Abstract
Approximately 80% of prostate cancers exhibit some degree of bone metastasis. The role of the bone marrow and the hematopoietic stem cell (HSC) niche in attracting metastatic cells and maintaining dormancy of disseminated tumor cells (DTCs) is an increasingly important topic towards the development of novel prostate cancer therapies. This paper reviews aspects of the HSC niche that lead to prostate cancer cell homing and dormancy in the bone marrow. This review also discusses the role of DTCs in the niche environment and discusses the role of erythropoietin in targeting DTCs within the HSC niche.
1. Introduction
Cancer cells disseminate from a primary tumor and enter the circulation, of which less than 0.01% survive and produce metastases [1]. Hematogenous circulation and lymphatic routes appear to be major routes through which disseminating tumor cells (DTCs) navigate. There are many challenges that tumor cells must overcome during the metastatic process including dissociation from neighboring cells of the primary tumor, extravasation, survival, and establishment in distant sites. DTCs have a number of different fates including death, dormancy, or proliferation [2]. The role of the microenvironment in tumor cell fate regulation has been reported as early as Paget's “seed and soil hypothesis” [3]. This hypothesis was expanded upon by Fidler [2], who suggested that tumor cells (i.e. seed) extravagate into circulation, survive, and establish in a distant site (i.e. soil), and their fate (death, dormancy, or growth) is directly influenced by the microenvironment of the distant site. The “seed and soil” hypothesis has been used to describe many different tumor-related diseases, including prostate cancer, which has a particular predilection for metastasis to bone which also houses the hematopoietic stem cell (HSC). Toward this end, 80% of advanced prostate cancer cases exhibit distant site metastasis in bone accompanied by a median survival of approximately 40 months [4]. Here, we discuss insights into the role of the HSC niche in prostate cancer (PCa) bone metastasis.
2. Homing of DTCs to the HSC niche
The HSC niche is a complex microenvironment comprised of many cell types, including endothelial cells, adipocytes, osteoclasts, osteomacs, and cells of osteoblastic lineage [5]. A healthy HSC niche provides homing signals to healthy HSCs in order to promote their normal function [6]. Shiozawa et al. [7] demonstrated that increasing the number of HSC niches promoted increased number of bone marrow DTCs, which indicated these same homing signals can be exploited in PCa metastasis. Two important mediators of the HSC microenvironment are the chemo-attractant stromal derived factor-1 (SDF-1 or CXCL12) and the cell attachment factor (Annexin2 or ANXA2). CXCL12 regulates HSC homing to the bone marrow as well as mobilization into circulation, while ANXA2 is likely involved in HSC binding to the osteoblastic niche, and may act as an anchor of CXCL12 and aid in localization to the niche [8]. Recently, it was shown that bone marrow stromal cells expressing enhanced levels of CXCL12 and ANXA2 increases recruitment of PCa cells into the bone marrow, promotes proliferation of PCa cells, and protects PCa cells from chemotherapy induced apoptosis [9]. These data suggest that DTCs may home to the HSC niche using similar mechanisms to the HSCs themselves in the regulation of cell fate.
3. DTC dormancy in the HSC niche
The concept of a dormancy supportive/permissive microenvironment in the bone marrow is an increasingly important and complex area of investigation in clinical oncology due to the general mechanism by which chemotherapeutics act to target mitotic cells [10]. There are many mechanisms by which DTC mitotic cycling is affected including regulation of the immune system, angiogenesis/nutrients, tumor extracellular matrix, and hormones [11]. In the HSC niche, cancer cells are subject to HSC quiescent signaling mechanisms, and a number of possible contributing chemokines have been identified [7], [12]. DTC dormancy can be achieved through lack of activating signals (e.g. Wnt, Notch) or directly due to inhibitory signals (BMPs) [13]. Aguirre-Ghiso et al. [14] demonstrated that the balance between p38 and ERK, both mitogen activated kinases (MAPKs), affect DTC mitotic state. When ERK is elevated compared to p38, proliferation is favored; conversely, elevation of p38 compared to ERK favors quiescence. The balance of ERK and p38 in vivo is further regulated by other ligands: down-regulation of urokinase receptor (uPAR) results in an ERKLow/p38High signaling ratio, inducing proliferative behavior in squamous carcinoma cells (HEp3) [15]. BMP7 has also been demonstrated to induce dormancy in PCa cells through the p38 MAPK pathway [16].
