Abstract
Objective
To describe and compare sudden unexpected infant death (SUID) investigations among states participating in the SUID Case Registry from 2010 through 2012.
Study design
We analyzed observational data from 770 SUID cases identified and entered into the National Child Death Review Case Reporting System. We examined data on autopsy and death scene investigation (DSI) components, including key information about the infant sleep environment. We calculated the percentage of components that were complete, incomplete, and missing/unknown.
Results
Most cases (98%) had a DSI. The DSI components most frequently reported as done were the narrative description of the circumstances (90%; range, 85%–99%), and witness interviews (88%, range, 85%–98%). Critical information about 10 infant sleep environment components was available for 85% of cases for all states combined. All 770 cases had an autopsy performed. The autopsy components most frequently reported as done were histology, microbiology, and other pathology (98%; range, 94%–100%) and toxicology (97%; range, 94%–100%).
Conclusions
This study serves as a baseline to understand the scope of infant death investigations in selected states. Standardized and comprehensive DSI and autopsy practices across jurisdictions and states may increase knowledge about SUID etiology and also lead to an improved understanding of the cause-specific SUID risk and protective factors. Additionally, these results demonstrate practices in the field showing what is feasible in these select states. We encourage pediatricians, forensic pathologists, and other medicolegal experts to use these findings to inform system changes and improvements in DSI and autopsy practices and SUID prevention efforts.
Death scene investigation (DSI) and autopsy findings provide essential information that may explain why some infants die suddenly and unexpectedly. There were 3434 reported sudden unexpected infant deaths (SUIDs) in the US in 2013, accounting for 14.6% of all infant deaths.1,2 Most SUIDs are unwitnessed, and autopsy findings alone are not usually enough to explain why these deaths occurred. A thorough DSI, including a detailed description of obstructions to the infant’s airway and potential hazards in the sleep environment, can help the death certifier distinguish between sudden infant death syndrome (SIDS) and other causes of death, such as accidental suffocation.3
Despite the critical role of the DSI and autopsy in determining cause of death, the importance of standardized protocols did not receive national attention until the 1980s. In 1986, Bass et al4 demonstrated that a DSI could inform the cause-of-death determination in cases of SUID. In 1989, an expert panel formally recognized the importance of the DSI and autopsy when they defined SIDS as “the sudden death of an infant under 1 year of age which remains unexplained after a thorough case investigation, including performance of a complete autopsy, examination of the death scene, and review of the clinical history.”3 In 1996, the Centers for Disease Control and Prevention (CDC) published guidelines and a reporting form, the Sudden Unexpected Infant Death Investigation Reporting Form (SUIDIRF), for the investigation of SUID.5,6 At the same time, several forensic pathologists from around the world developed an international SUID autopsy protocol.7 After the publication of the SUIDIRF and the international autopsy protocol (beginning around 1998), death certifiers reported fewer SIDS cases and attributed more cases to unknown causes and accidental suffocation.8,9 It is likely that improved case investigations influenced this shift in SIDS classification.9
Recognition of the importance of DSI and autopsy in the 1980s and protocol development in the 1990s did not result immediately in universal investigation practices. In 1992, although most SIDS cases (about 90%) had autopsies, the number of SIDS determinations that were made without a DSI was unknown. In addition, autopsy and DSI practices varied by jurisdiction and only 4 states had written protocols specific to SUID.10 By 2004, fewer than two-thirds of US medical examiner and coroner offices reported having a DSI (60.9%) or autopsy (63.9%) policy for SUID.11 As a result of these findings, in 2006 the CDC revised the 1996 SUIDIRF, developed educational materials,5,6,12,13 and conducted training that subsequently reached >23 000 medicolegal professionals.14 In 2007, the National Association of Medical Examiners outlined the “bare minimum” of a complete SUID death investigation.15 Despite the numerous efforts to improve SUID investigation practice, we are unaware of any formal evaluation to assess the extent of variation in DSI and autopsy practices across states and jurisdictions. Although death certificates typically document if an autopsy was completed, they do not capture whether a DSI was performed or which components of the autopsy and DSI were completed. The SUID Case Registry is a cooperative agreement between the CDC and funded grantees to enhance existing child death review (CDR) programs’ ability to conduct comprehensive population-based surveillance of SUID data, including information on DSI and autopsy practices. We describe and compare the frequency of DSI and autopsy performance practices for SUID cases in 7 states participating in the CDC’s SUID Case Registry.
