Abstract
The vasculature of the skeletal system regulates osteogenesis and hematopoiesis, in addition to its primary function as a transportation network. Recent studies suggest that the vasculature in bone regulates multiple steps involved in the metastatic cascade. Matrix and growth factor abundant vascular microenvironments in bone not only provide a fertile soil for the metastatic growth but also support the dormancy of Disseminated Tumour Cells (DTCs). Interestingly, vasculature also seems to direct the reactivation of dormant DTCs. Targeting such early steps of bone metastasis by directing therapies against vascular niches can lead to the development of effective therapeutic strategies that delay or even prevent the metastatic relapse. However, this would require a detailed understanding of the regulatory mechanisms that govern the interaction between endothelial cells and DTCs in the early stages of bone metastasis. This review aims to highlight the importance of vascular niches and outline their newly identified roles during bone metastasis.
Keywords: Endothelial cells, Metastasis, Dormancy, Disseminated tumour cells, Angiogenesis
1. Introduction
The skeleton is the most prevalent site of metastasis for several cancer types. Skeletal metastasis is associated with a reduced quality of life owing to prolonged pain, poor therapeutic success and low survival rate [1], [2]. The initial stages that underpin this poor outcome is laid by the extravasation and lodging of circulating tumour cells in the bone marrow microenvironment, a process that in most patients precedes the primary tumour detection. Disseminated tumour cells (DTCs) may survive within the bone marrow microenvironment in the state of dormancy (cell cycle arrest) for very long periods. While a subset of patients develop detectable metastases as late as two-to-three decades post primary tumour detection, others show no signs of relapse despite the detection of DTCs in bone postmortem [2], [3], [4]. Such clinical evidence argue in favor of targeting DTCs within the bone microenvironment, rather than targeting the initial steps of tumour cell extravasation and dissemination. Designing strategies to target DTCs within the bone marrow microenvironment is likely to facilitate the development of therapeutic regimes that delay or prevent metastatic relapse. However, this requires fundamental understanding of the mechanisms that lead to dormancy and subsequent reactivation of cancer cells. Existing therapeutic strategies target and slow down progression in the late stage of the disease; but these therapies are not curative [1], [2], [3], [4]. Despite its tremendous impact on the therapeutic outcome, our understanding of the early stage (survival, quiescence, migration and proliferation of cancer cells in bone) of the disease remains poor. Multiple lines of evidence demonstrate that tumour cells are regulated in a non-cell-autonomous manner. Notably, recent findings highlight the important roles of the microenvironment in determining the fate of DTCs [5], [6].
Extensive vascularization of the skeletal tissue suggests the importance of blood vessels in regulating its physiological functions. Endothelium, the innermost cellular layer of blood vessels forms a central component of the bone marrow vascular microenvironment [7]. Recent evidence support that a pivotal role is played by the vascular niche during the early stages of bone metastasis [5]. During the later stages of the disease, blood vessels enhance the metastatic outgrowth by mediating the delivery of oxygen, nutrients and growth factors. This implies that altering the vascular microenvironment of DTCs during the early stages of metastasis can lead to the elimination of DTCs; thereby preventing the relapse, while targeting the vasculature at the later stages can slow down the metastatic growth. This review highlights the significance of vascular niches during skeletal metastases and discusses the potential therapeutic interventions for targeting the vascular niche.
2. Properties of the bone vasculature
Organ specificity of the vasculature and heterogeneity among vascular beds has been suggested to guide the differential extravasation of tumour cells to various organs. Therefore, understanding the morphological, cellular, molecular and functional properties of the bone vasculature is critical for deciphering the role of the vascular niche during skeletal metastases [8]. Like vascular beds in other organs, endothelial cells in the skeletal system are also organized as a widespread hierarchical network of blood vessels that perform multiple functions. Apart from being a transport network, blood vessels in the skeletal system provide inductive signals to regulate skeletal development, homeostasis and remodeling [7]. Emerging studies reveal that the vasculature of the skeletal system plays crucial roles in osteogenesis (bone formation) and haematopoiesis through direct cellular interactions and paracrine (angiocrine) signalling pathways [9], [10], [11]. Haematopoietic Stem Cells (HSCs) are frequently detected within vascular microenvironments, which involve different vessel subtypes comprising of both endothelial and perivascular cells. Specific vascular microenvironments are required to support HSC homing, self-renewal and quiescence [10]. The skeletal vascular microenvironment is thus often denoted as the “bone-marrow vascular niche”. Consequently, there has been a tremendous interest in understanding the structural and functional properties of the skeletal endothelium. However, the progress has been hampered by the lack of understanding of the precise organization of the vasculature. Bone vasculature is vaguely defined as a network of sinusoids and arterioles. The discontinuous and fenestrated sinusoidal endothelium constitutes the predominant vascular surface in the skeletal system [12]. We have recently unravelled the fundamental aspects of the basic hierarchical organization of bone arteries, capillaries and veins. We found that the molecular signature, metabolic activity and functional properties vary between metaphysis and diaphysis blood vessels. In addition, we have identified and characterized a distinct capillary and endothelial cell subpopulations, one of which (termed type H) plays critical roles in the regulation of osteoprogenitor cells and thereby bone formation [9], [11].
