at late stages, can disrupt general body homeostasis, thereby leading to the morbidities afflicting relapsing patients. Because of the irreversible and complex nature of late stage disease, earlier detection of metastatic events is likely to result in higher treatment efficacy and to increase the survival of these patients. Thus, characterizing the early determinants of skeletal metastasis is necessary to uncover novel targets and strategies to eradicate metastatic cells at early stages.
Studies focused on the early determinants of skeletal metastasis are difficult for many reasons, including challenges in detecting, tracing, and studying small numbers of metastatic cells within the skeleton and the lack of good preclinical models of early bone metastasis. One strategy to progress in this area, however, is to search for specific conditions that can favor skeletal metastasis or tumor recurrence in patients with metastatic cancers. Answers to this question could lead to the discovery of specific target(s) that could be used to prevent or limit skeletal metastasis, or to increase the exposure of metastatic cells to cytotoxic drugs.
Chronic stress and depression are prevalent in individuals with low socioeconomic status. These conditions are associated with higher cancer recurrence and reduced survival in women with breast cancer [8], [9], [10], [11], [12], [13], [14], and also share the common activation of a defense mechanism characterized by the release of catecholamines by sympathetic nerves and glucocorticoids by the adrenal glands. This is an adaptive and physiological response to acute stress, which can become pathological in the setting of chronic stress, depression, and other traumatic emotional events that trigger prolonged sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenal (HPA) axis activation. What links these observations to the bone marrow environment and bone metastasis is that sympathetic nerves richly innervate the skeleton and release norepinephrine (NE) as a neuromediator to regulate bone remodeling and bone homeostasis [15]. Specifically, osteoblasts express the β2-adrenergic receptor (β2AR), and its stimulation by NE leads to the synthesis of RANKL, a well-known osteoclastogenic cytokines with promigratory properties toward cancer cells [16], [17], [18], [19]. Other studies also revealed that the mobilization of hematopoietic stem cell from the bone marrow environment into the blood circulation was under the control of sympathetic signals and SDF1/CXCL12, a cytokine also involved in the homing of metastatic cells into the skeleton [20]. These observations thus suggested that psychosocial factors known to induce SNS and HPA chronic activation impact the bone marrow environment to transform it into a “fertile soil” favoring cancer cell metastasis.
To experimentally address this hypothesis, one first needs models of bone metastasis. The intracardiac injection model of cancer cells allows the investigation of metastatic events ulterior to growth and egress from the primary tumor. One also needs mouse models of depression or chronic stress, which are associated with SNS and HPA activation. These models are numerous and can be classified based on exposure and response to various types of acute or chronic stressors. Learned helplessness, chronic mild stress, or social defeat stress are widely used mouse models of depression and stress. A pharmacologic alternative to these models is the use of βAR agonists such as isoproterenol (Iso), which simulates SNS activation specifically, although it should be noted that the duration and intensity of the Iso signal may differ from the endogenous βAR ligands NE and epinephrine because of endogenous release kinetics and differences in metabolism. These tools allowed independent groups to provide experimental evidence supporting the hypothesis that sympathetic activation induced by chronic stress or severe depression favors metastasis to soft organs and bones. Using intracardiac injections of osteotropic MDA-MD-231 and 4T1 breast cancer cells, and a mouse model of learned helplessness (chronic immobilization stress, CIS), we have shown that SNS activation for two weeks prior to cancer cell inoculation increases the number of metastatic foci into the skeleton as well as bone destruction [21]. In addition, blockade of SNS action on target tissues by the β-blocker propranolol inhibits this CIS stimulatory effect on bone metastasis, suggesting that sympathetic activation and the βAR in osteoblasts mediate this pathological response to emotional stimuli [21].
