Abstract
Adenosine triphosphate (ATP) is a universal mediator of metabolism and signaling across unicellular and multicellular species. There is a fundamental interdependence between the dynamics of ATP and the physiology that occurs inside and outside the cell. Characterizing and understanding ATP dynamics provides valuable mechanistic insight into processes that range from neurotransmission to the chemotaxis of immune cells. Therefore, we require the methodology to interrogate both temporal and spatial components of ATP dynamics from the subcellular to organismal levels in live specimens. Over the last several decades, a number of molecular probes that are specific for ATP have been developed. These probes have been combined with imaging approaches, particularly optical microscopy, to enable qualitative and quantitative detection of this critical molecule. In this review, we survey current examples of technologies that are available to visualize ATP in living cells and identify areas where new tools and approaches are needed to expand our capabilities.
Keywords: ATP, imaging, energy metabolism, purinergic signaling, fluorescence, sensors, biosensors
INTRODUCTION
Significant efforts have been made over the decades to directly visualize adenosine triphosphate (ATP) in living systems. ATP is a molecule at the center of metabolism and signaling both inside and outside the living cell, and its universal importance in biology reaches well beyond its most familiar role as an energy metabolite.
In energy transduction, ATP hydrolysis provides a thermodynamic driving force for cellular chemistry (Westheimer, 1987; Kamerlin et al., 2013). It remains ambiguous why ATP evolved such a central and universal role (Plattner and Verkhratsky, 2016), but it is clear that energy-dependent reactions are critical for numerous cellular processes from the maintenance of neuronal membrane potential (Magistretti and Allaman, 2015) to organelle transport (Zala et al., 2013) to nucleocytosolic translocation (Dzeja et al., 2002). In fact, hydrolysis of other nucleotides, such as GTP, and other phosphate metabolites can also provide a driving force for specific energy-dependent processes. Despite the energetic contribution of these alternatives, ATP still plays a central role through the action of nucleotide diphosphate kinases and enzymes that create a phosphotransfer network involved in the distribution of bioenergetics (Shugar, 1996; Dzeja and Terzic, 2003).
Beyond energy transduction, ATP plays a central role in signaling. ATP serves as a phosphate-group donor for substrate activation in metabolic reactions and as the coenzyme for a large number of kinases. In the regulation of protein function, ATP can serve as both a phosphate- and an adenylyl-group donor for post-translational modifications. ATP is also required for the biosynthesis of cyclic adenosine monophosphate (cAMP), which is a critical second messenger in signal transduction. Furthermore, ATP itself can serve as a bona fide signaling ligand for ATP-sensitive or purinergic ionotropic and G-protein coupled receptors. With its vast repertoire of biological functions, the spatial and temporal dynamics of ATP broadly affect both intracellular and extracellular processes that determine normal physiology as well as pathological states. To this end, we briefly review examples demonstrating the importance of ATP in brain energy metabolism and inflammation before surveying a number of ATP detection and imaging methods that are currently available.
Intracellular ATP in Metabolism and Signaling: Brain energy metabolism
In the healthy brain, ATP-consuming processes that are involved in electrochemical signaling impose significant energy demands, which is reflected in the high rates of cerebral glucose and oxygen consumption (Harris et al., 2012; Howarth et al., 2012; Magistretti and Allaman, 2015). The dynamics of brain energy metabolism are complex, in part, because ATP production must respond to these energy demands in an activity-dependent manner. For example, neuronal excitability relies on sufficient energy production to maintain neuronal ion gradients and membrane potentials in the face of continuous action potential generation and signaling in the brain. ATP production must also support ancillary processes associated with neurotransmission such as biosynthesis of neurotransmitters, vesicle loading, and axonal transport, to name just a few (Howarth et al., 2012). Many open questions remain concerning the precise mechanisms by which fuel utilization and ATP production are coordinated in the brain, both within cells and between cells. For example, while ATP production is typically viewed as requiring efficient mitochondrial respiration in neurons, aerobic glycolysis occurs under physiological conditions in certain regions of the brain such as the parietal cortex and prefrontal cortex, especially during development (Vaishnavi et al., 2010; Goyal et al., 2014). Observation of regional aerobic glycolysis may be related to the compartmentation of metabolism between neurons and glia. It has been hypothesized that astrocytes support neuronal activity by performing aerobic glycolysis and shuttling lactate to neurons for mitochondrial respiration and ATP generation (Bélanger et al., 2011). Furthermore, the complex morphology of dendritic arbors and elongated axons requires local ATP generation for synaptic function, which may be provided by motile glycolytic machinery or mitochondria (Zala et al., 2013; Rangaraju et al., 2014).
Because of the high numbers and activities of ATP-consuming processes in the brain, bioenergetics fundamentally influence cognition and behavior. As a result, the physiology underlying activity-dependent ATP production is closely linked to and ultimately dictates the utility of methods such as 18F-fluorodeoxyglucose positron emission tomography, magnetic resonance imaging (MRI) of cerebral metabolic rates, and blood oxygen level dependent functional MRI of cerebral blood flow (Raichle and Mintun, 2006). These methods have, in turn, demonstrated the vulnerability of the brain to metabolic insult. Bioenergetic deficits occur in aging, injury, and neurological diseases such as Alzheimer’s and Parkinson’s diseases (Johnson et al., 2012; Surmeier et al., 2012). In brain ischemia and stroke, for example, attenuated cerebral blood flow causes loss of glucose and oxygen availability, intracellular ATP depletion, and collapse of ion gradients within minutes (Dreier and Reiffurth, 2015). Additionally, ischemic injury results in the release of ATP to the pericellular space, initiating an extracellular cascade of events, including purinergic signaling, chemotaxis of immune cells, and inflammation (Pedata et al., 2015).
Extracellular ATP in Metabolism and Signaling: Inflammation
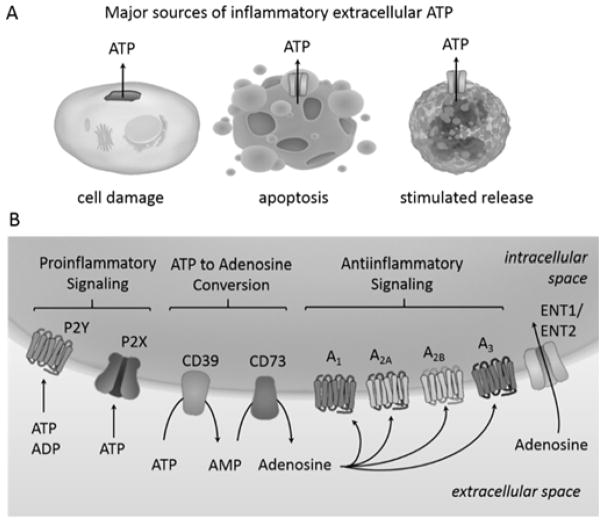
Leakage of intracellular ATP into the pericellular space can result from disruption of the plasma membrane, which may be precipitated by mechanical damage or necrotic cell death. In addition to this unregulated release, cells can promote ATP secretion through the expression of protein channels that connect the cytoplasm to the extracellular space, as has been observed in cells undergoing apoptosis and in activated inflammatory immune cells (Figure 1). In both cases, the rapid ATP egress is driven by the gradient from high intracellular ATP concentration to low extracellular ATP concentration. If extracellular ATP levels become significantly elevated, activation of membrane bound nucleotide receptors on neighboring cells incites a strong proinflammatory response (Idzko et al., 2014). In this context, extracellular ATP acts as a damage associated molecular pattern (DAMP) molecule and is interpreted by immune cells as a danger signal (Eltzschig et al., 2012).
Figure 1.
Extracellular ATP. (A) Mechanical damage of the cell membrane can cause uncontrolled ATP release whereas ATP release from apoptotic or inflammatory cells is mediated by transmembrane channels. (B) Extracellular ATP and ADP act as signaling molecules by binding P2 receptors, which are classified into 2 families based on their structure. P2Y receptors are GPCRs whereas P2X receptors are nucleotide-gated ion channels. Activation of P2Rs is generally proinflammatory. Extracellular ATP and ADP are converted to adenosine by the action of the ectonucleotidases CD39 and CD73. Adenosine signaling can occur through four structurally related GPCRs and generally leads to decreased inflammation. Adenosine is cleared from the extracellular space by the equilibrative nucleoside transporters 1 and 2 (ENT1, ENT2).
The receptors activated by ATP (Figure 1), which are broadly expressed on immune and other cells, are members of the P2 class of receptors for extracellular nucleotides and are classified into two families, P2Y and P2X (Burnstock and Boeynaems, 2014). The P2Y family of receptors are G-protein coupled receptors (GPCRs), and eight members have been identified to date. P2X receptors are ligand-gated ion channels. The P2 receptors vary in their responsiveness to a range of nucleotides, with P2Y receptors being optimally responsive to endogenous nucleotides such as ATP, ADP, UTP, etc. depending on the receptor subtype and species. On the other hand, ATP is the preferred endogenous agonist for all subtypes of P2X receptors.