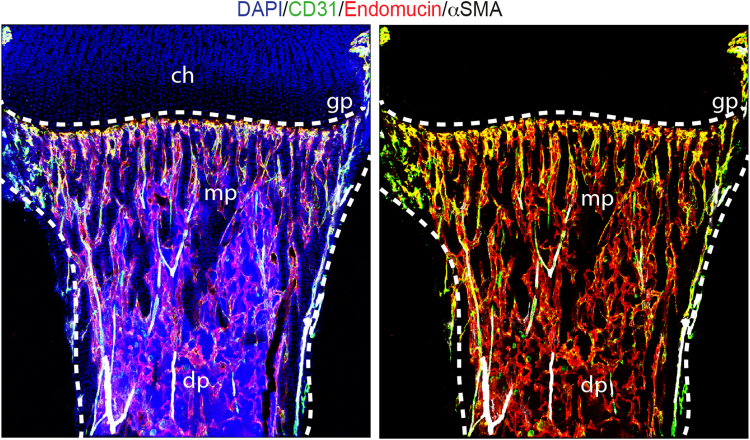
Different vessel subtypes have distinct functional roles in the vertebrate skeletal system. Veins drain the sinusoids and type H capillaries, while arteries deliver oxygen-rich blood and terminate into the type H capillaries. Columnar type H capillaries oriented towards the growth plate are characterized by high expression of markers CD31 and Endomucin and are physically connected to distal arterioles in the metaphysis and endosteum of long bone (Fig. 1). Type H capillaries are surrounded by bone-forming osteoprogenitor cells and release pro-osteogenic growth factors. In contrast, type L (CD31lo Endomucinlo) blood vessels, which correspond to the highly branched network of sinusoidal endothelium of the bone marrow cavity, are not directly connected to arterioles and lack association with bone forming osteoprogenitor cells [9], [11]. Such unique properties of the bone vasculature combined with its heterogeneous nature and expression of growth factors suggest the existence of distinct microenvironments in bone which may support the early events during skeletal metastasis and can also accelerate disease progression in the late stage.
Fig. 1.
Organization of blood vessels in bone. Tile scan confocal images showing metaphysis (mp) and diaphysis (dp) regions of the mouse long bone (tibia) immunostained for CD31 (green), Endomucin (red) and α-SMA (white). Linear CD31hi/Endomucinhi type H blood vessels are abundant in the metaphysis while a highly branched network of sinusoidal endothelium (type L) forms the predominant vascular surface in the diaphysis. CD31hi (green) Endomucin− arteries with α-SMA+ coverage are directly connecting to type H capillaries but not to type L blood vessels. Dashed lines mark the growth plate (gp) and compact bone. Chondrocytes; ch. Nuclei are stained with DAPI (blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3. Dissemination-permissive characteristics of the BM vascular niche
Sinusoids (type L capillaries) are the most abundant blood vessels in bone and widely distributed throughout the bone marrow cavity [8]. Sinusoids in bone are discontinuous single layer of endothelial cells devoid of pericytes. Due to the absence of a consistent vessel wall and low level expression of tight junction molecules, sinusoidal endothelium is highly permeable in nature [8]. Additionally, the sinusoidal wall is specialized to facilitate easy two-way trafficking of hematopoietic cells [8]. These characteristics of bone sinusoids are known to expedite invasion, extravasation and docking of circulating tumour cells within the bone marrow (Fig. 2A). In comparison to other capillary beds, sinusoidal vessels have exceptionally large diameter [8]. As a result of the large diameter and voluminous nature of these sinusoidal vessels, blood flow within bone is reduced dramatically. This leads to trapping and arrest of circulating tumour cells in the skeletal system [5], [13]. Furthermore, the expression profile of the skeletal vasculature is largely different as compared to blood vessels in other tissues. Of note, the vasculature in bone expresses and secretes high levels of CXCL12 (stromal cell-derived factor-1 (SDF-1)), a member of the CXC family of chemokines, which is known to play a key role in homing and retention of HSCs in the bone marrow microenvironment [14]. High levels of these ligands might draw the circulating tumour cells expressing its receptor, CXCR4 to the bone (Fig. 2A). Therefore, circulating tumour cells commandeer HSC homing pathways to land and establish their footholds within the foreign bone marrow microenvironment. Other players in HSC homing pathways are also predicted to be hijacked by DTCs to facilitate their landing in bone such as matrix molecules like integrin family members, and adhesion molecules like E-selectin and Robo4 [15]. Thus, the morphological, structural, adhesive and paracrine/angiocrine properties of the skeletal vasculature appear to direct the dissemination of cancer cells to bone.