Other HSC-mediated factors, such as low oxygen [17], angiogenic [18], or additional secreted factors can also control dormancy. Shiozawa et al. [19] reported that growth arrest specific-6 (GAS6), a ligand of TYRO3, AXL, and MER tyrosine kinase receptors produced by osteoblasts in the HSC niche, supported PCa cell dormancy with increased survival and additionally prevented proliferation. Additionally, angiogenic simulators c-myc, vascular endothelial factor (VEGF), and fibroblast growth factor 2(FGF2) may be involved in exit of DTCs from dormancy [20], [21]. Understanding the mechanisms of DTC dormancy may lead to better targeted therapies for metastatic disease.
4. The role of DTCs in niche formation
Following localization to the bone marrow, DTCs or their progeny can have osteoblastic or osteolytic effects, or both activities [22]. Osteoblastic lesions stimulate osteoblast formation and promote bone formation, albeit poorly woven bone [4]. Osteolytic lesions stimulate osteoclastic activity, which results in bone loss. In the instance of PCa, the impact of DTCs have been observed to be both osteoblastic and osteolytic in nature [23]. Joseph et al. [24] demonstrated that hematopoietic progenitor cells (HPCs) from mice inoculated with LNCaP-derived C4-2B induced osteoblastic differentiation of bone marrow stromal cells in co-culture through production of BMP-2. Conversely, these authors reported HSCs from mice inoculated with the PCa cell line, PC3, induced osteoclastic activity resulting in predominantly osteolytic lesions through an IL-6 mediated signaling pathway. Applications of this research include using HSC/HPC targeted therapy to limit the effects of bone metastasis. However, more research is needed to understand the precise mechanisms through which metastatic lesions may direct niche formation.
5. Erythropoietin and the HSC niche
Many molecules produced both locally and systemically, including erythropoietin (Epo) and adrenergic catecholamines, also appear to affect HSC niche and metastatic lesions in the bone marrow. EPO is a hematopoietic hormone produced predominantly in the kidneys in response to hypoxia. EPO functions through binding to a preformed homodimer transmembrane receptor (Epo-R). Interestingly, Epo-R is expressed in both hematopoietic and non-hematopoietic tissues, suggesting that the role of Epo may be more widespread beyond hematopoiesis [25]. The mRNA expression of Epo-R has been reported in tissues of the brain, testes, placenta, heart, lungs, bone marrow, spleen, and even tumor cells [26], [27], [28].
In cancer, Epo has both direct and indirect actions on DTCs in the HSC niche. First, Epo may directly act on tumor cells located within the bone marrow. Some studies provide evidence that Epo/Epo-R axis activation leads to tumor cell proliferation; while other studies demonstrate Epo/Epo-R activation did not support tumor cell growth specifically [29]. Interestingly, Shiozawa et al. [30] demonstrated that Epo did not stimulate PCa tumor cell (using PCa cell lines) proliferation in vitro, or enhance metastasis in vivo; however, these authors did demonstrate an increased tumor cell resistance to apoptosis resulting in PCa cell survival. Similarly, Todaro et al. [31] reported that Epo stimulation increased resistance of breast cancer stem-like cells (BCSC) isolated from patient tumors to chemotherapeutics, doxorubicin and 5-FU, in vitro and in a subcutaneous mouse model. Further, they reported stimulation with Epo upregulated known survival pathway mediators—Akt, Erk, and Bcl-xL—which may explain the mechanism by which BCSC cells achieved protection from chemotherapy [31]. These remain among the possible mechanisms that affect cancer therapy and may impact patient survival, through resistance of DTCs within the bone marrow to current chemotherapeutics [32].