The P2X7 receptor (P2X7R) is the most studied member from the ionotropic P2X receptor family, and its activation by ATP is an important proinflammatory signal for a number of immune cells, including macrophages, microglia, and dendritic cells (Di Virgilio, 2007). ATP binding to P2X7R causes channel opening and membrane depolarization via Na+ and Ca2+ influx and K+ efflux. P2X7R activates the NLRP3 inflammasome, which is a multiprotein assembly that enables the caspase-1 catalyzed release of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18. In addition, the P2X7 receptor undergoes a conformational change that widens the channel to allow passage of larger molecules, including ATP. However, the mechanism and role of channel widening are debated, and it is not necessary for NLRP3 activation as it has been shown that a decrease in intracellular potassium is necessary and sufficient (Muñoz-Planillo et al., 2013). P2X7R activation and the resulting IL-1β signaling has been shown to be important in bacterial pathogen clearance by macrophages (Coutinho-Silva and Ojcius, 2012) and cancer antigen presentation to lymphocytes by dendritic cells (Ghiringhelli et al., 2009). However, when dysregulated or activated inappropriately this pathway contributes to a number of inflammatory disorders, including inflammatory bowel disease (Kurashima et al., 2012) and asthma (Müller et al., 2011).
From the metabotropic P2Y receptor family, P2Y2R has been shown to be an important mediator of inflammation and active in the recruitment of immune cells to sites of infection and wound healing (Elliott et al., 2009). ATP released from damaged or infected cells that binds P2Y2R on leukocytes acts as a chemoattractant to draw in neutrophils and macrophages, which can then phagocytose the ailing cells and help resolve the inflammation. Interestingly, neutrophils have been shown to use directional, autocrine ATP signaling as a mechanism to amplify their migration response to chemoattractants. Specifically, Chen and coworkers reported that stimulation with the chemoattractant N-formyl-Met-Leu-Phe (FMLP) causes human neutrophils to release ATP that is localized near the stimulus (Chen et al., 2006). The released ATP then binds P2Y2Rs expressed on the neutrophil cell surface and promotes chemotaxis along the FMLP gradient. Neutrophils from mice deficient in P2Y2R showed significantly impaired chemotaxis.
The proinflammatory signaling of extracellular ATP is terminated by nucleotidases active in the interstitial fluid as well as the membrane-bound ectonucleotidases CD39 and CD73 (Figure 1) (Idzko et al., 2014). CD39 catalyzes the conversion of ATP first to ADP and then to AMP, and it can also accept ADP as a substrate for conversion to AMP. Subsequently, CD73 converts AMP to adenosine, and thus a decrease in extracellular ATP results in an increase in extracellular adenosine. Extracellular adenosine can engage P1 receptors, of which there are four subtypes, A1, A2A, A2B, A3. Whereas ATP signaling generally upregulates the immune response, signaling at adenosine receptors is associated with the dampening and resolution of inflammation. Adenosine signaling is terminated by cellular uptake of adenosine through equilibrative nucleoside transporters 1 and 2 (ENT1, ENT2). Following cellular uptake, adenosine is metabolized to either AMP by adenosine kinase or to inosine by adenosine deaminase.
Examples from brain energetics and inflammation illustrate why the “how, when, and where” of ATP production and consumption clearly impact physiology. These examples also highlight the importance of studying the spatial and temporal dynamics of ATP. Because ATP levels can respond rapidly and locally to physiological changes, real time imaging in live specimens is essential for: (1) fully capturing how changes in ATP levels are distributed throughout cells and tissues, and (2) providing experimental access to dissect the mechanisms and machinery responsible for ATP’s roles in metabolism and signaling.
METHODS FOR DETECTION AND IMAGING ATP
A number of well-established and newly developed methods can measure ATP, though there are far fewer methods that can image ATP in live specimens. Chemical and physical methods, ranging from liquid chromatography to mass spectrometry (Khlyntseva et al., 2009), can offer superb specificity. For example, extracellular ATP has been measured using enzyme-coated platinum microelectrodes (Kueng et al. 2004; Llaudet et al. 2005) or optical fibers (Wang et al., 2013). However, these methods typically lack spatiotemporal resolution or lack compatibility with live specimens. Chemical and physical methods that involve de-proteinization to measure intracellular concentrations also release bound forms of adenine nucleotides (Harris et al., 1973). This type of sample preparation results in measurements of total rather than free ATP and ADP concentrations that may skew equilibrium and energy status estimates (Veech et al., 1979; Mörikofer-Zwez and Walter, 1989; Tantama et al., 2013). As an alternative to instrumental analysis, molecular probes that are specific to free ATP offer the opportunity for real-time analysis with greater resolution or compatibility with live specimens or both. Thus, we present examples of both non-imaging and imaging methods for detecting ATP, in order to contrast their advantages.
A number of non-imaging approaches to ATP measurement have been developed. For example, in an electrophysiological approach, the ligand sensitivity of P2X receptors can be exploited because, as discussed, they are ATP-gated cation channels and thus produce ATP-dependent currents. When P2X receptors are expressed in the plasma membrane of PC12 cells (Praetorius and Leipziger, 2009) or HEK-293 cells (Hayashi et al., 2004), patch-clamp electrophysiology in outside-out or whole-cell mode can be used to detect ATP in proximity to the electrode-immobilized membrane or cell. This technique has been used to study ATP release from a variety of cells; for example, Hazama et al. were able to measure ATP concentrations released from pancreatic β cells in real time by measuring P2X current amplitudes via whole-cell patch-clamp (Hazama et al., 1998). Also exploiting an electrophysiological approach, nucleotide-calibrated measurements of ATP-sensitive potassium (KATP) channel currents have been used to calculate submembrane concentrations of ATP and test for local gradients in COSm6 monkey kidney cells and in Xenopus oocytes (Gribble et al., 2000). In a contrasting non-imaging spectroscopic approach, Vancraenenbroeck recently published a sensor based on malonyl-coenzyme A synthetase, an enzyme that undergoes a conformational change upon ATP binding. Two molecules of tetramethylrhodamine are conjugated to the synthetase in a manner such that ATP binding causes a substantial increase in fluorescence intensity, with high selectivity for ATP and micromolar sensitivity (Vancraenenbroeck and Webb, 2015). These and other non-imaging methods provide important means to quantify ATP, and in many cases are ideal for assaying cells or extracts with high sensitivity and high throughput; however, non-imaging methods are unable to provide the same high level of spatial and time-resolved information content achieved with imaging methods.
Several methods to image ATP have been developed over the past many decades (Figure 2) (Table 1). While the ideal approach would be quantitative and compatible with non-invasive, probe-free visualization of ATP in live specimens with high spatial and temporal resolution, a single method satisfying these criteria does not yet exist. Magnetic resonance spectroscopy techniques that exploit phosphorous magnetization transfer can non-invasively quantify ATP in vivo, but practical considerations, such as long acquisition times per voxel and the limited availability of the instrumentation to many biologists, have limited its applicability to imaging (Du et al., 2008; Chaumeil et al., 2009; Befroy et al., 2012). Instead, methods to visualize ATP primarily rely on optical microscopy paired with molecular probes, which provide signal readouts that are specific for ATP. These molecular probes have been developed using a variety of physical formats from small organic indicators to nanoparticles, and they exploit both indirect and direct ATP detection mechanisms.
Figure 2.
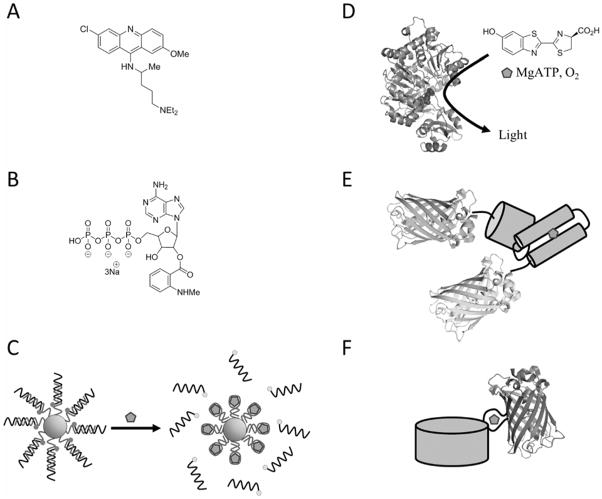
Tools for ATP Visualization. (A) Quinacrine is a fluorescent dye that can stain vesicular ATP. (B) Mant-ATP is a fluorescent analogue. (C) Nanoflare using an ATP aptamer attached to a gold nanoparticle. In the absence of ATP, a competitor strand that is conjugated to a fluorescent dye is bound to the aptamer, but fluorescence is quenched by proximity to the gold. When ATP binds, the competitor strand is released and fluorescence is de-quenched. (D) Luciferase chemiluminescence requires a luciferin substrate and ATP. (E) The ATeam sensors use the F1F0 ATP synthase ε subunit as an ATP binding domain attached to a FRET pair of fluorescent proteins. (F) The Perceval sensors use the PII family protein GlnK as an ATP binding domain attached to a circularly permuted fluorescent protein. For (A) – (D) the sensor or a substrate must be exogenously supplied and loaded into cells or tissue. For (E) – (F) the sensors are completely genetically-encoded.