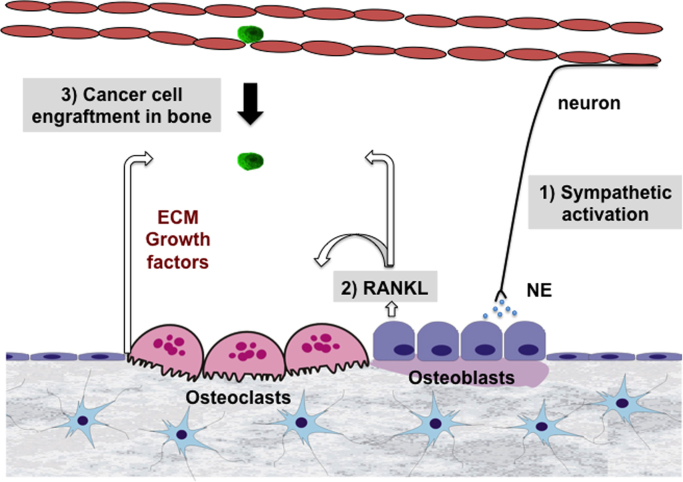
The results of additional experiments suggested that the pro-migratory properties of RANKL mediate this effect of CIS on the host bone marrow environment and breast cancer cell bone metastasis. Indeed, βAR stimulation in osteoblasts leads to a strong increase in RANKL expression, and the conditioned medium of these cells could stimulate breast cancer cell migration in vitro. This could be inhibited by OPG, the RANKL decoy receptor. Most importantly, knock down of the RANKL receptor RANK in MDA-MD-231 bone metastatic breast cancer cells reduced the effect of CIS on bone metastasis in vivo [21]. These data thus suggested that sympathetic activation via an indirect effect on the bone stroma, and specifically via NE and the βAR expressed in bone cells, promotes breast cancer cell engraftment within the bone marrow in a RANKL-dependent fashion (Fig. 1). The existence of RANK-negative cells in bone metastatic tumors, however, suggests that additional mechanisms exist [22]. In addition, a direct effect of NE on cancer cells is suspected (most of these cells express the βARs). Studies using ovarian cancer cells for instance indicated that CIS/SNS activation promotes the growth and invasion properties of these tumors [23], [24], [25]. There is also evidence that sympathetic activation in the primary tumor causes remodeling of the host stroma, leading to an environment that is favorable for cancer cell egress and dissemination to distant organs [26]. The contribution of sympathetic and parasympathetic nerves to the skeletal metastasis and growth of prostate cancer cells has also been demonstrated in animal models [27]. Taken together, these studies provide experimental evidence for a significant contribution of the sympathetic nervous system to priming the bone microenvironment for cancer metastasis.
Fig. 1.
Schematic representation of the RANKL-dependent mechanism by which sympathetic nerve activation might promote the engraftment of metastatic breast cancer cells into the bone marrow microenvironment.
These studies in mice suggest that interventions aimed at reducing sympathetic activation, βAR signaling, or RANKL expression or signaling might limit breast cancer cell metastatic engraftment in the skeleton and improve the prognosis of women with early breast cancer diagnosis. This is supported by several clinical studies. Longer disease-free survival [28] and lower metastasis development and tumor recurrence were observed in women on β-blockers [29], [30]. Another study reported less advanced disease at diagnosis and lower cancer-specific mortality in patients who had taken propranolol 1 year before diagnosis [31], thus the use of β-blockers at early stage of the disease seems to be critical to observe a protective effect on mortality [32], [33], [34]. A beneficial effect of β-blockers on patient survival was detected for other types of cancer than breast cancer, including ovarian cancer [35], and RANKL blockade increased metastasis-free survival in men with castration-resistant prostate cancer [36]. In conclusion, both clinical association data and preclinical data in mice support a stimulatory role of the sympathetic nervous system in the skeletal establishment of metastatic cancer cells, which appears relevant to a number of solid and possibly blood cancers.
Outstanding questions:
-
•
Does β2AR stimulation in breast cancer cells, in addition to osteoblastic β2AR stimulation, contribute to their engraftment into the skeleton?
-
•
Do skeletal sympathetic nerves contribute in establishing a “fertile soil” in bone via host β2AR-expressing osteoblasts only, or via other cells such as immune or endothelial cells known to be responsive to sympathetic signals?