There is also evidence that Epo increases niche formation, which may indirectly affect cancer cells or DTCs residing within the bone marrow niche [33], [34]. It was previously reported that blood loss stimulates HSCs and may activate osteoprogenitor cells [35]. Further, Jung et al. [33] demonstrated that HSCs isolated from animals subjected to an acute bleed, showed increased capacity to induce osteoblastic differentiation, due to increased HSC-derived BMP-2 and BMP-6. Later, Shiozawa et al. [36] reported blood loss induced Epo production, causing BMP production in HSCs through a Jak/Stat signaling mechanism. In addition Epo regulates the bone microenvironment through direct action on mesenchymal cells, inducing osteoblast differentiation. Thus demonstrating that Epo can regulate bone metabolism through induction of osteoblast differentiation or production of BMPs by HSCs [34], [36]. Further investigation showed increased angiogenesis as well as elevated numbers of red blood cells in the peripheral blood, and mesenchymal cells and hematopoietic stem cells in the bone marrow [34]. Taken together, understanding the role of Epo on the niche, within which DTCs reside may aid in the development of effective treatment modalities for cancer patients.
Together these data demonstrate interconnectivity of hematopoiesis and tumor metastasis. On one side the HSC niche is targeted by circulating tumor cells to facilitate the establishment of DTCs in marrow. Once there, DTCs are likely to undergo one of three fate decisions: apoptosis, dormancy or proliferation. Each of these fate choices are likely regulated by the niche by soluble and non-soluble factors. The niche, as an integrator of systemic demands for hematopoiesis, also is likely able to regulate DTC fate when occupied by these molecular parasites. Yet how this occurs, remains poorly understood. Similarly, progeny of DTCs are able to produce osteoblastic, osteolytic lesions or mixed. How this occurs is also not well understood and how the niche participates or regulates these activities is an active area of investigation (Fig. 1).
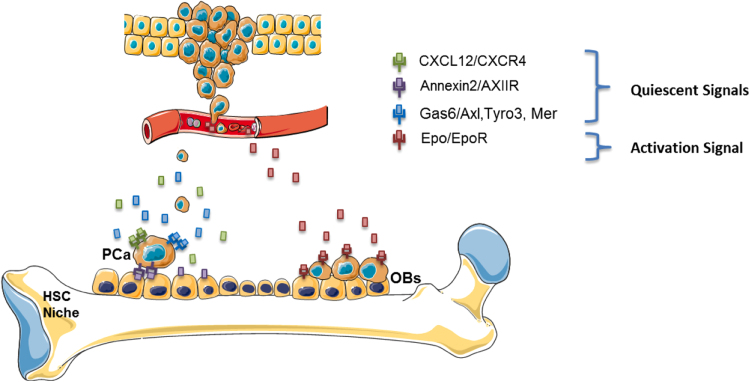
Fig. 1.
Model of Dormancy and Activation of PCa metastasis within the bone marrow niche. The bone marrow niche is a functionally dynamic component in the activity of metastatic cancer cells. PCa cells express receptors for CXCL12, Annexin2, and Gas6, which instigate dormancy and reduce mitotic activity. Conversely, dormant DTCs can be reactivated through external signals, such as Epo. PCa=Prostate Cancer; OBs=osteoblasts.
6. 10 outstanding questions in the field
-
1.
What is dormancy – is it a lack of proliferation or does proliferation and cell death balance out for a zero gain of cell numbers?
-
2.
Does signaling remain the same if metastatic tumor cells replace HSCs in the niche?
-
3.
What mechanisms govern dormancy and disease recurrence in the bone marrow?
-
4.