Table 1.
Examples of tools for adenosine triphosphate (ATP) visualization.
| Technology | Detection Mechanisms | Imaging Parameters |
|---|---|---|
| Quinacrinea | Fluorescent dye that binds peptide-bound ATP found in intracellular granules; intensiometric* | Fluorescence λex = 420–488 nm; λem = 490–510 nm |
| Mant-ATPb | Fluorescent ATP analogue that can be used as a tracer of ATP pools; intensiometric | Fluorescence λex = 356 nm; λem = 428–448 nm |
| ATP Aptamer Nanoflarec | ATP binding to DNA aptamers bound to a gold nanoparticles causes release of Cy5 conjugated “reporter” strand whose fluorescence “turns-on” upon release; intensiometric | Fluorescence (Dye Dependent) Cy5: λex = 649 nm; λem = 666 nm |
| Luciferased | Chemiluminescent enzyme metabolizes its substrate luciferin using ATP, resulting in ATP-dependent luminescence; intensity-based signal | Chemiluminescence (substrate dependent) D-luciferin: λem = 560 nm |
| Syn-ATPe | Luciferase-mCherry bioluminescence resonance energy transfer (BRET) sensor targeted to neuronal synapses; both luminescence and red fluorescence; ratiometric | Chemiluminescence D-luciferin: λem = 560 nm Fluorescence λex = 587 nm; λem = 610 nm |
| ATeamf | ATP binding causes an increase in Förster resonance energy transfer (FRET) between a CFP and a YFP; ratiometric | CFP Donor Fluorescence λex = 435 nm; λem = 475 nm YFP Acceptor Fluorescence λex = 515 nm; λem = 527 nm FRET λex = 435 nm; λem = 527 nm |
| QUEENg | ATP binding causes a change in the excitation spectrum of a circularly-permuted green fluorescent protein (cpEGFP); ratiometric | A-Band Fluorescence λex = 400 nm; λem = 513 nm B-Band Fluorescence λex = 494 nm; λem = 513 nm |
| PercevalHRh | ATP binding causes a change in the excitation spectrum of a circularly-permuted yellow fluorescent protein (cpVenus); ratiometric | A-Band Fluorescence λex = 420 nm; λem = 515 nm B-Band Fluorescence λex = 500 nm; λem = 515 nm |
Intensiometric sensors exhibit a change in intensity without a drastic change in spectrum: for luminescence a single emission wavelength is monitored, and for fluorescence, a single excitation (λex) wavelength and emission wavelength (λem) pair is monitored. Intensiometric signals depend not only on ATP but also can be affected by dye concentration, expression level, and photobleaching. Ratiometric signals obtain two readouts that are divided to provide a normalized response. Ratiometric signals are advantageous because they normalize for dye concentration and expression level and reduce signal drift from photobleaching. A-Band fluorescence: excitation of the protonated chromophore form of the fluorescent protein. B-Band fluorescence: excitation of the deprotonated chromophore form of the fluorescent protein; CFP, cyan fluorescent protein; Cy5, cyanine dye; YFP, yellow fluorescent protein.
Magnesium Green, for example, is a magnesium-sensitive small organic fluorophore that can be used to indirectly detect ATP hydrolysis (Leyssens et al., 1996). The majority of intracellular ATP is complexed with divalent magnesium ions; however, ADP has a lower affinity for magnesium ions relative to ATP. Thus, hydrolysis of MgATP causes an increase in free magnesium ion concentration and subsequent increase in Magnesium Green fluorescence. By invoking binding equilibrium, Magnesium Green has been used in non-imaging studies to determine the ATP-ADP exchange rate through the mitochondrial adenine nucleotide transporter in isolated mitochondria (Chinopoulos et al., 2009) and permeabilized cells (Kawamata et al., 2010). In an imaging study exploiting fluorescence confocal microscopy with isolated hair cells loaded with Magnesium Green, Shin et al. indirectly visualized a higher contribution of creatine kinase activity to ATP generation in hair bundles versus their soma (Shin et al., 2007). While sensitive to ATP hydrolysis, Magnesium Green has the disadvantage that it is not perfectly specific, as it has moderate affinity for calcium. On the other hand, Magnesium Green is advantageous over other magnesium indicators because its fluorescence can be excited with illumination in visible range, reducing phototoxicity compared to indicators such as Mag-Fura-2 and Mag-Indo-1, which require ultraviolet (UV) excitation. However, Magnesium Green shows a simple increase in fluorescence intensity upon binding magnesium ions without a ratiometric shift in excitation or emission peaks. Thus, unlike ratiometric probes, the Magnesium Green signal is also dependent on dye concentration, making it challenging to use for quantitative studies because loading efficiency and photobleaching can cause variations in signal unrelated to changes in ATP hydrolysis (Leyssens et al., 1996).
In order to directly visualize ATP pools, fluorescent analogues can be used as tracers once loaded into the tissue, cell, or organelle of interest. Analogues can be synthesized by conjugating fluorescent groups such as a methylanthraniloyl (mant) or a coumarin (deac) to the nucleobase, ribose or phosphate groups of ATP. The analogues mant-ATP and deac-ATP have been extensively used to study kinase and ATPase activities in solution (Fili and Toseland, 2014). For example, mant-ATP has been used to monitor vesicular ATP release from dopaminergic neurons (Ho et al., 2015), and deac-ATP has been used to study the kinetics of myosin Va movement on actin using total internal reflection fluorescence (TIRF) microscopy (Sakamoto et al., 2008). Analogues of ATP modified with Cy3 or BODIPY fluorescent dyes are also available, which shift excitation bands into the visible range to reduce phototoxicity.
Additional synthetic ATP analogs have been developed to detect ATP hydrolysis. These analogs exploit Förster-type resonance energy transfer (FRET) between a donor fluorophore covalently attached to a γ-phosphate group and an acceptor fluorophore linked to the base or ribose (Hardt et al., 2013). Cleavage of the phosphodiester bond allows the donor and acceptor to diffuse away from one another, causing a drastic loss of FRET and an increase in donor fluorescence. Hacker et al. have used such an analog to monitor ATP consumption in real time during ubiquitin activation by UBA1, a human E1 enzyme (Hacker et al., 2013). Similarly, a FRET-based ATP analogue with the organic fluorophore Sulfo-Cy3 dye linked to the γ-phosphate and a quencher linked to the ribose C2 position was used by Gutiérrez Acosta et al. to study acetone degradation in D. biacutus cell extract (Gutiérrez Acosta et al., 2014). While these FRET-based ATP analogues have not yet been used for imaging, in principle they could be used similarly to mant-ATP. It is important to recognize with these ATP analogues that the fluorescent dye group can be of equivalent or greater mass than ATP itself. Therefore, the covalent modification could change the behavior of the analogue in unpredictable ways, and functional assays are critical to validate the use of such analogues.
Small molecule sensors for ATP as an analyte have also been reported. For example, quinacrine is a fluorescent acridine derivative that stains peptide-bound ATP found in high concentrations in intracellular granules (Irvin and Irvin, 1954; Bodin and Burnstock, 2001). In this manner, quinacrine has been used to image vesicular ATP release in endothelial and epithelial cells (Bodin and Burnstock, 2001; Feranchak et al., 2010; Akopova et al., 2012). Alternatively, Pak et al. developed an imidazolium based ratiometric sensor for ATP with a pyrene excimer clamp. When ATP binds to this sensor, it forms a pyrene-adenine-pyrene sandwich by π-π stacking. Formation of the complex results in an increase in pyrene emission at 375 nm and a decrease in emission at 487 nm. This pyrene-based sensor was used in HeLa cells to monitor the decrease in ATP levels upon addition of the ATP synthase inhibitor oligomycin and upon the hydrolysis of ATP to ADP by apyrase (Pak et al., 2015).