Methods
We used data from the National Child Death Review Case Reporting System (NCDR-CRS), which captures information reported by CDR teams. Our retrospective review consisted of SUID cases reported by state and local CDR teams participating in the initial years of the SUID Case Registry. CDR and the SUID Case Registry programs and practices have been described elsewhere.16,17 Briefly, CDR teams in states participating in the SUID Case Registry gather and review information about SUID cases from multiple data sources, including death certificates, autopsy reports, coroner/medical examiner records, law enforcement reports, emergency medical services reports, photographs and reports from doll reenactments, child protective services records, hospital reports, medical records, death scene photos, and pathology reports. Multidisciplinary CDR teams summarize findings and recommendations from reviews and enter this information into the NCDR-CRS. Data then undergo quality assurance measures at the state level. Data use agreements with participating states allow us to use these data and ensure that the state’s data remain confidential.
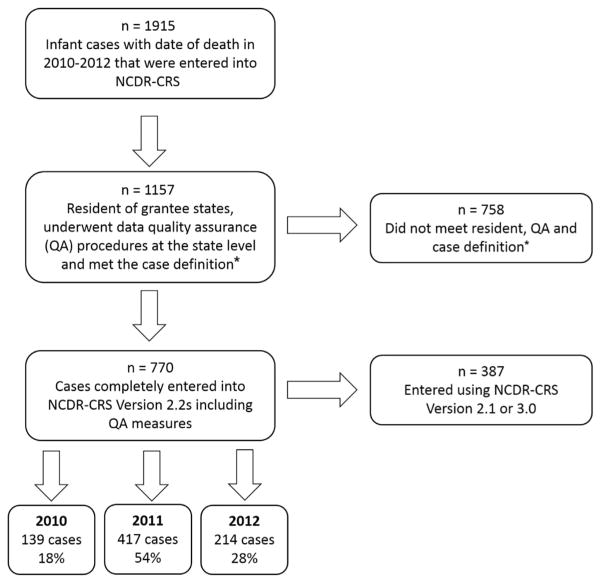
For this analysis, we selected SUID cases that occurred from 2010 through 2012 and were entered into the NCDR-CRS Version 2.2s (Figure; available at www.jpeds.com). Eligible cases were resident infant deaths (<365 days old) with any of the following causes reported on the death certificate: unknown, undetermined, SIDS, SUID, unintentional sleep-related asphyxia/suffocation/strangulation, unspecified suffocation, cardiac or respiratory arrest without other well-defined causes, or ill-defined causes with potentially contributing unsafe sleep factors, except if manner of death was reported as homicide. Case numbers varied across study years. Two states joined the SUID Case Registry in 2011 and therefore did not contribute any cases for 2010. Additionally, 2 states had not submitted the 2012 death year cohort at the time of this analysis. Our final sample included 770 cases (Figure).
Figure.
Description of the study population. *Cases that met the case definition were infant deaths (<365 days old) reported on the death certificate as cause unknown, undetermined, SIDS, SUID, unintentional sleep-related asphyxia/suffocation/strangulation, unspecified suffocation, cardiac or respiratory arrest without other well-defined causes, or ill-defined causes with potentially contributing unsafe sleep factors, except if manner of death was reported as homicide.
We examined variables related to DSI, infant sleep environment, and autopsy available in the NCDR-CRS Version 2.2s. These variables are described in the NCDR-CRS data dictionary.18 Most study variables were derived from single questions in the NCDR-CRS. For the remaining study variables, we created composite variables by combining multiple questions (Table I).
Table I.