Is dormancy or tumor reactivation a stochastic or a cell autonomous event.
-
5.
Is dormancy and the exit from regulated by multiple signals and how are they integrated?
-
6.
What happens to DTCs that do not engage the HSC niche? Are these cells more likely to undergo apoptosis, awakening, or do they simply fail to survive?
-
7.
Relapse following curative therapy is often the result of a few DTC clones which become reactivated. How is this coordinated?
-
8.
Why does EPO/EPO-R axis stimulation differ among tumor cell type populations?
-
9.
How can the positive clinical effects of EPO be maintained, while eliminating the harmful effects of EPO on tumor cells?
-
10.
What is the mechanism by which Epo stimulates DTC reactivation in the niche?
Acknowledgments
This work is directly supported by the National Cancer Institute (CA093900, CA163124, and CA143803), the U.S. Department of Defense (W81XWH-11-1-0636 and W81XWH-15-1-0637), and the Prostate Cancer Foundation. R.S Taichman receives support as the Major McKinley Ash Collegiate Professor.
References
- 1.Fidler I.J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125I-5-iodo-2′-deoxyuridine. J. Natl. Cancer Inst. 1970;45:773–782. [PubMed] [Google Scholar]
- 2.Fidler I.J. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 3.Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;133:571–573. [PubMed] [Google Scholar]
- 4.Sturge J., Caley M.P., Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat. Rev. Clin. Oncol. 2011;8:357–368. doi: 10.1038/nrclinonc.2011.67. [DOI] [PubMed] [Google Scholar]
- 5.Taichman R.S. Prospective identification and skeletal localization of cells capable of multilineage differentiation in vivo. Stem Cells Dev. 2010;19:1557–1570. doi: 10.1089/scd.2009.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whetton A.D., Graham G.J. Homing and mobilization in the stem cell niche. Trends Cell Biol. 1999;9:233–238. doi: 10.1016/s0962-8924(99)01559-7. [DOI] [PubMed] [Google Scholar]
- 7.Shiozawa Y. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J. Clin. Investig. 2011;121:1298. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung Y. Annexin-2 is a regulator of stromal cell-derived factor–1/CXCL12 function in the hematopoietic stem cell endosteal niche. Exp. Hematol. 2011;39:151–166. doi: 10.1016/j.exphem.2010.11.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung Y. Annexin 2–CXCL12 interactions regulate metastatic cell targeting and growth in the bone marrow. Mol. Cancer Res. 2015;13:197–207. doi: 10.1158/1541-7786.MCR-14-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yumoto K. Molecular pathways: niches in metastatic dormancy. Clin. Cancer Res. 2014;20:3384–3389. doi: 10.1158/1078-0432.CCR-13-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaked Y. Tumor dormancy and the angiogenic switch: possible implications of bone marrow-derived cells. Curr. Pharm. Des. 2014;20:4920–4933. doi: 10.2174/1381612819666131125153536. [DOI] [PubMed] [Google Scholar]
- 12.Sosa M.S., Bragado P., Aguirre-Ghiso J.A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer. 2014;14:611–622. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giancotti F.G. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–764. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aguirre-Ghiso J.A., Ossowski L., Rosenbaum S.K. Green fluorescent protein tagging of extracellular signal-regulated kinase and p38 pathways reveals novel dynamics of pathway activation during primary and metastatic growth. Cancer Res. 2004;64:7336–7345. doi: 10.1158/0008-5472.CAN-04-0113. [DOI] [PubMed] [Google Scholar]