Aptamers are single-stranded DNA or RNA oligonucleotides which can be easily modified to attain high-affinity and specificity for their targets. Aptamers are extensively used to study small molecule metabolites (Paige et al., 2012; Feng et al., 2014) and are analyzed by variety of methods such as fluorescence spectroscopy (Sun et al., 2010; Park et al., 2015b; Wang et al., 2015; Song et al., 2016) electrochemistry (Mukherjee et al., 2015; Zhao et al., 2015), surface plasmon resonance (Park et al., 2015a) and colorimetry (Huo et al., 2016). Aptamers can be engineered to detect ATP in nanomolar to millimolar ranges; however, cell permeability and degradation issues limit their use in live-cell imaging (Wang et al., 2014). To overcome this problem, nanoparticles have been used to deliver and protect aptamers from degradation by DNases and RNases in cells while modifying their fluorescent properties. For example, Qiang et al. reported a polydopamine nanosphere-linked aptamer hybrid that protects the aptamer and quenches its fluorescence (Qiang et al., 2015). Addition of ATP releases the aptamer resulting in an increase in the fluorescence. The aptamer is highly selective and sensitive, detecting ATP in 0.01–2mM range. Changes in ATP concentration could be measured in HeLa cells upon treatment with oligomycin or Ca2+. Similarly, nanoparticles have been used to construct nanoflares (Zheng et al., 2009) to image ATP. Aptamers bound to gold nanoparticles are hybridized with fluorescent DNA strands that are quenched by proximity to the nanoparticle. Binding of ATP to the aptamers releases the fluorescent strands, and the increase in fluorescence can be used to quantitate ATP in live cells (Zheng et al., 2009; Torabi and Lu, 2014). One drawback of these biosensors is that the aptamers have been engineered for adenine selectivity, which can make it challenging to distinguish between adenine derivatives (Ozalp et al., 2010). To overcome this drawback, Sassanfar et al. synthesized RNA aptamers specific for ATP (Sassanfar and Szostak, 1993), and Ozalp et al. (2010) developed DNA aptamers selective for ATP (apparent KD: 3.2 mmol l−1) and ADP (apparent KD: 4.4 mmol l−1).
While both small organic indicators and aptamer-based biosensors have found utility in imaging studies, they pose a challenge to sample preparation because they require cell penetration or cell loading of the exogenous reagent. In simple model systems, such as monolayer cell cultures, introduction of these ATP imaging reagents can typically be achieved by sustained incubations, electroporation, or microinjection. However, these preparatory requirements limit their application in many cell types and complex or thick tissues. In contrast to these technologies, genetically-encoded indicators are protein-based reagents that are in part or wholly encoded by an appropriate gene sequence. Thus, genetically-encoded imaging reagents offer the advantage of being compatible with a wide variety of specimens when the appropriate gene transfer or expression method is available, such as transfection reagents, viral transduction, and tissue-specific expression in transgenic mice. Recent examples of genetically-encoded ATP imaging reagents have utilized luciferases and fluorescent proteins.
Luciferases are used to produce bioluminescence in order to visualize cellular activities as well as track cell populations in vivo. Firefly luciferase and its exogenously-supplied substrate luciferin can be used to measure ATP because the chemiluminescent reaction is ATP dependent. Adenylation of luciferin by luciferase activates the substrate for conversion to oxyluciferin, and thus the resulting luminescence is proportional to ATP concentration (Manfredi et al., 2002; Lundin, 2014). Currently, commercial and academic efforts have produced a variety of luciferases that have been engineered and codon-optimized from different species for bioluminescence imaging in live samples (Thome et al., 2010; Hall et al., 2012), but the ATP-dependent firefly and beetle luciferases are most commonly used to measure ATP (Manfredi et al., 2002; Lundin, 2014). Brachini et al. have recently developed a luciferase with higher activity and quantum yield by fusing the N-terminal domain of the Photinus pyralis luciferase joined to the C-terminal domain of the Luciola italica luciferase. The chimeric luciferase was further optimized to show 3-fold higher sensitivity in live cells compared to the commercial Luc2 variant (Branchini et al., 2015). Mutagenesis has also produced luciferase variants with red-shifted luminescence (Branchini et al., 2010). While these emission variants typically exhibit lower luminescence yields, they offer the advantage that red luminescence suffers less tissue absorbance, making them good reporters for in vivo imaging (Liang et al., 2012). There are also important caveats to the quantitation of ATP by luciferase. For example, optimization is critical because of the inherent dependence on oxygen concentration and the often underappreciated complication of product inhibition as well as inhibition by cellular factors or pharmacological agents (Leitão and Esteves da Silva, 2010; Lundin, 2014).
Despite difficulties with absolute quantitation, luciferase has been used monitor ATP changes in variety of cells such as HEK-293 cells, cardiomyocytes and neurons (Brovko, 2010). Bell et al. (2007) were able to use wild-type luciferase inside the cell to monitor differences in intracellular ATP responses in mitochondria and the cytosol of cardiomyocytes after stimulation. Free cytosolic and membrane-tethered luciferase have also been used to study the possibility of compartmentalization of submembrane ATP and control of KATP channel activity in pancreatic β-cells and hypothalamic neurons (Kennedy et al., 1999; Ainscow et al., 2002). In detection of intracellular ATP, luciferase is introduced into mammalian cells by gene transfection, microinjection or viral vectors, though Lee et al. developed luciferase fused to a protein transduction domain to facilitate direct transport of the luciferase protein into the cell (Lee et al., 2012). Even with genetic encoding, however, the exogenous luciferin substrate must be supplied and must penetrate tissues and cells. While this has proven achievable in live cells and animals, there is still the possibility of variable substrate access and cell-type specific toxicity which must be taken into account (Rangaraju et al., 2014). In the detection of extracellular ATP, luciferase has also been used to study ATP release by tethering the enzyme to the extracellular face of the plasma membrane via conjugation to primary IgG antibodies that can bind to surface antigens, via streptavidin-biotin tags, and via glycophosphatidylinositol (GPI) lipid anchors (pmeLuc) (Praetorius and Leipziger, 2009). For example, HEK-293 cells stably transfected with pmeLuc could detect micromolar levels of ATP in the tumor microenvironment while extracellular ATP was undetectable in healthy tissues (Pellegatti et al., 2008). The luciferase-luciferin system can offer a low-background, low-toxicity method to monitor ATP; however, low luminescence limits its applicability in real time ATP imaging. Bioluminescence imaging typically requires long exposure times that limit spatiotemporal resolution, and in some applications it can require specialized equipment (Bell et al., 2007; Brovko, 2010). For example, Furuya et al. were able to improve the temporal resolution to 100-ms by using a cooled electron multiplying charge coupled device (EMCCD) camera coupled with an image intensifier to compensate for low photon emission rates (Furuya et al., 2014). They used it along with low-magnification, high numerical aperture objectives to monitor ATP release from a single cell with 10 nM detection sensitivity. Furthermore, interference from the sample matrix can occur due to inhibitory levels of various anions and salts, ion channel inhibitors, and P2 receptor antagonists (Praetorius and Leipziger, 2009).
An alternative to luciferase-based detection is the use of fluorescent protein-based ATP sensors. Fluorescent protein-based sensors do not require addition of an exogenous luciferin substrate, and they are convenient due to ease of manipulation at the DNA level and subsequent ease of expression in cells and in vivo. For example, as an alternative to the electrophysiological approach (Gribble et al., 2000), ATP level changes in HEK-293 cells have been visualized by imaging the ATP-dependent conformational change of KATP channels fused to an ECFP-EYFP cyan and yellow fluorescent protein FRET pair (Tsuboi et al., 2004). Imamura et al. developed a family of sensors named the ATeams that are also based on FRET between a donor fluorescent protein and acceptor fluorescent protein. In the original ATeams the ε subunit from the F0F1-ATP synthase is linked to the CFP and YFP FRET pair (Imamura et al., 2009). The ε subunit is a 14 kDa protein subunit, composed of a N-terminal β-barrel and 2 C-terminal helices. It undergoes a large conformational change upon binding of ATP, which is the mechanism by which the ε subunit regulates F0F1 ATPase activity based on intracellular ATP levels. The ATeam sensors exhibit apparent dissociation constants ranging from 7.4 μM to 3.3 mM, with at least 10- to 100-fold greater selectivity for ATP over ADP, and response kinetics on the timescale of seconds.
The ATeam sensors have been used to study ATP changes in bacterial cells, neurons, and a number of different cell types in different species (Imamura et al., 2009; Toloe et al., 2014). In unicellular organisms, Maglica et al. (2015) used ATeam sensors to monitor antibiotic induced cell death by single cell tracking of ATP levels in Mycobacterium smegmatis, demonstrating its potential as an important technology in drug discovery for antibiotic screening and mechanism of action imaging assays. In multicellular organisms, Ozawa et al. (2015) found that glycolysis dependent ATP production was necessary for lamellipodia formation in podocytes. In contrast, the cortical subcellular region of podocytes produces ATP by both glycolysis and oxidative phosphorylation in mitochondria. These metabolic studies demonstrate how the ATeam sensors can provide important insight into podocyte cell biology, potentially advancing our understanding of the role of metabolic dysfunction in chronic kidney diseases (Ozawa et al., 2015).