-
•
Does the increase in glucocorticoids induced by HPA activation in response to chronic stress contributes to the observed effect of SNS activation on breast cancer cell metastasis to the skeleton?
-
•
Do β-blockers or RANKL blockade improve patient survival by reducing skeletal metastasis or by limiting early metastatic growth?
-
•
Although high RANK levels in primary breast cancer tumors are predictive of bone metastasis and worst prognosis, the mechanism by which RANK-negative breast cancer cells metastasize into the skeleton is unknown.
Acknowledgments
Research reported in this publication was supported by NCI of the National Institutes of Health under award number R01 CA168717 to FE. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Manders K., van de Poll-Franse L.V., Creemers G.J., Vreugdenhil G., van der Sangen M.J., Nieuwenhuijzen G.A., Roumen R.M., Voogd A.C. Clinical management of women with metastatic breast cancer: a descriptive study according to age group. BMC Cancer. 2006;6:179. doi: 10.1186/1471-2407-6-179. PMC1534056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman R.E. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clinical Cancer Res. : Off. J. Am. Assoc. Cancer Res. 2006;12:6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 3.Ren G., Esposito M., Kang Y. Bone metastasis and the metastatic niche. J. Mol. Med. 2015;93:1203–1212. doi: 10.1007/s00109-015-1329-4. (PMC4636917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferron M., Hinoi E., Karsenty G., Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. USA. 2008;105:5266–5270. doi: 10.1073/pnas.0711119105. (PMC2278202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinoi E., Gao N., Jung D.Y., Yadav V., Yoshizawa T., Kajimura D., Myers M.G., Jr., Chua S.C., Jr., Wang Q., Kim J.K., Kaestner K.H., Karsenty G. An osteoblast-dependent mechanism contributes to the leptin regulation of insulin secretion. Ann. N. Y. Acad. Sci. 2009;1173(Suppl 1):E20–E30. doi: 10.1111/j.1749-6632.2009.05061.x. [DOI] [PubMed] [Google Scholar]
- 6.Karsenty G., Oury F. Regulation of male fertility by the bone-derived hormone osteocalcin. Mol. Cell Endocrinol. 2014;382(521–526):3850748. doi: 10.1016/j.mce.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waning D.L., Mohammad K.S., Reiken S., Xie W., Andersson D.C., John S., Chiechi A., Wright L.E., Umanskaya A., Niewolna M., Trivedi T., Charkhzarrin S., Khatiwada P., Wronska A., Haynes A., Benassi M.S., Witzmann F.A., Zhen G., Wang X., Cao X., Roodman G.D., Marks A.R., Guise T.A. Excess TGF-beta mediates muscle weakness associated with bone metastases in mice. Nat. Med. 2015;21:1262–1271. doi: 10.1038/nm.3961. (PMC4636436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chida Y., Hamer M., Wardle J., Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat. Clin. Pract. Oncol. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 9.Spiegel D., Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol. Psychiatry. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 10.Burgess C., Cornelius V., Love S., Graham J., Richards M., Ramirez A. Depression and anxiety in women with early breast cancer: five year observational cohort study. Br. Med. J. 2005;330(702):555631. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross K. Mapping pathways from stress to cancer progression. J. Natl. Cancer Inst. 2008;100(914–915):917. doi: 10.1093/jnci/djn229. [DOI] [PubMed] [Google Scholar]
- 12.Giese-Davis J., Wilhelm F.H., Conrad A., Abercrombie H.C., Sephton S., Yutsis M., Neri E., Taylor C.B., Kraemer H.C., Spiegel D. Depression and stress reactivity in metastatic breast cancer. Psychosom. Med. 2006;68:675–683. doi: 10.1097/01.psy.0000238216.88515.e5. [DOI] [PubMed] [Google Scholar]