Variable descriptions
| Study variables | Question in the NCDR-CRS | Valid responses |
|---|---|---|
| DSI | ||
| Performed | DSI performed? | Yes |
| Agencies that conducted a scene investigation? | Medical examiner, coroner, medical examiner investigator, coroner investigator, law enforcement, fire investigator, emergency medical services, child protective services, other, unknown | |
| For infants, which of the following data sources were available at the review? | CDC’s SUIDI reporting form, jurisdictional equivalent of the CDC SUIDI reporting form | |
| SUIDIRF or a jurisdictional equivalent | For infants, which of the following data sources were available at the review? | CDC’s SUIDI reporting form, jurisdictional equivalent of the CDC SUIDI reporting form |
| If a DSI was performed, which of the following DSI components were completed? | CDC’s SUIDI reporting form or jurisdictional equivalent | |
| Narrative description of circumstances | If a DSI was performed, which of the following DSI components were completed? | Narrative description of circumstances completed |
| Scene photos | If a DSI was performed, which of the following DSI components were completed? | Scene photos |
| Any scene re-creation | If a DSI was performed, which of the following DSI components were completed? | Scene re-creation with doll, scene recreation without doll |
| Scene re-creation with a doll | If a DSI was performed, which of the following DSI components were completed? | Scene re-creation with doll |
| Witness interviews | If a DSI was performed, which of the following DSI components were completed? | Witness interviews |
| Infant sleep environment | ||
| Incident sleep place | Incident sleep place: | Crib, bassinette, adult bed, waterbed, playpen/other play structure but no portable crib, couch, chair, floor, car seat, stroller, other |
| Position placed | Child put to sleep: | On back, on stomach, on side |
| Position found | Child found: | On back, on stomach, on side |
| Usual sleep place | Usual sleep place: | On back, on stomach, on side |
| Crib, bassinette, or port-a-crib in the home | Was there a crib, bassinette, or port-a-crib in the home for child? | Yes, no |
| New or different environment than usual | Child in a new or different environment than usual? | Yes, no |
| Airway when found | Child’s airway was: | Unobstructed by person or object, fully obstructed by person or object, partially obstructed by person or object |
| Caregiver/supervisor fell asleep while feeding child | Caregiver/supervisor fell asleep while feeding child? | Yes, no |
| Same room as caregiver/supervisor at time of death | Child sleeping in the same room as caregiver/supervisor at time of death? | Yes, no |
| Same surface with person(s) or animal(s) | Child on same surface with person(s) or animal(s)? | Yes, no |
| Autopsy | ||
| Autopsy performed | Autopsy performed? | Yes |
| If investigation found evidence of abuse, from what source? | From autopsy | |
| For infants, which of the following data sources were available at the review? | Autopsy/pathology reports | |
| Toxicology screen? | Yes | |
| For infants, histology conducted? | Yes | |
| For infants, blood chemistry conducted? | Yes | |
| Radiographs taken? | Yes | |
| For infants, microbiology conducted? | Yes | |
| For infants, other pathology conducted? | Yes | |
| Toxicology | Toxicology screen? | Yes |
| If autopsy performed, were the following assessed in the autopsy? | Routine toxicology for ethanol, sedatives and/or stimulants, toxicology for suspected drugs if investigation suspects exposure | |
| Microbiology, histology, and other pathology | If autopsy performed, were the following assessed in the autopsy? | Microbiology, microscopic examination of brain, heart, lung, airway, or liver |
| For infants, microbiology conducted? | Yes | |
| For infants, histology conducted? | Yes | |
| For infants, other pathology conducted? | Yes | |
| Blood chemistry | For infants, blood chemistry conducted? | Yes |
| Radiograph | Radiographs taken? | Yes |
| If autopsy performed, were the following assessed in the autopsy? | Radiograph-single, radiograph-skeletal series, CT scan | |
| Genetic testing | If autopsy performed, were the following assessed in the autopsy? | Genetic testing |
| Metabolic testing | If autopsy performed, were the following assessed in the autopsy? | Metabolic screening |
Statistical Analyses
We calculated the percentage complete, incomplete, and missing/unknown for each DSI and autopsy component. For the sleep environment variables, we calculated the percentage of cases with documented information about each selected sleep environment component. Data about the infant sleep environment was reported for the 759 cases that occurred in a sleep environment. For all analyses, we examined each state and component individually and all states and components combined. Because we aimed to compare differences among states and not to identify lower performing states, we represent states with letters (A–G) and do not reveal the numbers of SUID cases or type of medicolegal system by state to maintain confidentiality per our data use agreements with the participating states.
Results
Based on the composite variable for DSI performed, 98% of cases had a DSI, ranging from 100% in states A, E, and G to 95% in state F (Table II). For all states combined, narrative description of the circumstances (90%) and witness interviews (88%) were reported most frequently. The SUIDIRF or jurisdictional equivalent (71%), scene photos (68%), any scene re-creation (45%), and scene re-creation with a doll (37%) were reported less frequently. The SUIDIRF or jurisdictional equivalent had the largest range when compared across states; ranging from 100% completion in state E to 24% completion in state A. Narrative description of circumstances had the narrowest range, from 99% completion in state A to 85% completion in state C. Variation in the percentages of DSI components reported as complete was observed depending on the state’s type of medicolegal system.
Table II.