When imaging fluorescent ATP sensors that excite and emit in the 400 nm to 500 nm spectral range, it is important to consider metabolism-dependent changes in autofluorescence background. For example, autofluorescence of flavins such as flavin adenine dinucleotide (FAD) have been long used to visualize metabolic changes in live samples and continues to be used as a “label-free imaging” option (Quinn et al., 2013; Jahn et al., 2015). This background could convolute fluorescent signals when using sensors at low expression levels, but at moderate sensor expression this is less likely because the lower extinction coefficient (~10,000 M−1·cm−1) and fluorescence quantum yield (< 0.01 to 0.06) of FAD (Valle et al., 2012). In order to mitigate problems with spectral overlap with FAD autofluorescence and to generate spectral diversity for multi-sensor imaging, Nakano et al. (2011) replaced the CFP and YFP in the original ATeam sensors with a different FRET pair. They used GFP (cp173-mEGFP) and OFP (mKOκ) to generate a red shifted ATeam named GO-ATeam. This allowed them to image Ca2+ level changes, using Ca2+ sensors that are excited with UV illumination, simultaneously along with ATP levels in HeLa cells. GO-ATeam is also more stable to acidification, an important advantage because metabolic stress can cause a drop in intracellular pH (Nakano et al., 2011). Subsequently, Rueda et al. used GO-ATeam to image ATP depletion in presence and absence of Ca2+ upon NMDA exposure in neurons (Rueda et al., 2015).
Zadran et al. developed an ATP sensor using a particular type of fluorescent protein FRET pair that can exhibit enhanced acceptor fluorescence. In their approach, a mutated variant of the ε subunit of B. subtilis F0F1-ATP synthase is coupled to GFP and YFP. Both GFP and YFP are excited at the same wavelength, and ATP binding results in enhanced YFP acceptor fluorescence signal as a result of increased FRET. Zadran’s sensor was shown to detect as low as 10 nM ATP with high specificity (Zadran et al., 2013), and subsequently, this sensor was used to monitor ATP flux in tumors and during transition to metastatic behavior (Zadran et al., 2014).
Yaginuma et al. recently developed the QUEEN family of sensors, which are related to the ATeam sensors, but with a different architecture that uses a circularly permutated fluorescent protein (cpFP). In the QUEEN architecture, a cpFP is inserted between 2 α helices of the F0F1 ATP synthase ε subunit with linkers. Two variants were designed with apparent affinities of 7 μM and 2 mM, called QUEEN-7mu and QUEEN-2m, respectively (Yaginuma et al., 2014). Using these sensors, they were able to quantify ATP distribution in individual E. coli cells.
Using a circular permutation strategy, Tantama et al. (2013) reported an ATP-to-ADP ratio sensor, PercevalHR, which is an improved version of the original Perceval (Berg et al., 2009). The PII family protein GlnK1, which can bind MgATP and ADP with high affinity, was modified with a cpFP insertion within a loop at the nucleotide binding site. MgATP binding causes a conformational change that alters the chromophore environment of the cpFP. As a result of the protein engineering, the sensor has two peaks in its excitation spectra at approximately 420 nm and 500 nm. MgATP binding increases the fluorescence intensity with 500 nm excitation while ADP binding increases the fluorescence intensity with 420 nm excitation. These nucleotide-dependent spectral features enable excitation ratiometric imaging, which offers the significant advantage of concentration independence that normalize the signal for protein expression and reduces artifacts from photobleaching. The fast association and dissociation rates as well as the high dynamic range of the sensor at physiologically relevant ATP-to-ADP ratio ranges make PercevalHR useful for studying energy metabolism in cells (Tantama et al., 2013). Although the Perceval sensors exhibit some pH sensitivity, the ATP response can be deconvoluted from changes in sample pH using a pH sensor and experimental calibration (Tantama et al., 2011; Tantama and Yellen, 2014). Using Perceval to measure ATP-to-ADP ratios in neurons, Zala et al. showed that mitochondrial trafficking is dependent on mitochondrial ATP, but not glycolysis (Zala et al., 2013). Using PercevalHR, Rueda et al. (2015) studied NMDA stimulation-dependent decreases in ATP-to-ADP ratio in neurons deficient in the SCaMC-3 mitochondrial calcium-dependent MgATP-phosphate exchanger.
Finally, luciferase and fluorescent protein technologies have been combined in ATP sensors that exploit bioluminescence resonance energy transfer (BRET). These BRET sensors have been developed to improve brightness and red-shifted, luciferase-based imaging (Chu et al., 2016). Saito et al. developed an ATP sensor, Nano-lantern (ATP1) in which a split Renilla luciferase (Rluc8) is modified with the ε subunit of the F0F1-ATP synthase and Venus (Saito et al., 2012). ATP binding results in complementation and reconstitution of the active Rluc8, which can efficiently transfer energy to Venus. It has an apparent ATP affinity of 0.3 mmol l−1 and is useful for imaging in tissues with high autofluorescence, and conveniently, Rluc8 does not consume any ATP in its chemiluminescent reaction, simplifying the detection mechanism and interpretation. Using this sensor, Saito et al. (2012) were able to visualize ATP increases in the mesophyll of leaf cells after light irradiation. Borghei et al. used firefly luciferase to develop a red shifted ATP sensor. They generated a fusion protein with firefly luciferase (X5) and the fluorescent protein, mCherry. Although the low quantum yield of mCherry (0.22) results in a weak signal, they could detect an ATP dependent increase in emission at 600 nm by mCherry (Borghei and Hall, 2014). Presumably, replacement of mCherry with a brighter red fluorescent protein would improve signal strength. Importantly, Rangaraju et al. used a synaptically-targeted engineered luciferase-mCherry sensor (Syn-ATP) to achieve ratiometric measurements of activity-dependent ATP consumption and production, although the Syn-ATP ratiometric signal was not obtained through BRET (Rangaraju et al., 2014).
Currently, there are a wide variety of ATP detection and imaging reagents that can be used to visualize both intracellular energy metabolism and extracellular purinergic signaling. The choice of technologies depends on the biological process under study, the specimen format, and any limitation of the imaging instrumentation. While we have presented several examples, it is beyond the scope of this review to evaluate all detection and imaging technologies for ATP here, and other examples have been reviewed elsewhere (for example see Praetorius and Leipziger, 2009; Khlynsteva et al., 2009).
DISCUSSION AND CONCLUSIONS
While a number of ATP imaging technologies currently exist, there are still gaps in our ability to fully probe ATP metabolism and signaling. Quantitative imaging could be improved by engineering sensors with (1) a variety of ATP affinity ranges, (2) faster ATP binding and response kinetics, (3) higher brightness and contrast ratios, (4) selectivity for ATP hydrolysis products, and (5) spectral color variation. Engineering sensors with different affinity ranges is important because ATP concentrations vary widely from nanomolar extracellular levels in some tissues to millimolar concentrations in cytosolic pools. Likewise, engineering sensors with faster kinetics is important to capture ATP concentration changes that may occur on the timescale of milliseconds, such as during the initial phases of vesicular ATP release. Improving the brightness of fluorescent sensors and the contrast ratio between unbound and ATP-bound states will improve signal-to-noise ratios, improve quantitation, and can improve spatial and temporal resolution in practice. Engineering sensors with selectivity for ATP hydrolysis products would expand the toolbox to include ADP, AMP, and adenosine as measurable analytes. Similarly, engineering sensors with spectral color variation will enable simultaneous imaging of multiple sensors, providing a means to directly correlate ATP with, for example Ca2+ signaling and kinase activities. Finally, engineering spectral variants with luminescence and fluorescence in the farred and infrared spectral ranges will ultimately enable ATP imaging to be achieved with specificity and subcellular resolution over subsecond to lifetime timescales in live animals.
With current technologies and future improvements, there is great potential to study ATP with a systems biology perspective of energy metabolism and purinergic signaling. Continued use of these methods to image activity-dependent bioenergetics will improve our mechanistic understanding of neurotransmission (Tantama et al., 2013; Rangaraju et al., 2014). Furthermore, bioenergetic deficits have been linked to a number of aging-related neurodegenerative diseases such as Huntington’s and Parkinson’s diseases, and use of imaging approaches will aid the study of neurodegenerative mechanisms (Surmeier et al., 2012; Zala et al., 2013; Rangaraju et al., 2014). Across cell types, these technologies can probe metabolic and mitochondrial function at the single-cell level, which is becoming important for understanding diseases such as cancer in which links to metabolism are increasingly being found (Mayers and Vander Heiden, 2015). Ultimately, in both healthy and disease state biology, live-cell imaging is becoming an integral approach, and our growing ability to quantitatively visualize ATP dynamics will allow us to link phenomenology and mechanism.