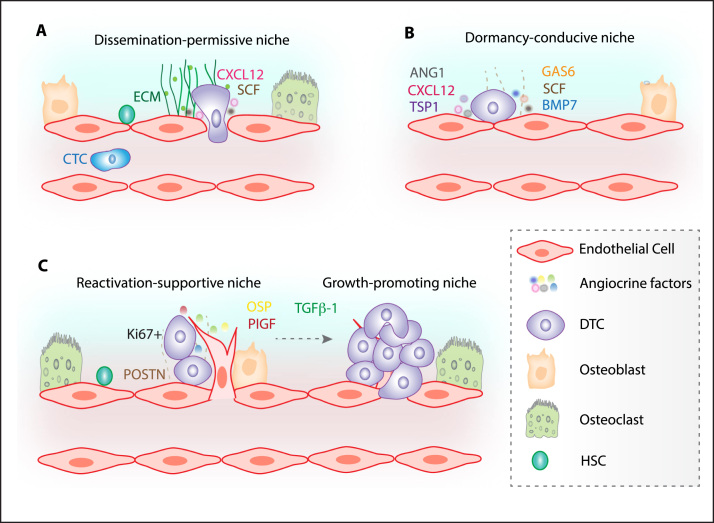
Fig. 2.
Bone marrow vascular niches regulate the fate of DTCs. The morphological, structural, adhesive and paracrine/angiocrine properties of the skeletal vasculature guide the dissemination of cancer cells to bone. Especially, vascular microenvironments in bone are exceptionally rich in extracellular matrix (ECM) proteins, growth factors like SCF and chemokines like CXCL12. Due to the absence of a consistent vessel wall sinusoidal endothelium is highly permeable and specialized to facilitate easy trafficking of haematopoietic cells. Circulating tumour cells (CTCs) commandeer HSC homing pathways to land on the bone marrow microenvironment (A). Subsequent to the successful landing of cancer cells into the bone marrow microenvironment these cells rarely proliferate instantly. DTCs exposed to the unfamiliar bone microenvironment survive in the dormant state and are frequently detected in close proximity to the vasculature. Endothelial cells from mature blood vessels secrete dormancy inducing factors like TSP1 and CXCL12 and thereby maintain DTCs in the dormant state (B). Interestingly, in contrast to the stable mature vasculature, sprouting vasculature reactivates (detected as Ki67 positive; a proliferation marker) dormant DTCs through the production of factors like periostin (POSTN) and transforming growth factor β-1 (TGFβ-1). Thus the vasculature of the skeletal system not only maintains the dormancy of DTCs but also drives the reactivation of dormant DTCs. Once reactivated and a micrometastasis is generated it promotes blood vessel ingrowth by the production of angiogenic factors further enhancing the metastatic growth (C).