Percentage of cases with a DSI and autopsies performed and percent of DSI and autopsy components performed, stratified by state, 2010–2012
| State | A | B | C | D | E | F | G | Total (N = 770) |
|---|---|---|---|---|---|---|---|---|
| DSI performed | ||||||||
| Yes | 100 | 98 | 98 | 96 | 100 | 95 | 100 | 98 |
| No | 0 | 1 | 2 | 4 | 0 | 5 | 0 | 2 |
| Missing/unknown | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
|
If DSI completed, components of the DSI performed
| ||||||||
| State | A | B | C | D | E | F | G | Total (N = 753) |
|
| ||||||||
| SUIDIRF or jurisdictional equivalent | ||||||||
| Yes | 24 | 87 | 70 | 62 | 100 | 88 | 80 | 71 |
| No | 18 | 5 | 5 | 32 | 0 | 11 | 20 | 10 |
| Missing/unknown | 58 | 8 | 25 | 6 | 0 | 1 | 0 | 18 |
| Narrative description of circumstances | ||||||||
| Yes | 99 | 90 | 85 | 89 | 92 | 92 | 97 | 90 |
| No | 0 | 1 | 1 | 6 | 0 | 2 | 2 | 1 |
| Missing/unknown | 1 | 9 | 14 | 4 | 8 | 6 | 2 | 8 |
| Scene photos | ||||||||
| Yes | 70 | 62 | 66 | 62 | 92 | 63 | 95 | 68 |
| No | 7 | 8 | 7 | 34 | 0 | 26 | 3 | 11 |
| Missing/unknown | 23 | 30 | 27 | 4 | 8 | 11 | 2 | 21 |
| Any scene re-creation | ||||||||
| Yes | 20 | 33 | 65 | 49 | 83 | 20 | 73 | 45 |
| No | 71 | 34 | 9 | 49 | 8 | 65 | 25 | 35 |
| Missing/unknown | 9 | 34 | 27 | 2 | 8 | 14 | 2 | 21 |
| Scene re-creation with a doll | ||||||||
| Yes | 13 | 27 | 56 | 43 | 58 | 19 | 56 | 37 |
| No | 77 | 39 | 16 | 55 | 33 | 66 | 42 | 41 |
| Missing/unknown | 10 | 34 | 29 | 2 | 8 | 14 | 2 | 22 |
| Witness interviews | ||||||||
| Yes | 97 | 85 | 85 | 92 | 92 | 85 | 98 | 88 |
| No | 1 | 1 | 3 | 6 | 0 | 6 | 0 | 2 |
| Missing/unknown | 2 | 14 | 12 | 2 | 8 | 9 | 2 | 9 |
| All DSI components* | ||||||||
| Yes | 62 | 71 | 74 | 71 | 9 | 70 | 89 | 72 |
| No | 19 | 10 | 5 | 25 | 2 | 22 | 10 | 12 |
| Missing/unknown | 19 | 19 | 21 | 4 | 6 | 8 | 2 | 15 |
|
| ||||||||
| State | A | B | C | D | E | F | G | Total (N = 770) |
|
| ||||||||
| Autopsy performed | ||||||||
| Yes | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| No | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Missing/unknown | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||
|
If autopsy completed, components of the autopsy performed
| ||||||||
| State | A | B | C | D | E | F | G | Total (N = 770) |
|
| ||||||||
| Toxicology | ||||||||
| Yes | 99 | 99 | 94 | 100 | 100 | 98 | 100 | 97 |
| No | 1 | 1 | 4 | 0 | 0 | 2 | 0 | 2 |
| Missing/unknown | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 1 |
| Microbiology, histology, and other pathology | ||||||||
| Yes | 98 | 99 | 97 | 100 | 100 | 94 | 98 | 98 |
| No | 2 | 0 | 1 | 0 | 0 | 6 | 0 | 1 |
| Missing/unknown | 0 | 1 | 2 | 0 | 0 | 0 | 2 | 1 |
| Blood chemistry | ||||||||
| Yes | 10 | 37 | 42 | 86 | 100 | 17 | 95 | 41 |
| No | 35 | 60 | 45 | 8 | 0 | 81 | 2 | 46 |
| Missing/unknown | 56 | 4 | 13 | 6 | 0 | 3 | 3 | 14 |
| Radiograph | ||||||||
| Yes | 62 | 96 | 85 | 98 | 100 | 84 | 100 | 87 |
| No | 11 | 3 | 10 | 2 | 0 | 16 | 0 | 7 |
| Missing/unknown | 27 | 1 | 6 | 0 | 0 | 1 | 0 | 6 |
| Genetic testing | ||||||||
| Yes | 0 | 68 | 6 | 12 | 33 | 3 | 32 | 23 |
| No | 27 | 27 | 88 | 88 | 67 | 94 | 64 | 63 |
| Missing/unknown | 73 | 6 | 6 | 0 | 0 | 3 | 3 | 14 |
| Metabolic testing | ||||||||
| Yes | 31 | 88 | 65 | 82 | 100 | 66 | 98 | 71 |
| No | 14 | 10 | 28 | 14 | 0 | 26 | 0 | 18 |
| Missing/unknown | 56 | 3 | 7 | 4 | 0 | 8 | 2 | 12 |
| All autopsy components | ||||||||
| Yes | 50 | 81 | 65 | 80 | 89 | 60 | 87 | 69 |
| No | 15 | 17 | 29 | 19 | 11 | 38 | 11 | 23 |
| Missing/unknown | 35 | 3 | 6 | 2 | 0 | 3 | 2 | 8 |
Note: Groups not equal to 100% are due to rounding. States are represented by letters to maintain confidentiality.