Acknowledgments
M.T. acknowledges support from the Showalter Foundation, the Purdue Research Foundation, and by National Institutes of Health grants NS092010 and EY026425.
ABBREVIATIONS
- BRET
bioluminescence resonance energy transfer
- cpFP
circularly-permuted fluorescent protein
- Cy
cyanine
- DAMP
damage associated molecular pattern
- deac
diethylaminocoumarin
- EMCCD
electron multiplying charge coupled device
- ENT
equilibrative nucleoside transporter
- FMLP
N-formyl-Met-Leu-Phe peptide
- FRET
Förster-type resonance energy transfer
- mant
(2′/3′-O-(N-Methylanthraniloyl)
- NMDA
N-methyl-D-aspartate
- TIRF
total internal reflection fluorescence
LITERATURE CITED
- Ainscow EK, Mirshamsi S, Tang T, Ashford MLJ, Rutter GA. Dynamic imaging of free cytosolic ATP concentration during fuela sensing by rat hypothalamic neurones: evidence for ATP-independent control of ATP-sensitive K+ channels. J Physiol. 2002;544:429–445. doi: 10.1113/jphysiol.2002.022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopova I, Tatur S, Grygorczyk M, Luchowski R, Gryczynski I, Gryczynski Z, Borejdo J, Grygorczyk R. Imaging exocytosis of ATP-containing vesicles with TIRF microscopy in lung epithelial A549 cells. Purinergic Signal. 2012;8:59–70. doi: 10.1007/s11302-011-9259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Befroy DE, Rothman DL, Petersen KF, Shulman GI. 31P-magnetization transfer magnetic resonance spectroscopy measurements of in vivo metabolism. Diabetes. 2012;61:2669–2678. doi: 10.2337/db12-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Bell CJ, Manfredi G, Griffiths EJ, Rutter GA. Luciferase expression for ATP imaging: application to cardiac myocytes. Methods Cell Biol. 2007;80:341–352. doi: 10.1016/S0091-679X(06)80017-8. [DOI] [PubMed] [Google Scholar]
- Berg J, Hung YP, Yellen G. A genetically encoded fluorescent reporter of ATP:ADP ratio. Nat Methods. 2009;6:161–166. doi: 10.1038/nmeth.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol. 2001;38:900–908. doi: 10.1097/00005344-200112000-00012. [DOI] [PubMed] [Google Scholar]
- Borghei G, Hall EA. BRET-linked ATP assay with luciferase. Analyst. 2014;139:4185–4192. doi: 10.1039/c4an00436a. [DOI] [PubMed] [Google Scholar]
- Branchini BR, Ablamsky DM, Davis AL, Southworth TL, Butler B, Fan F, Jathoul AP, Pule MA. Red-emitting luciferases for bioluminescence reporter and imaging applications. Anal Biochem. 2010;396:290–297. doi: 10.1016/j.ab.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Branchini BR, Southworth TL, Fontaine DM, Kohrt D, Talukder M, Michelini E, Cevenini L, Roda A, Grossel MJ. An enhanced chimeric firefly luciferase-inspired enzyme for ATP detection and bioluminescence reporter and imaging applications. Anal Biochem. 2015;484:148–153. doi: 10.1016/j.ab.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovko L. Bioluminescence and Fluorescence for In Vivo Imaging. SPIE; 2010. Bioluminescence and Fluorescence Imaging for In Vivo Real-Time Monitoring of Key Metabolites and the Intracellular Environment; pp. 87–111. [Google Scholar]
- Burnstock G, Boeynaems J. Purinergic signalling and immune cells. Purinergic Signal. 2014;10:529–564. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumeil MM, Valette J, Guillermier M, Brouillet E, Boumezbeur F, Herard AS, Bloch G, Hantraye P, Lebon V. Multimodal neuroimaging provides a highly consistent picture of energy metabolism, validating 31P MRS for measuring brain ATP synthesis. Proc Natl Acad Sci U S A. 2009;106:3988–3993. doi: 10.1073/pnas.0806516106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Vajga S, Csanády L, Mándi M, Mathe K, Adam-Vizi V. A novel kinetic assay of mitochondrial AT-ADP exchange rate mediated by the ANT. Biophys J. 2009;96:2490–2504. doi: 10.1016/j.bpj.2008.12.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu J, Oh Y, Sens A, Ataie N, Dana H, Macklin JJ, Laviv T, Welf ES, Dean KM, Zhang F, Kim BB, Tang CT, Hu M, Baird MA, Davidson MW, Kay MA, Fiolka R, Yasuda R, Kim DS, Ng HL, Lin MZ. A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo. Nat Biotechnol. 2016;34:760–767. doi: 10.1038/nbt.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho-Silva R, Ojcius DM. Role of extracellular nucleotides in the immune response against intracellular bacteria and protozoan parasites. Microbes Infect. 2012;14:1271–1277. doi: 10.1016/j.micinf.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Reiffurth C. The stroke-migraine depolarization continuum. Neuron. 2015;86:902–922. doi: 10.1016/j.neuron.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, Chen W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc Natl Acad Sci U S A. 2008;105:6409–6414. doi: 10.1073/pnas.0710766105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzeja PP, Bortolon R, Perez-Terzic C, Holmuhamedov EL, Terzic A. Energetic communication between mitochondria and nucleus directed by catalyzed phosphotransfer. Proc Natl Acad Sci USA. 2002;99:10156–10161. doi: 10.1073/pnas.152259999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A. Phosphotransfer networks and cellular energetics. J Exp Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig H, Sitkovsky M, Robson S. Purinergic Signaling during Inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Dai S, Wang L. Optical aptasensors for quantitative detection of small biomolecules: a review. Biosens Bioelectron. 2014;59:64–74. doi: 10.1016/j.bios.2014.03.014. [DOI] [PubMed] [Google Scholar]
- Feranchak AP, Lewis MA, Kresge C, Sathe M, Bugde A, Luby-Phelps K, Antich PP, Fitz JG. Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium. J Biol Chem. 2010;285:8138–8147. doi: 10.1074/jbc.M109.065482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fili N, Toseland CP. Fluorescence and Labelling: How to Choose and What to Do. In: Toseland CP, Fili N, editors. Fluorescent Methods for Molecular Motors. Springer; Basel: 2014. pp. 1–24. [DOI] [PubMed] [Google Scholar]
- Furuya K, Sokabe M, Grygorczyk R. Real-time luminescence imaging of cellular ATP release. Methods. 2014;66:330–344. doi: 10.1016/j.ymeth.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Génin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, André F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME. Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab. 2014;19:49–57. doi: 10.1016/j.cmet.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble FM, Loussouarn G, Tucker SJ, Zhao C, Nichols CG, Ashcroft FM. A novel method for measurement of submembrane ATP concentration. J Biol Chem. 2000;275:30046–30049. doi: 10.1074/jbc.M001010200. [DOI] [PubMed] [Google Scholar]
- Gutiérrez Acosta OB, Hardt N, Hacker SM, Strittmatter T, Schink B, Marx A. Thiamine pyrophosphate stimulates acetone activation by Desulfococcus biacutus as monitored by a fluorogenic ATP analogue. ACS Chem Biol. 2014;9:1263–1266. doi: 10.1021/cb500152y. [DOI] [PubMed] [Google Scholar]
- Hacker SM, Pagliarini D, Tischer T, Hardt N, Schneider D, Mex M, Mayer TU, Scheffner M, Marx A. Fluorogenic ATP analogues for online monitoring of ATP consumption: observing ubiquitin activation in real time. Angew Chem Int Ed Engl. 2013;52:11916–11919. doi: 10.1002/anie.201304723. [DOI] [PubMed] [Google Scholar]
- Hall MP, Unch J, Binkowski BF, Valley MP, Butler BL, Wood MG, Otto P, Zimmerman K, Vidugiris G, Machleidt T, Robers MB, Benink HA, Eggers CT, Slater MR, Meisenheimer PL, Klaubert DH, Fan F, Encell LP, Wood KV. Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem Biol. 2012;7:1848–1857. doi: 10.1021/cb3002478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt N, Hacker SM, Marx A. Synthesis and fluorescence characteristics of ATP-based FRET probes. Org Biomol Chem. 2013;11:8298–8305. doi: 10.1039/c3ob41751d. [DOI] [PubMed] [Google Scholar]
- Harris DA, Rosing J, Van de Stadt RJ, Slater EC. Tight binding of adenine nucleotides to beef-heart mitochondrial ATPase. Biochim Biophys Acta. 1973;314:149–153. doi: 10.1016/0005-2728(73)90130-8. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Hazama A, Dutta AK, Sabirov RZ, Okada Y. Detecting ATP release by a biosensor method. Sci STKE. 2004;2004:pl14. doi: 10.1126/stke.2582004pl14. [DOI] [PubMed] [Google Scholar]