4. Dormancy-conducive characteristics of the BM vascular niche
Cancer cells ingression into the bone vasculature and their admission within the marrow cavity does not guarantee their retention and subsequent colonization in bone. Even though many cancer cells circulate through bone and succeed in entering the bone marrow cavity, only a few persist for metastatic colonization. Subsequent to their successful landing, cancer cells rarely proliferate instantly (Fig. 2B). These DTCs now exposed to the unfamiliar bone microenvironment, survive in the so-called dormant (quiescent/growth-arrested) state for long time period. Recent studies suggest that signals and pathways governing HSC quiescence are also involved in maintaining the dormancy of cancer cells in bone [14], [15]. Quiescent HSCs have been found in close proximity to the vasculature and vascular niches are crucial for maintaining HSCs in a quiescent state [10]. Likewise it is possible that specific vascular niches regulate the dormancy of cancer cells in bone. In line with this notion, Ghajar et al. identified that dormant cancer cells in bone are localized in close proximity to the stable vasculature [16]. To gain mechanistic insight into this endothelial and cancer cell interactions authors generated in vitro 3D cultures with human umbilical vein endothelial cells and bone marrow-derived mesenchymal cells. Cancer cells cultured on this organotypic microvasculature exhibited reduced proliferation and growth. Further analysis of the endothelial extracellular matrix revealed up-regulation of thrombospondin-1 (TSP-1), which was secreted by endothelial cells, as a suppressor of cancer cell growth. Antibody driven blocking of TSP-1 to impede cancer cell interaction with endothelium resulted in increased proliferation and growth of cancer cells. This interesting study provides crucial mechanistic insights into the regulation of cancer cell dormancy [16]. Since the study relied on findings from the in vitro 3D co-culture system, further research is needed to delineate and understand the in vivo situation. Another example of angiocrine factors regulating cancer cell dormancy in bone is the chemokine CXCL12. A critical component of the HSC niche, CXCL12 expressed by the bone vasculature may regulate the dormancy of DTCs in bone [17]. Further, stromal cells in bone also contribute to the dormancy of tumour cells by secreting microRNAs (miRs) that target CXCL12 expression. These anti-CXCL12 miRs transported from stromal cells to breast tumour cells induce cell cycle arrest [17]. However, it remains unstudied whether endothelial cells also express these miRs. Similarly, other known regulators of HSC quiescence in the bone marrow such as growth arrest-specific protein 6 (GAS6), transforming growth factor-β2 (TGFβ2), bone morphogenetic protein 4 (BMP4), BMP7 also induce dormancy of DTCs [18]. Interestingly, morphogenetic cues like BMPs and TGFβ family members that induce dormancy in DTCs are expressed by the bone vasculature. Overall, above studies emphasize the importance of bone endothelial cells in supporting the dormancy of DTCs (Fig. 2B), though the detailed identity of functional vascular niches for DTCs remains anonymous.
5. Growth-supportive characteristics of the BM vascular niche
Similar to normal tissues, tumours also need functional blood vessels to support their growth via the delivery of oxygen and nutrients. When a DTC starts proliferating into a micrometastasis, it drives angiogenesis to build new blood vessels. If angiogenesis fails and new vasculature is not recruited, micrometastasis remains deprived of oxygen and nutrients. Consequently, cell death balances proliferation thereby preventing the development of clinically detectable metastases [18]. Remarkably, it has been demonstrated that while stable vasculature supports dormancy of DTCs, proliferating cancer cells are selectively localized in close proximity to the sprouting vasculature (Fig. 2C). In vitro 3D co-culture experiments with cancer cells and actively growing microvasculature demonstrated that cancer cell proliferation positively correlated with the sprouting microvasculature. These neovascular tips were characterized by high expression of periostin, fibronectin, tenascin, versican, and active transforming growth factor-β1 (TGF-β1) [16]. All these factors have been previously implicated to contribute to the development of the metastatic niche. Further the bone marrow cavity is hypoxic and bone matrix is exceptionally rich in growth factors, cytokines and bone resorbing factors. Endothelial cells secrete several of these growth factors including TGFβs, IGFs, FGFs, PDGFs and BMPs. Blocking of an angiocrine factor – placental growth factor (PlGF), using anti-mouse-PlGF antibodies, resulted in decreased bone metastasis [19]. Additional endothelial factors that can stimulate proliferation and growth of cancer cells in bone include osteopontin, SCF and CXCL12 [5], [10], [11]. The hypoxic nature of bone, combined with its abundant resource of growth factors and cytokines alter the phenotype of tumour cells to produce aggressive metastatic lesions. Above evidences suggest that, interfering with endothelial cell-cancer cell interactions during early stages of metastatic growth may lead to the development of effective therapeutic strategies to delay or even prevent the metastatic relapse.
6. Therapeutic targeting of the BM vascular niche
The most encouraging perspective of above observations is the potential of disrupting cancer cell-endothelial cell interactions and interfering with the bone marrow vascular niche during the early stage of the disease to sensitize cancer cells to the therapeutic regimes. Lessons from hematological malignancies indeed provide the proof of principle for targeting cancer cell-endothelial cell interactions [20]. This strategy has led to the successful eradication of leukaemic stem cells from patients and substantial improvement in survival rates. Mobilization of cancer cells out of their niche microenvironments render these cells vulnerable to chemotherapy. For instance, treatment with a CXCR4 antagonist AMD3100 sensitizes acute myeloid leukaemic cells to chemotherapy [20]. Another evidence suggests that endothelial Delta-like 4 (DLL4), a Notch signalling ligand induces exit of dormancy in T-ALL cells by interacting with NOTCH3 receptors on these cells. Moreover, vascular endothelial growth factor A (VEGFA) promotes overexpression of DLL4 [18]. Therefore, modulating endothelial cell-DTC interactions by CXCR4 antagonist or VEGF inhibitors offers the possibility to eradicate dormant DTCs. Even though, waking dormant tumour cells offers an exciting opportunity to eradicate them, simultaneous targeting and sensitization of these cells with existing therapies is the key to a successful outcome and the disease free survival.