Includes all variables in table except for scene re-creation with a doll because this variable is a subset of any scene re-creation.
Within each state, the consistency of reporting across DSI components varied. State E had the highest percentages of reported DSI components, ranging from 100% completion of the SUIDIRF or jurisdictional equivalent to 58% completion of a scene re-creation with a doll. State A had the lowest percentages and the most variation in reported DSI components, ranging from 99% completion of a narrative description of circumstances to 13% completion for scene re-creation with a doll. For all states and DSI components combined, the average missing/unknown was 15%, ranging from state A with 58% missing/unknown for SUIDIRF or jurisdictional equivalent to states E and G with 0% missing/unknown for SUIDIRF or jurisdictional equivalent. States B and C had the highest average missing/unknown for all DSI components combined (22%).
Sleep Environment
The majority of cases (98%) occurred in a sleep environment (Table III). Information about the infant sleep environment was available 85% of the time for all variables and states combined. Incident sleep place (98%), same surface with person(s) or animal(s) (96%), caregiver/supervisor fell asleep while feeding child (94%), new or different sleep environment than usual (92%), position found (86%), position placed (85%), and crib, bassinette, or port-a-crib in home (81%) were reported as available most frequently for all states combined. Usual sleep place (73%), airway when found (73%), and usual sleep position (63%) were reported as available least frequently for all states combined. Airway when found had the most variation, ranging from 95% availability in state G to 35% availability in state F. Incident sleep place, caregiver/supervisor fell asleep while feeding child, and same surface with person(s) or animal(s) had the least variation, ranging from 100% to 90%.
Table III.
Percentage of cases reporting information about sleep environment among deaths that occurred in the sleep environment, stratified by state,* 2010–2012
| State | A | B | C | D | E | F | G | Total (N = 759) |
|---|---|---|---|---|---|---|---|---|
| Incident sleep place | 100 | 100 | 98 | 100 | 100 | 90 | 98 | 98 |
| Position placed | 78 | 88 | 85 | 85 | 92 | 76 | 97 | 85 |
| Position found | 86 | 89 | 84 | 87 | 92 | 76 | 97 | 86 |
| Usual sleep place | 71 | 67 | 79 | 77 | 92 | 53 | 97 | 73 |
| Usual sleep position | 60 | 58 | 70 | 53 | 83 | 44 | 97 | 63 |
| Crib, bassinette, or port-a-crib in the home | 88 | 71 | 88 | 68 | 75 | 78 | 93 | 81 |
| New or different environment than usual | 99 | 91 | 93 | 98 | 100 | 75 | 97 | 92 |
| Airway when found | 78 | 79 | 72 | 85 | 58 | 35 | 95 | 73 |
| Caregiver/supervisor fell asleep while feeding child | 98 | 94 | 90 | 98 | 100 | 91 | 98 | 94 |
| Same room as caregiver/supervisor at time of death | 99 | 88 | 93 | 98 | 100 | 90 | 98 | 93 |
| Same surface with person(s) or animal(s) | 98 | 97 | 95 | 100 | 100 | 90 | 98 | 96 |
| Total | 87 | 84 | 86 | 86 | 90 | 72 | 97 | 85 |
Note: Groups not equal to 100% are due to rounding. States are represented by letters to maintain confidentiality.