- 13.Giese-Davis J., Wilhelm F.H., Conrad A., Abercrombie H.C., Sephton S., Yutsis M., Neri E., Taylor C.B., Kraemer H.C., Spiegel D. Depression and stress reactivity in metastatic breast cancer. Psychosom. Med. 2006;68:675–683. doi: 10.1097/01.psy.0000238216.88515.e5. [DOI] [PubMed] [Google Scholar]
- 14.Heffner K.L., Loving T.J., Robles T.F., Kiecolt-Glaser J.K. Examining psychosocial factors related to cancer incidence and progression: in search of the silver lining. Brain Behav. Immun. 2003;17(Suppl 1):S109–S111. doi: 10.1016/s0889-1591(02)00076-4. [DOI] [PubMed] [Google Scholar]
- 15.Patel M.S., Elefteriou F. The new field of neuroskeletal biology. Calcif. Tissue Int. 2007;80:337–347. doi: 10.1007/s00223-007-9015-3. [DOI] [PubMed] [Google Scholar]
- 16.Elefteriou F., Ahn J.D., Takeda S., Starbuck M., Yang X., Liu X., Kondo H., Richards W.G., Bannon T.W., Noda M., Clement K., Vaisse C., Karsenty G. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong A.P., Miller R.E., Jones J.C., Zhang J., Keller E.T., Dougall W.C. RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate. 2008;68:92–104. doi: 10.1002/pros.20678. [DOI] [PubMed] [Google Scholar]
- 18.Chen L.M., Kuo C.H., Lai T.Y., Lin Y.M., Su C.C., Hsu H.H., Tsai F.J., Tsai C.H., Huang C.Y., Tang C.H. RANKL increases migration of human lung cancer cells through intercellular adhesion molecule-1 up-regulation. J. Cell. Biochem. 2011;112:933–941. doi: 10.1002/jcb.23009. [DOI] [PubMed] [Google Scholar]
- 19.Jones D.H., Nakashima T., Sanchez O.H., Kozieradzki I., Komarova S.V., Sarosi I., Morony S., Rubin E., Sarao R., Hojilla C.V., Komnenovic V., Kong Y.Y., Schreiber M., Dixon S.J., Sims S.M., Khokha R., Wada T., Penninger J.M. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 20.Katayama Y., Battista M., Kao W.M., Hidalgo A., Peired A.J., Thomas S.A., Frenette P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 21.Campbell J.P., Karolak M.R., Ma Y., Perrien D.S., Masood-Campbell S.K., Penner N.L., Munoz S.A., Zijlstra A., Yang X., Sterling J.A., Elefteriou F. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10:e1001363. doi: 10.1371/journal.pbio.1001363. (PMC3398959) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santini D., Perrone G., Roato I., Godio L., Pantano F., Grasso D., Russo A., Vincenzi B., Fratto M.E., Sabbatini R., Della Pepa C., Porta C., Del Conte A., Schiavon G., Berruti A., Tomasino R.M., Papotti M., Papapietro N., Onetti Muda A., Denaro V., Tonini G. Expression pattern of receptor activator of NFkappaB (RANK) in a series of primary solid tumors and related bone metastases. J. Cell. Physiol. 2011;226:780–784. doi: 10.1002/jcp.22402. [DOI] [PubMed] [Google Scholar]
- 23.Thaker P.H., Han L.Y., Kamat A.A., Arevalo J.M., Takahashi R., Lu C., Jennings N.B., Armaiz-Pena G., Bankson J.A., Ravoori M., Merritt W.M., Lin Y.G., Mangala L.S., Kim T.J., Coleman R.L., Landen C.N., Li Y., Felix E., Sanguino A.M., Newman R.A., Lloyd M., Gershenson D.M., Kundra V., Lopez-Berestein G., Lutgendorf S.K., Cole S.W., Sood A.K. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat. Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 24.Sood A.K., Bhatty R., Kamat A.A., Landen C.N., Han L., Thaker P.H., Li Y., Gershenson D.M., Lutgendorf S., Cole S.W. Stress hormone-mediated invasion of ovarian cancer cells. Clinical Cancer Res.: Off. J. Am. Assoc. Cancer Res. 2006;12(369–375):3141061. doi: 10.1158/1078-0432.CCR-05-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sood A.K., Armaiz-Pena G.N., Halder J., Nick A.M., Stone R.L., Hu W., Carroll A.R., Spannuth W.A., Deavers M.T., Allen J.K., Han L.Y., Kamat A.A., Shahzad M.M., McIntyre B.W., Diaz-Montero C.M., Jennings N.B., Lin Y.G., Merritt W.M., DeGeest K., Vivas-Mejia P.E., Lopez-Berestein G., Schaller M.D., Cole S.W., Lutgendorf S.K. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J. Clin. Investig. 2010;120(1515–1523):2860925. doi: 10.1172/JCI40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sloan E.K., Priceman S.J., Cox B.F., Yu S., Pimentel M.A., Tangkanangnukul V., Arevalo J.M., Morizono K., Karanikolas B.D., Wu L., Sood A.K., Cole S.W. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(7042–7052):2940980. doi: 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnon C., Hall S.J., Lin J., Xue X., Gerber L., Freedland S.J., Frenette P.S. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341:1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]
- 28.Melhem-Bertrandt A., Chavez-Macgregor M., Lei X., Brown E.N., Lee R.T., Meric-Bernstam F., Sood A.K., Conzen S.D., Hortobagyi G.N., Gonzalez-Angulo A.M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powe D.G., Voss M.J., Zanker K.S., Habashy H.O., Green A.R., Ellis I.O., Entschladen F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 2010;1:628–638. doi: 10.18632/oncotarget.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Botteri E., Munzone E., Rotmensz N., Cipolla C., De Giorgi V., Santillo B., Zanelotti A., Adamoli L., Colleoni M., Viale G., Goldhirsch A., Gandini S. Therapeutic effect of beta-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res. Treat. 2013;140:567–575. doi: 10.1007/s10549-013-2654-3. [DOI] [PubMed] [Google Scholar]
- 31.Barron T.I., Connolly R.M., Sharp L., Bennett K., Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J. Clin. Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 32.Cardwell C.R., Coleman H.G., Murray L.J., Entschladen F., Powe D.G. Beta-blocker usage and breast cancer survival: a nested case-control study within a UK clinical practice research datalink cohort. Int. J. Epidemiol. 2013;42:1852–1861. doi: 10.1093/ije/dyt196. [DOI] [PubMed] [Google Scholar]
- 33.Shah S.M., Carey I.M., Owen C.G., Harris T., Dewilde S., Cook D.G. Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br. J. Clin. Pharmacol. 2011;72(157–161):3141198. doi: 10.1111/j.1365-2125.2011.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sorensen G.V., Ganz P.A., Cole S.W., Pedersen L.A., Sorensen H.T., Cronin-Fenton D.P., Garne J.P., Christiansen P.M., Lash T.L., Ahern T.P. Use of beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and risk of breast cancer recurrence: Danish nationwide prospective cohort study. J. Clin. Oncol. 2013;31(2265–2272):3677839. doi: 10.1200/JCO.2012.43.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watkins J.L., Thaker P.H., Nick A.M., Ramondetta L.M., Kumar S., Urbauer D.L., Matsuo K., Squires K.C., Coleman R.L., Lutgendorf S.K., Ramirez P.T., Sood A.K. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer. 2015;121:3444–3451. doi: 10.1002/cncr.29392. (PMC4575637) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith M.R., Saad F., Coleman R., Shore N., Fizazi K., Tombal B., Miller K., Sieber P., Karsh L., Damiao R., Tammela T.L., Egerdie B., Van Poppel H., Chin J., Morote J., Gomez-Veiga F., Borkowski T., Ye Z., Kupic A., Dansey R., Goessl C. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379:39–46. doi: 10.1016/S0140-6736(11)61226-9. (PMC3671878) [DOI] [PMC free article] [PubMed] [Google Scholar]