7. Concluding remarks
Emerging evidences indicate that vascular niches in bone control the fate of DTCs and thereby influence multiple steps within the metastatic cascade. Our current knowledge of the cellular and molecular mechanisms governing cancer cell dormancy and subsequent reactivation in bone suggests that DTCs exploit the molecular machinery used by HSCs to establish their footholds in bone. Nevertheless, the specific roles of vascular niches during the early steps of skeletal metastasis remain little understood. A better understanding of the DTC-endothelial cell interactions is required for targeting the early steps of skeletal metastasis. HSC niches have been shown to be composed of multiple cell populations including osteoblasts and mesenchymal stem cells. Similarly, the fate of DTCs in bone may be regulated by complex microenvironments involving multiple cell populations and vessel subtypes. Characterizing these niches and understanding molecular pathways coupling the behaviour of DTCs and niche cells can lead to the development of effective therapeutic strategies that delay or even prevent the metastatic relapse.
References
- 1.Coleman R.E. Bone cancer in 2011: prevention and treatment of bone metastases. Nat. Rev. Clin. Oncol. 2012;9:76–78. doi: 10.1038/nrclinonc.2011.198. [DOI] [PubMed] [Google Scholar]
- 2.Suva L.J., Washam C., Nicholas R.W., Griffin R.J. Bone metastasis: mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 2011;7:208–218. doi: 10.1038/nrendo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 4.Psaila B., Lyden D. The metastatic niche: adapting the foreign soil. Nat. Rev. Cancer. 2009;9:285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bussard K.M., Gay C.V., Mastro A.M. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008;27:41–55. doi: 10.1007/s10555-007-9109-4. [DOI] [PubMed] [Google Scholar]
- 6.Coleman R.E., Gregory W., Marshall H., Wilson C., Holen I. The metastatic microenvironment of breast cancer: clinical implications. Breast. 2013;22(Suppl 2):S50–S56. doi: 10.1016/j.breast.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Ramasamy S.K., Kusumbe A.P., Adams R.H. Regulation of tissue morphogenesis by endothelial cell-derived signals. Trends Cell Biol. 2015;25:148–157. doi: 10.1016/j.tcb.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugsley M.K., Tabrizchi R. The vascular system. An overview of structure and function. J. Pharm. Toxicol. Methods. 2000;44:333–340. doi: 10.1016/s1056-8719(00)00125-8. [DOI] [PubMed] [Google Scholar]
- 9.Ramasamy S.K., Kusumbe A.P., Wang L., Adams R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendelson A., Frenette P.S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat. Med. 2014;20:833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopp H.G., Hooper A.T., Avecilla S.T., Rafii S. Functional heterogeneity of the bone marrow vascular niche. Ann. N. Y. Acad. Sci. 2009;1176:47–54. doi: 10.1111/j.1749-6632.2009.04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mastro A.M., Gay C.V., Welch D.R. The skeleton as a unique environment for breast cancer cells. Clin. Exp. Metastasis. 2003;20:275–284. doi: 10.1023/a:1022995403081. [DOI] [PubMed] [Google Scholar]
- 14.Shiozawa Y., Pienta K.J., Taichman R.S. Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin. Cancer Res. 2011;17:5553–5558. doi: 10.1158/1078-0432.CCR-10-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winkler I.G., Barbier V., Nowlan B., Jacobsen R.N., Forristal C.E., Patton J.T. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat. Med. 2012;18:1651–1657. doi: 10.1038/nm.2969. [DOI] [PubMed] [Google Scholar]
- 16.Ghajar C.M., Peinado H., Mori H., Matei I.R., Evason K.J., Brazier H. The perivascular niche regulates breast tumour dormancy. Nat. Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim P.K., Bliss S.A., Patel S.A., Taborga M., Dave M.A., Gregory L.A. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 18.Sosa M.S., Bragado P., Aguirre-Ghiso J.A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer. 2014;14:611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coenegrachts L., Maes C., Torrekens S., Van Looveren R., Mazzone M., Guise T.A. Anti-placental growth factor reduces bone metastasis by blocking tumor cell engraftment and osteoclast differentiation. Cancer Res. 2010;70:6537–6547. doi: 10.1158/0008-5472.CAN-09-4092. [DOI] [PubMed] [Google Scholar]
- 20.Nervi B., Ramirez P., Rettig M.P., Uy G.L., Holt M.S., Ritchey J.K. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–6214. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]