Cases that did not occur in a sleeping environment were excluded from this analysis (n = 11).
Within each state, the availability of information about the infant sleep environment varied. State G had the highest percentage of available information. State F had the lowest percentage of available data and the largest range between variables, from 91% for caregiver/supervisor fell asleep while feeding child to 35% for airway when found.
Autopsy
All cases (100%) had an autopsy performed as indicated by the composite variable (Table II). Among autopsy components, histology, microbiology and other pathology (98%), toxicology (97%), and radiographs (87%) were performed most frequently for all states combined. Metabolic testing (71%), blood chemistry (41%), and genetic testing (23%) were performed less frequently. Blood chemistry had the largest range when comparing states, from 100% completion in state E to 10% completion in state A. Toxicology, and microbiology, histology, and other pathology had the narrowest range, from 100% to 94%. We observed variation in the percentages of autopsy components reported as complete depending on the state’s type of medicolegal system.
Within each state, the consistency in reporting varied across autopsy components. State E had the highest percentages of reported autopsy components; all components were reported 100% of the time with the exception of genetic testing (33%). State A had the lowest percentages of reported autopsy components, the highest average missing/unknown (35%), and the greatest range, from 99% completion of toxicology to 0% completion of genetic testing. For all states, 8% of the combined autopsy components were missing/unknown. Multiple states reported several autopsy components with 0% missing/unknown. Genetic testing in state A had the highest missing/unknown (73%).
Discussion
Implementation of thorough and consistent DSI and autopsy practices increases understanding of the circumstances surrounding SUID and improves accuracy in diagnosing the causes of SUID. Having reliable and accurate data about SUID cases improves the ability to monitor trends and develop effective prevention strategies, ultimately leading to a decrease in SUID. This report is a baseline study to understand the scope of infant death investigations in selected US states. We found that almost all SUID cases had an autopsy (100%) and DSI (98%) performed, but that the components of the investigation varied. The autopsy components conducted least often were blood chemistry (41%) and genetic testing (23%). However, these tests may only be conducted as needed and depending on the circumstances.15 The perceived usefulness of tests influence actual performance of testing, as highlighted in a recent study about infectious disease testing for SUID.19 The DSI components conducted least often include usual sleep place (73%) and usual sleep position (63%). Changes in sleep place and position are associated with an increased risk of SIDS,20 making usual sleep place and position important variables to document. However, the variable new or different environment than usual was available for 92% of cases and may be used by death certifiers when usual sleep place and position are unavailable.
Although the performance of a DSI and an autopsy were nearly universal in our study, a marked improvement from earlier decades,10 we note that there were still some DSI components for which the percentage of performance is not optimal. Any scene re-creation (45%), especially one with a doll (37%), was available for fewer than one-half of cases and can be an integral part in understanding airway obstruction and the role of potential hazards in the sleep environment.6,15 Additionally, there was a lack of information about the infant’s airway when found, available for 73% of cases, which is also important in understanding the circumstance surrounding the death. This information may be unavailable because parents or emergency medical services personnel move the infant quickly without noting the infant’s position.15,20
Because the analysis of missing/unknown responses revealed high data quality (ie, few missing and unknown responses), observed variations in reporting performance of DSI and autopsy components between states likely reflect true practice. The data used in these analyses are compiled from various sources, and are not collected through primary data collection. As such, understanding the reasons for the variations in reporting and missing/unknown responses are outside of the scope of these data but could be attributed to a number of factors, for example, differing protocols for autopsies and DSIs, variation in the capacity of the CDR teams collecting and entering data, availability of resources for investigations, and differences in medicolegal systems. Study states represent county coroner systems, county medical examiner systems, regional offices overseen by a state medical examiner, combined county medical examiner and coroner systems, state medical examiner systems, and combined state and county medical examiner systems.21 Although slight variation was observed in the percentages of autopsy components reported depending on the type of medicolegal system, this study shows that documenting complete and comprehensive autopsies and DSIs is feasible across all types of systems.
A limitation is that our study only represents 7 states and may lack generalizability to the US as a whole. In addition, we relied on information reported by state and local CDR teams, and data have not been validated. However, it is likely that reported data were accurate because most reported information was based on abstracted and summarized data from several sources and discussions among multidisciplinary professionals who often had first-hand knowledge of the cases. Moreover, our ability to accurately evaluate the completeness of DSI and autopsy components was improved because we created composite variables considering responses to several related questions. Additionally, if there were reporting biases, we would anticipate that DSI and autopsy components were underreported, because it is more likely that a component was conducted and not documented as opposed to documented and not conducted.