- 15.Ghiso J.A.A., Kovalski K., Ossowski L. Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J. Cell. Biol. 1999;147:89–104. doi: 10.1083/jcb.147.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi A. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp. Med. 2011;208:2641–2655. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almog N. Molecular mechanisms underlying tumor dormancy. Cancer Lett. 2010;294:139–146. doi: 10.1016/j.canlet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Cabarcas S.M., Mathews L.A., Farrar W.L. The cancer stem cell niche—there goes the neighborhood? Int. J. Cancer. 2011;129:2315–2327. doi: 10.1002/ijc.26312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiozawa Y. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12:116. doi: 10.1593/neo.91384. IN4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plaks V., Kong N., Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favaro E., Amadori A., Indraccolo S. Cellular interactions in the vascular niche: implications in the regulation of tumor dormancy. Apmis. 2008;116:648–659. doi: 10.1111/j.1600-0463.2008.01025.x. [DOI] [PubMed] [Google Scholar]
- 22.Suva L.J. Bone metastasis: mechanisms and therapeutic opportunities. Nat. Rev. Endocrinol. 2011;7:208–218. doi: 10.1038/nrendo.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sottnik J.L., Dai J., Zhang H., Campbell B., Keller Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res. 2015;75:2151–2158. doi: 10.1158/0008-5472.CAN-14-2493. doi: 10.1158/0008-5472.CAN-14-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph J. Disseminated prostate cancer cells can instruct hematopoietic stem and progenitor cells to regulate bone phenotype. Mol. Cancer Res. 2012;10:282–292. doi: 10.1158/1541-7786.MCR-11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiozawa Y., Taichman R.S. Bone: elucidating which cell erythropoietin targets in bone. Nat. Rev. Endocrinol. 2015;11:263–264. doi: 10.1038/nrendo.2015.32. [DOI] [PubMed] [Google Scholar]
- 26.Broxmeyer H.E. Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration. J. Exp. Med. 2013;210:205–208. doi: 10.1084/jem.20122760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGee S. Effects of erythropoietin on the bone microenvironment. Growth Factors. 2012;30:22–28. doi: 10.3109/08977194.2011.637034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szenajch J. The role of erythropoietin and its receptor in growth, survival and therapeutic response of human tumor cells: from clinic to bench—a critical review. Biochim. Biophys. Acta (BBA) – Rev. Cancer. 2010;1806:82–95. doi: 10.1016/j.bbcan.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Debeljak N., Solár P., Sytkowski A.J. Erythropoietin and cancer: the unintended consequences of anemia correction. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiozawa Y. Erythropoietin supports the survival of prostate cancer, but not growth and bone metastasis. J. Cell. Biochem. 2013;114:2471–2478. doi: 10.1002/jcb.24592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todaro M. Erythropoietin activates cell survival pathways in breast cancer stem–like cells to protect them from chemotherapy. Cancer Res. 2013;73:6393–6400. doi: 10.1158/0008-5472.CAN-13-0248. [DOI] [PubMed] [Google Scholar]
- 32.Quayle L., Ottewell P.D., Holen I. Bone metastasis: molecular mechanisms implicated in tumour cell dormancy in breast and prostate cancer. Curr. Cancer Drug Targets. 2015;15:469–480. doi: 10.2174/1568009615666150506092443. [DOI] [PubMed] [Google Scholar]
- 33.Jung Y. Hematopoietic stem cells regulate mesenchymal stromal cell induction into osteoblasts thereby participating in the formation of the stem cell niche. Stem Cells. 2008;26:2042–2051. doi: 10.1634/stemcells.2008-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun H. Erythropoietin modulates the structure of bone morphogenetic protein 2–engineered cranial bone. Tissue Eng. A. 2012;18:2095–2105. doi: 10.1089/ten.tea.2011.0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucas T.S. Stimulation of systemic bone formation induced by experimental blood loss. Clin. Orthop. Relat. Res. 1997;340:267–275. doi: 10.1097/00003086-199707000-00034. [DOI] [PubMed] [Google Scholar]
- 36.Shiozawa Y. Erythropoietin couples hematopoiesis with bone formation. PLoS One. 2010;5:e10853. doi: 10.1371/journal.pone.0010853. [DOI] [PMC free article] [PubMed] [Google Scholar]