- Hazama A, Hayashi S, Okada Y. Cell surface measurements of ATP release from single pancreatic beta cells using a novel biosensor technique. Pflugers Arch. 1998;437:31–35. doi: 10.1007/s004240050742. [DOI] [PubMed] [Google Scholar]
- Ho T, Jobling AI, Greferath U, Chuang T, Ramesh A, Fletcher EL, Vessey KA. Vesicular expression and release of ATP from dopaminergic neurons of the mouse retina and midbrain. Front Cell Neurosci. 2015;9:389. doi: 10.3389/fncel.2015.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J Cereb Blood Flow Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo Y, Qi L, Lv XJ, Lai T, Zhang J, Zhang ZQ. A sensitive aptasensor for colorimetric detection of adenosine triphosphate based on the protective effect of ATP-aptamer complexes on unmodified gold nanoparticles. Biosens Bioelectron. 2016;78:315–320. doi: 10.1016/j.bios.2015.11.043. [DOI] [PubMed] [Google Scholar]
- Idzko M, Ferrari D, Eltzschig H. Nucleotide signalling during inflammation. Nature. 2014;509:310–317. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura H, Nhat KP, Togawa H, Saito K, Iino R, Kato-Yamada Y, Nagai T, Noji H. Visualization of ATP levels inside single living cells with fluorescence resonance energy transfer-based genetically encoded indicators. Proc Natl Acad Sci U S A. 2009;106:15651–15656. doi: 10.1073/pnas.0904764106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin JL, Irvin EM. The interaction of quinacrine with adenine nucleotides. J Biol Chem. 1954;210:45–56. [PubMed] [Google Scholar]
- Jahn K, Buschmann V, Hille C. Simultaneous fluorescence and phosphorescence lifetime imaging microscopy in living cells. Sci Rep. 2015;5:14334. doi: 10.1038/srep14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Fox NC, Sperling RA, Klunk WE. Brain imaging in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006213. doi: 10.1101/cshperspect.a006213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamerlin SCL, Sharma PK, Prasad RB, Warshel A. Why nature really chose phosphate. Q Rev Biophys. 2013;46:1–132. doi: 10.1017/S0033583512000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata H, Starkov AA, Manfredi G, Chinopoulos C. A kinetic assay of mitochondrial ADP-ATP exchange rate in permeabilized cells. Anal Biochem. 2010;407:52–57. doi: 10.1016/j.ab.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ, Pouli AE, Ainscow EK, Jouaville LS, Rizzuto R, Rutter GA. Glucose generates sub-plasma membrane ATP microdomains in single islet β-cells. J Biol Chem. 1999;274:13281–13291. doi: 10.1074/jbc.274.19.13281. [DOI] [PubMed] [Google Scholar]
- Khlyntseva SV, Bazel YR, Vishnikin AB, Andruch V. Methods for the determination of adenosine triphosphate and other adenine nucleotides. J Anal Chem. 2009;64:657–673. [Google Scholar]
- Kueng A, Kranz C, Mizaikoff B. Amperometric ATP biosensor based on polymer entrapped enzymes. Biosens Bioelectron. 2004;19:1301–1307. doi: 10.1016/j.bios.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Kurashima Y, Amiya T, Nochi T, Fujisawa K, Haraguchi T, Iba H, Tsutsui H, Sato S, Nakajima S, Iijima H, Kubo M, Kunisawa J, Kiyono H. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat Commun. 2012;3:1034. doi: 10.1038/ncomms2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaudet E, Hatz S, Droniou M, Dale N. Microelectrode biosensor for real-time measurement of ATP in biological tissue. Anal Chem. 2005;77:3267–3273. doi: 10.1021/ac048106q. [DOI] [PubMed] [Google Scholar]
- Lee MS, Park WS, Kim YH, Ahn WG, Kwon SH, Her S. Intracellular ATP assay of live cells using PTD-conjugated luciferase. Sensors (Basel) 2012;12:15628–15637. doi: 10.3390/s121115628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão JMM, Esteves da Silva JCG. Firefly luciferase inhibition. J Photochem Photobiol B: Biol. 2010;101:1–8. doi: 10.1016/j.jphotobiol.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Leyssens A, Nowicky AV, Patterson L, Crompton M, Duchen MR. The relationship between mitochondrial state, ATP hydrolysis, [Mg2+]i and [Ca2+]i studied in isolated rat cardiomyocytes. J Physiol. 1996;496:111–128. doi: 10.1113/jphysiol.1996.sp021669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Walczak P, Bulte JW. Comparison of red-shifted firefly luciferase Ppy RE9 and conventional Luc2 as bioluminescence imaging reporter genes for in vivo imaging of stem cells. J Biomed Opt. 2012;17:016004. doi: 10.1117/1.JBO.17.1.016004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin A. Optimization of the firefly luciferase reaction for analytical purposes. Adv Biochem Eng Biotechnol. 2014;145:31–62. doi: 10.1007/978-3-662-43619-6_2. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Maglica Ž, Özdemir E, McKinney JD. Single-cell tracking reveals antibiotic-induced changes in mycobacterial energy metabolism. MBio. 2015;6:e02236–02214. doi: 10.1128/mBio.02236-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi G, Yang L, Gajewski CD, Mattiazzi M. Measurement of ATP in mammalian cells. Methods. 2002;26:317–326. doi: 10.1016/S1046-2023(02)00037-3. [DOI] [PubMed] [Google Scholar]
- Mayers JR, Vander Heiden MG. Famine versus feast: understanding the metabolism of tumors in vivo. Trends Biochem Sci. 2015;40:130–140. doi: 10.1016/j.tibs.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mörikofer-Zwez S, Walter P. Binding of ADP to rat liver cytosolic proteins and its influence on the ratio of free ATP/free ADP. Biochem J. 1989;259:117–124. doi: 10.1042/bj2590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Meshik X, Choi M, Farid S, Datta D, Lan Y, Poduri S, Sarkar K, Baterdene U, Huang CE, Wang YY, Burke P, Dutta M, Stroscio MA. A Graphene and Aptamer Based Liquid Gated FET-Like Electrochemical Biosensor to Detect Adenosine Triphosphate. IEEE Trans Nanobioscience. 2015;14:967–972. doi: 10.1109/TNB.2015.2501364. [DOI] [PubMed] [Google Scholar]
- Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Vieira RP, Grimm M, Dürk T, Cicko S, Zeiser R, Jakob T, Martin SF, Blumenthal B, Sorichter S, Ferrari D, Di Virgillio F, Idzko M. A potential role for P2X7R in allergic airway inflammation in mice and humans. Am J Respir Cell Mol Biol. 2011;44:456–464. doi: 10.1165/rcmb.2010-0129OC. [DOI] [PubMed] [Google Scholar]
- Nakano M, Imamura H, Nagai T, Noji H. Ca2+ regulation of mitochondrial ATP synthesis visualized at the single cell level. ACS Chem Biol. 2011;6:709–715. doi: 10.1021/cb100313n. [DOI] [PubMed] [Google Scholar]
- Ozalp VC, Nielsen LJ, Olsen LF. An aptamer-based nanobiosensor for real-time measurements of ATP dynamics. Chembiochem. 2010;11:2538–2541. doi: 10.1002/cbic.201000500. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Ueda S, Imamura H, Mori K, Asanuma K, Yanagita M, Nakagawa T. Glycolysis, but not Mitochondria, responsible for intracellular ATP distribution in cortical area of podocytes. Sci Rep. 2015;5:18575. doi: 10.1038/srep18575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige JS, Nguyen-Duc T, Song W, Jaffrey SR. Fluorescence imaging of cellular metabolites with RNA. Science. 2012;335:1194. doi: 10.1126/science.1218298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak YL, Swamy KM, Yoon J. Recent Progress in Fluorescent Imaging Probes. Sensors (Basel) 2015;15:24374–24396. doi: 10.3390/s150924374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Byun JY, Shim WB, Kim SU, Kim MG. High-sensitivity detection of ATP using a localized surface plasmon resonance (LSPR) sensor and split aptamers. Biosens Bioelectron. 2015a;73:26–31. doi: 10.1016/j.bios.2015.05.043. [DOI] [PubMed] [Google Scholar]
- Park JW, Park Y, Kim BH. Quencher-free molecular aptamer beacons (QF-MABs) for detection of ATP. Bioorg Med Chem Lett. 2015b;25:4597–4600. doi: 10.1016/j.bmcl.2015.08.052. [DOI] [PubMed] [Google Scholar]
- Pedata F, Dettori I, Coppi E, Melani A, Fusco I, Corradetti R, Pugliese AM. Purinergic signalling in brain ischemia. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.11.007. In Press. [DOI] [PubMed] [Google Scholar]
- Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One. 2008;3:e2599. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plattner H, Verkhratsky A. Inseparable tandem: Evolution chooses ATP and Ca2+ to control life, death, and cellular signaling. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150419. doi: 10.1098/rstb.2015.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal. 2009;5:433–446. doi: 10.1007/s11302-009-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W, Hu H, Sun L, Li H, Xu D. Aptamer/Polydopamine Nanospheres Nanocomplex for in Situ Molecular Sensing in Living Cells. Anal Chem. 2015;87:12190–12196. doi: 10.1021/acs.analchem.5b03075. [DOI] [PubMed] [Google Scholar]
- Quinn KP, Sridharan GV, Hayden RS, Kaplan DL, Lee K, Georgakoudi I. Quantitative metabolic imaging using endogenous fluorescence to detect stem cell differentiation. Sci Rep. 2013;3:3432. doi: 10.1038/srep03432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Mintun MA. Brain work and brain imaging. Annu Rev Neurosci. 2006;29:449–476. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- Rangaraju V, Calloway N, Ryan TA. Activity-driven local ATP synthesis is required for synaptic function. Cell. 2014;156:825–835. doi: 10.1016/j.cell.2013.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda CB, Traba J, Amigo I, Llorente-Folch I, González-Sánchez P, Pardo B, Esteban JA, del Arco A, Satrústegui J. Mitochondrial ATP-Mg/Pi carrier SCaMC-3/Slc25a23 counteracts PARP-1-dependent fall in mitochondrial ATP caused by excitotoxic insults in neurons. J Neurosci. 2015;35:3566–3581. doi: 10.1523/JNEUROSCI.2702-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Chang YF, Horikawa K, Hatsugai N, Higuchi Y, Hashida M, Yoshida Y, Matsuda T, Arai Y, Nagai T. Luminescent proteins for high-speed single-cell and whole-body imaging. Nat Commun. 2012;3:1262. doi: 10.1038/ncomms2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto T, Webb MR, Forgacs E, White HD, Sellers JR. Direct observation of the mechanochemical coupling in myosin Va during processive movement. Nature. 2008;455:128–132. doi: 10.1038/nature07188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassanfar M, Szostak JW. An RNA motif that binds ATP. Nature. 1993;364:550–553. doi: 10.1038/364550a0. [DOI] [PubMed] [Google Scholar]
- Shin JB, Streijger F, Beynon A, Peters T, Gadzala L, McMillen D, Bystrom C, Van der Zee CE, Wallimann T, Gillespie PG. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron. 2007;53:371–386. doi: 10.1016/j.neuron.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shugar D. The NTP phosphate donor in kinase reactions: Is ATP a monopolist? Acta Biochim Pol. 1996;43:9–24. [PubMed] [Google Scholar]
- Song Q, Peng M, Wang L, He D, Ouyang J. A fluorescent aptasensor for amplified label-free detection of adenosine triphosphate based on core-shell Ag@SiO2 nanoparticles. Biosens Bioelectron. 2016;77:237–241. doi: 10.1016/j.bios.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Sun Y, Seo S, Provence S, Periasamy A, So P, Konig K. Comparison of FRET microscopy imaging techniques for studying protein-protein interactions in living cells using FRET standards. Proc SPIE 7569, Multiphoton Microscopy in the Biomedical Sciences X. 2010:75690Z. [Google Scholar]
- Surmeier DJ, Guzman JN, Sanchez J, Schumacker PT. Physiological phenotype and vulnerability in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009290. doi: 10.1101/cshperspect.a009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantama M, Hung YP, Yellen G. Imaging intracellular pH in live cells with a genetically encoded red fluorescent protein sensor. J Am Chem Soc. 2011;133:10034–10037. doi: 10.1021/ja202902d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantama M, Martínez-François JR, Mongeon R, Yellen G. Imaging energy status in live cells with a fluorescent biosensor of the intracellular ATP-to-ADP ratio. Nat Commun. 2013;4:2550. doi: 10.1038/ncomms3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantama M, Yellen G. Imaging changes in the cytosolic ATP-to-ADP ratio. Methods Enzymol. 2014;547:355–371. doi: 10.1016/B978-0-12-801415-8.00017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome N, Inglese J, Auld DS. Illuminating insights into firefly luciferase and other bioluminescent reporters used in chemical biology. Chem Biol. 2010;17:646–657. doi: 10.1016/j.chembiol.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toloe J, Mollajew R, Kügler S, Mironov SL. Metabolic differences in hippocampal ‘Rett’ neurons revealed by ATP imaging. Mol Cell Neurosci. 2014;59:47–56. doi: 10.1016/j.mcn.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Torabi SF, Lu Y. Functional DNA nanomaterials for sensing and imaging in living cells. Curr Opin Biotechnol. 2014;28:88–95. doi: 10.1016/j.copbio.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Lippiat JD, Ashcroft FM, Rutter GA. ATP-dependent interaction of the cytosolic domains of the inwardly rectifying K+ channel Kir6.2 revealed by fluorescence resonance energy transfer. Proc Natl Acad Sci U S A. 2004;101:76–81. doi: 10.1073/pnas.0306347101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle L, Vieyra FEM, Borsarelli CD. Hydrogen-bonding modulation of excited-state properties of flavins in a model of aqueous confined environment. Photochem Photobiol Sci. 2012;11:1051–1061. doi: 10.1039/c2pp05385c. [DOI] [PubMed] [Google Scholar]
- Vancraenenbroeck R, Webb MR. A Fluorescent, Reagentless Biosensor for ATP, Based on Malonyl-Coenzyme A Synthetase. ACS Chem Biol. 2015;10:2650–2657. doi: 10.1021/acschembio.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veech RL, Lawson JWR, Cornell NW, Krebs HA. Cytosolic phosphorylation potential. J Biol Chem. 1979;254:6538–6547. [PubMed] [Google Scholar]
- Wang C, Huang CY, Lin WC. Optical ATP biosensor for extracellular ATP measurement. Biosens Bioelectron. 2013;43:355–361. doi: 10.1016/j.bios.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Liao J, Yang X, Zhao M, Chen M, Yao W, Tan W, Lan X. A label-free aptasensor for highly sensitive detection of ATP and thrombin based on metal-enhanced PicoGreen fluorescence. Biosens Bioelectron. 2015;63:172–177. doi: 10.1016/j.bios.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang L, Li Z, Lin Y, Li J. In situ simultaneous monitoring of ATP and GTP using a graphen oxide nanosheet-based sensing platform in living cells. Nat Protoc. 2014;9:1944–1955. doi: 10.1038/nprot.2014.126. [DOI] [PubMed] [Google Scholar]
- Westheimer FH. Why nature chose phosphates. Science. 1987;235:1173–1178. doi: 10.1126/science.2434996. [DOI] [PubMed] [Google Scholar]
- Yaginuma H, Kawai S, Tabata KV, Tomiyama K, Kakizuka A, Komatsuzaki T, Noji H, Imamura H. Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci Rep. 2014;4:6522. doi: 10.1038/srep06522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadran S, Arumugam R, Herschman H, Phelps ME, Levine RD. Surprisal analysis characterizes the free energy time course of cancer cells undergoing epithelial-to-mesenchymal transition. Proc Natl Acad Sci U S A. 2014;111:13235–13240. doi: 10.1073/pnas.1414714111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadran S, Sanchez D, Zadran H, Amighi A, Otiniano E, Wong K. Enhanced-acceptor fluorescence-based single cell ATP biosensor monitors ATP in heterogeneous cancer populations in real time. Biotechnol Lett. 2013;35:175–180. doi: 10.1007/s10529-012-1065-6. [DOI] [PubMed] [Google Scholar]
- Zala D, Hinckelmann MV, Yu H, Lyra da Cunha MM, Liot G, Cordelières FP, Marco S, Saudou F. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Zhao T, Liu R, Ding X, Zhao J, Yu H, Wang L, Xu Q, Wang X, Lou X, He M, Xiao Y. Nanoprobe-Enhanced, Split Aptamer-Based Electrochemical Sandwich Assay for Ultrasensitive Detection of Small Molecules. Anal Chem. 2015;87:7712–7719. doi: 10.1021/acs.analchem.5b01178. [DOI] [PubMed] [Google Scholar]
- Zheng D, Seferos DS, Giljohann DA, Patel PC, Mirkin CA. Aptamer nano-flares for molecular detection in living cells. Nano Lett. 2009;9:3258–3261. doi: 10.1021/nl901517b. [DOI] [PMC free article] [PubMed] [Google Scholar]