Because SUID Case Registry grantees evaluate the gaps in case investigations, they work with CDR teams and medicolegal professionals to implement activities to improve DSI and autopsy consistency and comprehensiveness. For example, some grantees provide resources to medical examiner offices to investigate and review SUID cases. Other grantees facilitate trainings for medical examiners, coroners, and death investigators to emphasize what data are important to document at an infant death scene, how to complete the SUIDIRF or jurisdictional equivalent, and how to perform a scene re-creation using a doll. Grantees have also distributed DSI kits with dolls and cameras for documenting scene re-creations. These tools allow investigators to record the most important information from the death scene, including the infant’s airway when found. Future studies using the SUID Case Registry data could monitor the impact of these activities on progress toward improving practices.
The importance of the DSI and autopsy in determining cause of death is well-established and much work has been done to improve case investigation practices. This study serves as a baseline to understand the scope of infant death investigations in selected states. Standardized and comprehensive DSI and autopsy practices across jurisdictions and states may increase knowledge about SUID etiology and lead to improved understanding of cause-specific SUID risk and protective factors. Additionally, these data, representing a variety of medicolegal systems, demonstrate that documenting complete and comprehensive autopsies and DSIs is feasible across many systems. We encourage pediatricians, forensic pathologists, and other medicolegal experts who review these findings to use them to inform system changes and improvements in DSI and autopsy practices and SUID prevention efforts. To improve practices, the medicolegal community could work to ensure that comprehensive protocols are further developed, such that a gold standard becomes universally accepted and implemented. There is a need to identify DSI and autopsy practices that are most effective and have the strongest influence on explaining why SUID occurs.22 In determining feasibility, one must consider available resources (eg, budget, staffing, equipment, and training needs).23 Future studies using the SUID Case Registry data can monitor resulting progress in improving standardized practices.
Acknowledgments
T.C.’s agency, the Michigan Public Health Institute, was supported by the Maternal and Child Infant Health Bureau of the Health Resources and Services Administration and the Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, US Centers for Disease Control and Prevention. A.E.L. was supported by a contract between DB Consulting and the Division of Reproductive Health, National Center for Chronic Disease Prevention and Health Promotion, US Centers for Disease Control and Prevention (200-2010-37208).
We acknowledge the National Center for the Review and Prevention of Child Death and the Child Death Review coordinators in the participating states (Colorado, Georgia, Michigan, Minnesota, New Hampshire, New Jersey, and New Mexico) for their support of this project by allowing access to the data contained in this report.
Glossary
- CDC
Centers for Disease Control and Prevention
- CDR
Child death review
- DSI
Death scene investigation
- NCDR-CRS
National Child Death Review-Case Reporting System
- SIDS
Sudden infant death syndrome
- SUID
Sudden unexpected infant death
- SUIDIRF
Sudden Unexpected Infant Death Investigation Reporting Form
Footnotes
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors declare no conflicts of interest.
References
- 1.Matthews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl Vital Stat Rep. 2015;64:1–30. [PubMed] [Google Scholar]
- 2. [Accessed September 1, 2015];Compressed Mortality File 1999–2013 on CDC WONDER Online Database. 2014 Oct; database on the Internet http://wonder.cdc.gov/cmf-icd10.html.
- 3.Willinger M, James LS, Catz C. Defining the sudden infant death syndrome (SIDS): deliberations of an expert panel convened by the National Institute of Child Health and Human Development. Pediatr Pathol. 1991;11:677–84. doi: 10.3109/15513819109065465. [DOI] [PubMed] [Google Scholar]
- 4.Bass M, Kravath RE, Glass L. Death-scene investigation in sudden infant death. N Engl J Med. 1986;315:100–5. doi: 10.1056/NEJM198607103150206. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. [Accessed November 1, 2015];Sudden Unexplained Infant Death Investigation Reporting Form. 2007 www.cdc.gov/sids/pdf/suidi-form2-1-2010.pdf.
- 6.Centers for Disease Control and Prevention. Hanzlick R, Jentzen J. [Accessed November 1, 2015];Sudden unexplained infant death investigation: Guidelines for the scene investigator. 2007 http://www.cdc.gov/sids/pdf/508suidiguidelinessingles_tag508.pdf.
- 7.Krous HF International Standardized Autopsy Protocol Committee of the Global Strategy Task Force. The international standardized autopsy protocol for sudden unexpected infant death. J Sudden Infant Death Syndrome Infant Mortal. 1996;1:203–46. [Google Scholar]
- 8.Malloy MH, MacDorman M. Changes in the classification of sudden unexpected infant deaths: United States, 1992–2001. Pediatrics. 2005;115:1247–53. doi: 10.1542/peds.2004-2188. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro-Mendoza CK, Tomashek KM, Anderson RN, Wingo J. Recent national trends in sudden, unexpected infant deaths: more evidence supporting a change in classification or reporting. Am J Epidemiol. 2006;163:762–9. doi: 10.1093/aje/kwj117. [DOI] [PubMed] [Google Scholar]
- 10.Combs DL, Parrish RG, Ing RT. Death investigation in the United States and Canada. Atlanta: US Department of Health and Human Services, Public Health Service, CDC; 1992. [Google Scholar]
- 11.Camperlengo LT, Shapiro-Mendoza CK, Kim SY. Sudden infant death syndrome: diagnostic practices and investigative policies, 2004. Am J Forensic Med Pathol. 2012;33:197–201. doi: 10.1097/PAF.0b013e3181fe33bd. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention’s National Steering Committee and Development Core Team for Sudden Unexplained Infant Death Investigation. [Accessed November 1, 2015];Sudden, unexplained infant death investigation: Curriculum guide. 2007 http://www.cdc.gov/sids/pdf/curriculumguide_tag508.pdf.
- 13.Centers for Disease Control and Prevention’s National Steering Committee and Development Core Team for Sudden Unexplained Infant Death Investigation. [Accessed November 1, 2015];Sudden unexplained infant death investigation: A systematic training program for the professional infant death investigation specialist. 2007 http://www.suidi.org/training/SUIDITextbook.pdf.
- 14.Camperlengo L, Shapiro-Mendoza CK, Gibbs F. Improving sudden unexplained infant death investigation practices: an evaluation of the Centers for Disease Control and Prevention’s SUID Investigation Training Academies. Am J Forensic Med Pathol. 2014;35:278–82. doi: 10.1097/PAF.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 15.Corey TS, Hanzlick R, Howard J, Nelson C, Krous H NAME Ad Hoc Committee on Sudden Unexplained Infant Death. A functional approach to sudden unexplained infant deaths. Am J Forensic Med Pathol. 2007;28:271–7. doi: 10.1097/01.paf.0000257385.25803.cf. [DOI] [PubMed] [Google Scholar]
- 16.Covington TM. The US National Child Death review case reporting system. Inj Prev. 2011;17(Suppl 1):i34–7. doi: 10.1136/ip.2010.031203. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro-Mendoza CK, Camperlengo LT, Kim SY, Covington T. The sudden unexpected infant death case registry: a method to improve surveillance. Pediatrics. 2012;129:e486–93. doi: 10.1542/peds.2011-0854. [DOI] [PubMed] [Google Scholar]
- 18.The National Center for the Review and Prevention of Child Death. [Accessed November 1, 2015];Child Death Review case reporting system data dictionary version 2.2S. 2011 https://cdrdata.org/Account/Login?ReturnUrl=%2F.
- 19.Brooks EG, Gill JR National Association of Medical Examiners, NAME Ad Hoc Committee for Bioterrorism Infectious Disease. Testing for infectious diseases in sudden unexpected infant death: a survey of medical examiner and coroner offices in the United States. J Pediatr. 2015;167:178–82. e1. doi: 10.1016/j.jpeds.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Pasquale-Styles MA, Tackitt PL, Schmidt CJ. Infant death scene investigation and the assessment of potential risk factors for asphyxia: a review of 209 sudden unexpected infant deaths. J Forensic Sci. 2007;52:924–9. doi: 10.1111/j.1556-4029.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 21.Randy L, Hanzlick M. A Perspective on medicolegal death investigation in the United States: 2013. Acad Forensic Pathol. 2014;4:2–9. [Google Scholar]
- 22.Garstang J, Ellis C, Sidebotham P. An evidence-based guide to the investigation of sudden unexpected death in infancy. Forensic Sci Med Pathol. 2015;11:345–57. doi: 10.1007/s12024-015-9680-x. [DOI] [PubMed] [Google Scholar]
- 23.Committee on Identifying the Needs of the Forensic Science Community and National Research Council of The National Academies. Strengthening forensic science in the United States: A path forward. Washington (DC): The National Academies Press; 2009. [Google Scholar]